
Correlation of pulmonary venous circulating tumor cells with clinicopathological parameters in patients with early-stage lung adenocarcinoma
Introduction
Lung cancer (LCa) is one of the most common global malignancies and a leading cause of cancer related death (1). Most LCa patients are diagnosed when tumor metastasis has already occurred, and despite curative treatment, postoperative metastases and recurrence are frequent. When diagnosed during the early stages (stage I), ~25–50% of patients experience recurrence or distant metastasis within 5 years (2,3). The progression of early-stage LCa is distinct and varied from micro to occult metastases and recurrence. Although serologic tumor markers, clinicopathological parameters, and radiologic modalities are commonly used in the clinical management of LCa patients, disease progression, surveillance, and therapeutic responses are difficult to monitor (4-6). In this regard, the identification of novel biomarkers for non-invasive LCa detection would permit the integration of tumor prediction and real-time surveillance, improving therapeutic and clinical outcomes.
Accumulating evidence indicates that circulating tumor cells (CTCs) from primary tumors serve as a primary driving force for tumor progression, metastasis, and chemotherapy resistance (7-12). CTCs can be detected in most solid tumors and correlate with prognosis, tumor metastasis, and relapse (13-19). To-date, CTC detection has been employed in colon cancer, breast cancer and prostate cancer studies (13-17). Despite their promising clinical relevance, only 23.7% of non-small cell lung cancer (NSCLC) patients exhibit positive CTC detection (>1 per 7.5 mL of blood) in Chinese and Western populations (20). CTCs were also undetectable in the peripheral blood (PB) of stage I LCa patients.
The analysis of CTC counts in PB vs. pulmonary venous blood (PVB) demonstrated higher numbers of CTCs from PVB samples, implying a higher sensitivity of CTC detection for early stage LCa (6,21,22). In this study, we prepared CTCs from PB and PVB in cohort of 120 subjects containing 24 healthy controls (collecting PB), 36 patients with benign lung tumors (collecting intraoperative PVB) and 60 early-stage lung adenocarcinoma (ESLA) patients (collecting intraoperative PVB), to evaluate the association between CTC counts and clinicopathological features. Herein, we highlight PVB CTCs as biomarkers for the real-time surveillance of LCa progression and therapy.
Methods
Patients and sample collection
A total of 60 early-stage lung adenocarcinoma patients diagnosed in our hospital from January 2017 to December 2017 were enrolled. The diagnosis of early-stage lung adenocarcinoma was based on the Guidelines on the Clinical Management of LCa (2017 edition). There were 32 males and 28 females, aged 37–77 years (median =55 years). Pathological stages were determined according to current tumor-node-metastasis (TNM) classification as revised in 2018 (23). Pathological staging included 10 cases in stage IA1, 18 cases in stage IA2, and 32 cases in stage IA3. The degree of infiltration was classified into adenocarcinoma in situ, micro-invasive adenocarcinoma, and invasive adenocarcinoma. From biopsy specimens, pathological cell types were classified according to the 2015 WHO classification criteria. Pathological cell morphology was divided into wall-like, acinar, micro-papillary, and papillary. The association between immunohistochemical staining and genetic mutations in Napsin A, TTF-1, Ki67 and epidermal growth factor receptor (EGFR) were also assessed.
The patients underwent lung resection via open thoracotomy or video-assisted thoracoscopic surgery, with wedge resection followed by lobectomy. PVB was drained from the affected lobe which was isolated using minimal manipulation of the lung. Up to 10 mL of PVB was removed using a 22-gauge needle attached to a 10 mL syringe (22-gauge spinal needle was employed for video-assisted thoracoscopic surgery) prior to removal of the lesion (intraoperative PV). Heparin was added to all blood samples to prevent coagulation and cancer cells were detected within 2 h of collection. PB samples were collected from the control group.
Enrichment and identification of early-stage lung adenocarcinoma patients CTCs
The enrichment of early-stage lung adenocarcinoma CTCs was performed as previously described (24,25) in a blood volume of 3.2 mL. Briefly, red blood cells (RBCs) in the PVB samples were lysed, and residual cell pellets were resuspended in PBS. Samples were labeled with anti-leukocyte-specific antibodies (CD45) monoclonal antibody coated magnetic beads for 30 min, followed by separation of the beads using a magnetic stand. LCa CTCs were identified by CD45-FISH which combined FISH labeling with chromosome 8 centromere probes and anti-CD45 monoclonal antibodies. Briefly, specimens were hybridized with human chromosome 8 specific sequence (CEP8) probes at 37 °C for 20 min and washed in 50% formamide at 43 °C for 20 min. Samples were immersed into 2 × SSC gradient alcohol, and washed twice with 0.2% bovine serum albumin (BSA). Specimens were labeled with anti-CD45 antibodies conjugated to Alexa Fluor 594 in 2% BSA for 1.5 h. Specimens were washed in 0.2% BSA and mounted in Vectashield containing 4',6-diamidino-2-phenylindole (DAPI). Fixed samples were imaged along the ‘‘S’’ track. For the classification of positive CTCs, cells must be hyperdiploid and CEP8+/DAPI+/CD45‒.
CTC quantification and positive cut off values
CTCs ≥2 suggested the presence of malignant cells, suspected malignant lesions, and a potential risk of metastasis or distant metastasis (25). CTCs <2 suggested normal levels and that the nature of the lesion was benign. We referred to existing clinical diagnosis and treatment standards for all comprehensive assessments (26).
Primary tissue collection, genotyping of EGFR gene mutations (ARMS)-polymerase chain reaction (PCR) assays
DNA was extracted from formalin-fixed, paraffin-embedded lung tumor tissues using QIAamp DNA FFPE Tissue Kits. EGFR mutations were detected using the AmoyDx Human EGFR Gene 29 mutation detection kit with fluorescence PCR. Assays were performed on an ABI7500 real-time PCR instrument. Primers were labeled with 6-carboxyfluorescein and HEX. The EGFR kit detects 29 mutations in exons 18 to 21, including T790M, L858R, L861Q, S768I, G719S, G719A, and G719C; three insertions in exon 20; and 19 deletions in exon 19. DNA was PCR amplified in a final volume of 25 µL. PCR reactions contained 5 µL of DNA, 25 mmol/L MgCl2, 25 mmol/L dNTP, 100 µmol/L of forward and reverse primers, 10 X Takara buffer, and 5 U/µL Takara HS-Taq. The first cycle of amplifications was performed with a 5 min initial denaturation at 95, followed by 30 cycles of 45 s at 95 °C, 45 s at 54 °C, 1 min at 72 °C, and a 6 min final extension at 72 °C. Products from the first cycle were amplified in secondary cycles using identical PCR conditions.
Statistical analysis
Individual variables were assessed by univariate analysis using the Chi squared test. Risk ratios were calculated for each variable to assess the predictive values for CTCs. Logistic regression analysis was used to assess the relationships between CTC counts and clinicopathological data. All statistical analyses were performed using SPSS 20.0 software (SPSS Inc., Chicago, IL, USA). Graphs were plotted using GraphPad Prism (Version 5.02. San Diego, CA, USA). The area under each ROC curve (AUC) was calculated to assess the discriminating power. The Youden index (sensitivity + specificity) was calculated to select the optimal cut-off values for CTC distribution. P<0.05 was considered a statistically significant difference.
Results
Identification and characterization of CTCs enriched from PVB
CTCs were isolated and characterized as previously reported. Briefly, CTCs enriched from PVB were subjected to immunofluorescence in situ hybridization (imFISH) staining with antibodies against CEP8 and CD45. Cell nuclei were stained with DAPI. CTCs were defined as cells showing a CEP8+/DAPI+/CD45‒ profile. CTC counts less than 2 were considered false positives (Figure 1A,B,C,D,E,F). Viability assessments suggested that the CTCs isolated from PVB were approximately similar to those of whole blood cells (Figure 1G). Following this criterion, no positive CTCs were detected in either healthy controls or benign lung disease patients. In contrast, CTCs were identified in up to 73.33% (44/60) of patients with early-stage lung adenocarcinoma (P<0.05; Table 1), indicating these cells as specific signatures for early stage lung adenocarcinoma (Figures 2,3).
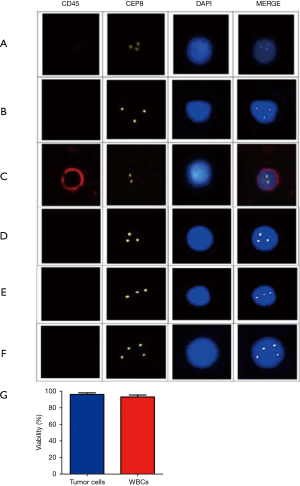
Table 1
| Variable | n | Positive/negative | Positive-CTC (%) | χ2 | P value |
|---|---|---|---|---|---|
| Early-lung adenocarcinoma group | 60 | 44/16 | 73.33 | 69.474 | <0.05 |
| Benign lung disease group | 36 | 0/36 | 0 | ||
| Healthy controls group | 24 | 0/24 | 0 |
CTC, circulating tumor cell.


CTC counts positively correlate with tumor invasion and pathological stage
Through the evaluation of CTC counts with clinicopathological features, CTCs were found to be associated with tumor infiltration (P=0.003) and pathological stage (P<0.000), (Table 2). The AUC analysis indicated that CTC counts >5 could predict tumor infiltration and higher pathological staging (specificity 85%, sensitivity 90%), whilst CTC counts <3 were associated with in situ IA1 staging. Of note, no correlation between CTCs and other factors such as gender, age, smoking history, pathological cell morphology, and immunohistochemical indicators were observed. These findings demonstrate that CTC counts are associated with tumor infiltration and pathological stage in patients with early stage lung adenocarcinoma.
Table 2
| Clinicopathological parameters | n | Positive/negative | Positive-CTC (%) | χ2 | P value |
|---|---|---|---|---|---|
| Gender | 0.097 | 0.491 | |||
| Male | 32 | 24/8 | 75.00 | ||
| Female | 28 | 20/8 | 71.43 | ||
| Age (years) | 0.128 | 0.473 | |||
| >55 | 36 | 27/9 | 75.00 | ||
| ≤55 | 24 | 17/7 | 70.83 | ||
| Smoking history | 0.302 | 0.402 | |||
| Never smoked | 26 | 20/6 | 76.92 | ||
| Have or are smoking | 34 | 24/10 | 70.59 | ||
| Degree of tumor infiltration | 11.851 | 0.003 | |||
| In situ cancer | 6 | 1/5 | 16.67 | ||
| Micro-infiltration | 22 | 16/6 | 72.73 | ||
| Infiltration | 32 | 27/5 | 84.38 | ||
| Pathological cell morphology | 5.313 | 0.150 | |||
| Wall-like | 8 | 4/4 | 50.00 | ||
| Acinar | 22 | 19/3 | 86.36 | ||
| Micro-papillary | 12 | 10/2 | 83.33 | ||
| Papillary | 18 | 11/7 | 61.11 | ||
| Pathological staging | 18.011 | 0.000 | |||
| IA1 | 10 | 2/8 | 20.00 | ||
| IA2 | 18 | 14/4 | 77.78 | ||
| IA3 | 32 | 28/4 | 87.50 | ||
| Immunohistochemical indicators | 3.561 | 0.313 | |||
| NapsinA(+)/TTF-1(+)/Ki67>20% (+) | 54 | 41/13 | 75.93 | ||
| NapsinA(+)/TTF-1(+)/Ki67>20% (–) | 0 | 0/0 | 0 | ||
| NapsinA(+)/TTF-1(–)/Ki67>20% (+) | 3 | 2/1 | 66.67 | ||
| NapsinA(–)/TTF-1(+)/Ki67>20% (+) | 2 | 1/1 | 50.00 | ||
| NapsinA(–)/TTF-1(–)/Ki67>20% (+) | 1 | 0/1 | 0 | ||
| NapsinA(+)/TTF-1(–)/Ki67<20% (–) | 0 | 0/0 | 0 | ||
| NapsinA(–)/TTF-1(+)/Ki67<20% (–) | 0 | 0/0 | 0 | ||
| NapsinA(–)/TTF-1(–)/Ki67<20% (–) | 0 | 0/0 | 0 |
CTC, circulating tumor cell.
Relationship between CTCs and EGFR gene mutations
EGFR mutations are commonly observed in early-stage lung adenocarcinoma patients (27). Inhibitors targeting the kinase domain of EGFR, particularly tyrosine kinase inhibitors (TKIs), are in clinical use and show therapeutic efficacy (28,29). We therefore examined the relationship between CTC counts and EGFR mutations. DNA was extracted from formalin-fixed, paraffin-embedded lung tumor tissues and EGFR mutations were detected using the AmoyDx Human EGFR Gene 29 mutations detection kit (Figure 4). The results showed positive CTC rates of 53.33% in patients with EGFR negative lung adenocarcinoma, and 93.55% positive CTC rates in patients with EGFR mutations (Table 3; P<0.05). CTCs were present in 26.67% of patients with L858R point mutations, and 28.33% of patients with exon 19 deletions (the two most common EGFR genetic alterations) (Table 4). No EGFR mutations were detected in healthy or benign control groups.
Table 3
| Clinicopathological parameters | n | Positive/negative | Positive-CTC (%) | χ2 | P value |
|---|---|---|---|---|---|
| EGFR | 17.332 | 0.000 | |||
| Mutant-type | 34 | 32/2 | 94.12 | ||
| Wild-type | 26 | 12/14 | 46.15 |
EGFR, epidermal growth factor receptor; CTC, circulating tumor cell.
Table 4
| Sample | Positive/negative-CTC | Mutant/wild type-EGFR | Exon 18 | Exon 19 | Exon 20 | Exon 21 |
|---|---|---|---|---|---|---|
| HA-1 | Negative | Mutant-type | – | – | – | L858R |
| HA-2 | Positive | Mutant-type | – | 19– del | – | – |
| HA-3 | Negative | Wild-type | – | – | – | – |
| HA-4 | Positive | Wild-type | – | – | – | – |
| HA-5 | Negative | Wild-type | – | – | – | – |
| HA-6 | Positive | Wild-type | – | 19– del | – | – |
| HA-7 | Positive | Mutant-type | – | 19– del | – | – |
| HA-8 | Positive | Wild-type | – | – | – | – |
| HA-9 | Positive | Mutant-type | – | – | – | L858R |
| HA-10 | Positive | Mutant-type | – | – | – | L858R |
| HA-11 | Negative | Wild-type | – | – | – | – |
| HA-12 | Positive | Mutant-type | – | – | – | L858R |
| HA-13 | Positive | Wild-type | – | – | – | – |
| HA-14 | Positive | Mutant-type | – | 19– del | – | – |
| HA-15 | Positive | Wild-type | – | – | – | – |
| HA-16 | Positive | Mutant-type | – | – | – | L858R |
| HA-17 | Positive | Mutant-type | – | 19-del | – | – |
| HA-18 | Negative | Wild-type | – | – | – | – |
| HA-19 | Positive | Mutant-type | – | – | – | L858R |
| HA-20 | Positive | Mutant-type | – | 19-del | – | – |
| HA-21 | Positive | Wild-type | – | – | – | – |
| HA-22 | Negative | Mutant-type | – | – | 20-ins | – |
| HA-23 | Negative | Wild-type | – | – | – | – |
| HA-24 | Negative | Wild-type | – | – | – | – |
| HA-25 | Positive | Mutant-type | – | – | – | L858R |
| HA-26 | Negative | Wild-type | – | – | – | – |
| HA-27 | Positive | Mutant-type | – | 19-del | – | – |
| HA-28 | Positive | Mutant-type | – | – | – | L858R |
| HA-29 | Positive | Mutant-type | – | 19-del | – | – |
| HA-30 | Positive | Mutant-type | – | 19-del | – | – |
| HA-31 | Positive | Mutant-type | – | – | – | L858R |
| HA-32 | Negative | Wild-type | – | – | – | – |
| HA-33 | Positive | Mutant-type | – | 19-del | – | – |
| HA-34 | Positive | Mutant-type | – | 19-del | – | – |
| HA-35 | Positive | Mutant-type | – | – | – | L858R |
| HA-36 | Positive | Wild-type | – | – | – | – |
| HA-37 | Positive | Mutant-type | – | 19-del | – | – |
| HA-38 | Negative | Wild-type | – | – | – | – |
| HA-39 | Positive | Mutant-type | – | – | – | L858R |
| HA-40 | Negative | Wild-type | – | – | – | – |
| HA-41 | Positive | Mutant-type | – | – | – | L858R |
| HA-42 | Negative | Wild-type | – | – | – | – |
| HA-43 | Positive | Mutant-type | – | 19-del | – | – |
| HA-44 | Positive | Wild-type | – | – | – | – |
| HA-45 | Positive | Mutant-type | – | – | – | L858R |
| HA-46 | Positive | Wild-type | – | – | – | – |
| HA-47 | Positive | Mutant-type | – | – | – | L858R |
| HA-48 | Positive | Wild-type | – | – | – | – |
| HA-49 | Positive | Mutant-type | – | 19-del | – | – |
| HA-50 | Negative | Wild-type | – | – | – | – |
| HA-51 | Positive | Wild-type | – | – | – | – |
| HA-52 | Positive | Wild-type | – | – | – | – |
| HA-53 | Positive | Mutant-type | – | – | – | L858R |
| HA-54 | Negative | Wild-type | – | – | – | – |
| HA-55 | Positive | Mutant-type | – | 19-del | – | – |
| HA-56 | Positive | Mutant-type | – | – | – | L858R |
| HA-57 | Positive | Mutant-type | – | – | – | L858R |
| HA-58 | Positive | Wild-type | – | – | – | – |
| HA-59 | Negative | Wild-type | – | – | – | – |
| HA-60 | Positive | Mutant-type | – | 19-del | – | – |
–, no mutation. EGFR, epidermal growth factor receptor; CTC, circulating tumor cell.
Discussion
Growing evidence has established that tumor metastasis contributes to a poor clinical outcome in LCa patients (30-32). The early detection of metastasis is critical for improved therapeutic responses (33-35). Despite early diagnosis and surgical resection, most patients experience postoperative recurrence or micro-metastasis within 5 years. This compromises imaging diagnostics, and highlights the urgent need for reliable tumor markers that facilitate real-time cancer surveillance, progression, and therapeutic predictions. CTCs are cells shed from the primary tumor into the circulation. The clinical relevance of CTCs regarding tumor metastasis, prognosis, and therapeutic responses have been described for many cancers, including LCa (31,36-38). However, methods to detect LCa CTCs requires improvement in terms of sensitivity and specificity.
In this study, we isolated and characterized CTCs from PVB, but not PV, since PVB has a higher abundance of CTCs in LCa. Immunomagnetic negative selection in addition to immunofluorescence analysis provided high CTC enrichment that was highly sensitive and specific (15,16). Using this strategy, we analyzed 60 cases of early-stage LCa, and found that 73.33% of patients display positive CTC enrichment, suggesting a higher sensitivity of CTC for LCa. We therefore demonstrate that CTC detection has clinical significance for the evaluation of tumor metastasis and therapeutic responses in follow-up studies.
Examination of the correlation of CTCs with clinical parameters suggested that CTC counts were significantly associated with pathological staging and tumor infiltration, which was in accordance with previous studies (22). Higher CTC counts and positive rates were observed in patients with stage IA3 and IA2 compared to IA1 (87.50% vs. 77.78% vs. 20.00%), further indicating the role of CTCs in tumor initiation and metastasis. Additionally, for distal metastasis in IA1 stage patients, CTC counts showed improved sensitivity, whilst traditional imaging methods failed to offer useful information. A large number of clinical studies show that traditional ultrasound, CT, MRI and ECT techniques have limitations for the clinical diagnosis of malignant tumors (39). CTCs thus represent potential sources of accurate and real-time LCa detection, with lower costs, higher sensitivity, and reliability. Further studies are now necessary to develop more effective strategies for CTC detection in patients with low CTC abundances. The identified CTCs also require evaluation to confirm they originated from primary tumor tissue.
Approximately 10–35% of patients with NSCLC have tumor associated EGFR mutations (27). These mutations occur within EGFR exons 18–21, which encodes a portion of the receptor kinase domain. EGFR mutations are typically heterozygous, with the mutant allele also amplified (40,41). Approximately 90% of these mutations are exon 19 deletions or exon 21 L858R point mutations (42) that increase EGFR kinase activity leading to the hyperactivation of downstream pro-survival signaling pathways (43). More importantly, TKIs are effective in patients harboring these activating mutations. It was therefore of interest to verify whether CTC counts serve as a biomarker for the prediction of EGFR mutations. The results revealed a strong association (P<0.05) as CTCs were positive in 94.12% of patients with EGFR mutations, and present in 26.67% and 28.33% of patients with L858R point mutations and exon 19 deletions, respectively. Since these represent the most common EGFR mutations in LCa, this highlights the power of CTC counts as prognostic markers in LCa patients.
The CD45-FISH-based strategy showed clinical value as a surrogate marker for therapeutic selection and monitoring in LCa. This approach is also beneficial for the putative isolation and verification of CTCs in other cancers. Despite these benefits, future studies should focus on the development of improved isolation methods with higher sensitivity and specificity, including chromosome enumeration probes, and tumor-specific antigens for the immunofluorescent analysis of CTCs.
Our findings strengthen the clinical relevance of CTCs as prognosis, tumor metastasis and recurrence markers (44-46). However, some limitations should be noted. The sample size was small and could be extended for further validations in future studies. In addition, more accurate and solid evidence of CTCs in LCa that integrate diagnosis, predictions, and the real-time surveillance of tumor progression and therapeutic responses are required. Large-scale, multicenter follow-up studies are required to fully explore the clinical value of CTCs as predictors of LCa prognosis, recurrence and metastasis. Such studies will lay the foundation for the rationalization and individualized treatment of LCa in the clinic.
Conclusions
This study revealed that CTC counts correlate with tumor invasion, pathological staging, and EGFR mutations, and thus provide a promising biomarker for the surveillance of lung adenocarcinoma progression. To clarify the clinical significance of PVB-CTC status, long-term follow-up studies should now be performed.
Acknowledgments
Funding: This work was financially supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.05.19). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Human Research Ethics Committee of the First Affiliated Hospital of University of Science and Technology of China (No. 2017-100). Written informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Kelsey CR, Marks LB, Hollis D, et al. Local recurrence after surgery for early-stage lung cancer: an 11-year experience with 975 patients. Cancer 2009;115:5218-27. [Crossref] [PubMed]
- Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271-89. [Crossref] [PubMed]
- Drapkin BJ, George J, Christensen CL, et al. Genomic and Functional Fidelity of Small Cell Lung Cancer Patient-Derived Xenografts. Cancer Discov 2018;8:600-15. [Crossref] [PubMed]
- Carter L, Rothwell DG, Mesquita B, et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nat Med 2017;23:114-9. [Crossref] [PubMed]
- Li Y, Cheng X, Chen Z, et al. Circulating tumor cells in peripheral and pulmonary venous blood predict poor long-term survival in resected non-small cell lung cancer patients. Sci Rep 2017;7:4971. [Crossref] [PubMed]
- Zhou L, Dicker DT, Matthew E, et al. Circulating tumor cells: silent predictors of metastasis. F1000Res 2017;6:1-8. [Crossref] [PubMed]
- Dasgupta A, Lim AR, Ghajar CM. Circulating and disseminated tumor cells: harbingers or initiators of metastasis? Mol Oncol 2017;11:40-61. [Crossref] [PubMed]
- Maheswaran S, Haber DA. Circulating tumor cells: a window into cancer biology and metastasis. Curr Opin Genet Dev 2010;20:96-9. [Crossref] [PubMed]
- Nakagawa T, Martinez SR, Goto Y, et al. Detection of circulating tumor cells in early-stage breast cancer metastasis to axillary lymph nodes. Clin Cancer Res 2007;13:4105-10. [Crossref] [PubMed]
- Huang X, Gao P, Song Y, et al. Meta-analysis of the prognostic value of circulating tumor cells detected with the CellSearch System in colorectal cancer. BMC Cancer 2015;15:202. [Crossref] [PubMed]
- Janni WJ, Rack B, Terstappen LW, et al. Pooled Analysis of the Prognostic Relevance of Circulating Tumor Cells in Primary Breast Cancer. Clin Cancer Res 2016;22:2583-93. [Crossref] [PubMed]
- Huh SJ, Liang S, Sharma A, et al. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res 2010;70:6071-82. [Crossref] [PubMed]
- Hristozova T, Konschak R, Stromberger C, et al. The presence of circulating tumor cells (CTCs) correlates with lymph node metastasis in nonresectable squamous cell carcinoma of the head and neck region (SCCHN). Ann Oncol 2011;22:1878-85. [Crossref] [PubMed]
- Spicer JD, McDonald B, Cools-Lartigue JJ, et al. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res 2012;72:3919-27. [Crossref] [PubMed]
- Shiota M, Bishop JL, Nip KM, et al. Hsp27 regulates epithelial mesenchymal transition, metastasis, and circulating tumor cells in prostate cancer. Cancer Res 2013;73:3109-19. [Crossref] [PubMed]
- Freitas VM, Hilfenhaus G, Iruela-Arispe ML. Metastasis of Circulating Tumor Cells: Speed Matters. Dev Cell 2018;45:3-5. [Crossref] [PubMed]
- Steinert G, Scholch S, Koch M, et al. Biology and significance of circulating and disseminated tumour cells in colorectal cancer. Langenbecks Arch Surg 2012;397:535-42. [Crossref] [PubMed]
- Bidard FC, Ferrand FR, Huguet F, et al. Disseminated and circulating tumor cells in gastrointestinal oncology. Crit Rev Oncol Hematol 2012;82:103-15. [Crossref] [PubMed]
- Zhang Z, Ramnath N, Nagrath S. Current Status of CTCs as Liquid Biopsy in Lung Cancer and Future Directions. Front Oncol 2015;5:209. [Crossref] [PubMed]
- Crosbie PA, Shah R, Krysiak P, et al. Circulating Tumor Cells Detected in the Tumor-Draining Pulmonary Vein Are Associated with Disease Recurrence after Surgical Resection of NSCLC. J Thorac Oncol 2016;11:1793-7. [Crossref] [PubMed]
- Reddy RM, Murlidhar V, Zhao L, et al. Pulmonary venous blood sampling significantly increases the yield of circulating tumor cells in early-stage lung cancer. J Thorac Cardiovasc Surg 2016;151:852-8. [Crossref] [PubMed]
- Akhurst T. Staging of Non-Small-Cell Lung Cancer. PET Clin 2018;13:1-10. [Crossref] [PubMed]
- Chen Q, Ge F, Cui W, et al. Lung cancer circulating tumor cells isolated by the EpCAM-independent enrichment strategy correlate with Cytokeratin 19-derived CYFRA21-1 and pathological staging. Clin Chim Acta 2013;419:57-61. [Crossref] [PubMed]
- Ning N, Zhan T, Zhang Y, et al. Improvement of specific detection of circulating tumor cells using combined CD45 staining and fluorescence in situ hybridization. Clin Chim Acta 2014;433:69-75. [Crossref] [PubMed]
- Chen YY, Xu GB. Effect of circulating tumor cells combined with negative enrichment and CD45-FISH identification in diagnosis, therapy monitoring and prognosis of primary lung cancer. Med Oncol 2014;31:240. [Crossref] [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Renaud S, Seitlinger J, Guerrera F, et al. Prognostic Value of Exon 19 Versus 21 EGFR Mutations Varies According to Disease Stage in Surgically Resected Non-small Cell Lung Cancer Adenocarcinoma. Ann Surg Oncol 2018;25:1069-78. [Crossref] [PubMed]
- Matam K, Goud I, Lakshmi MA, et al. a Hospital-Based Study. Asian Pac J Cancer Prev 2015;16:7071-6. [Crossref] [PubMed]
- Yan X, Jiao SC, Zhang GQ, et al. Tumor-associated immune factors are associated with recurrence and metastasis in non-small cell lung cancer. Cancer Gene Ther 2017;24:57-63. [Crossref] [PubMed]
- Cheng M, Liu L, Yang HS, et al. Circulating tumor cells are associated with bone metastasis of lung cancer Asian Pac J Cancer Prev 2014;15:6369-74. [Crossref] [PubMed]
- Lee YJ, Jung EJ, Lee SH, et al. Concurrent diagnosis of pulmonary metastasis of malignant mixed mullerian tumor and small cell lung cancer. Tuberc Respir Dis (Seoul) 2012;73:56-60. [Crossref] [PubMed]
- O'Dowd EL, Baldwin DR. Early diagnosis pivotal to survival in lung cancer. Practitioner 2014;258:21-4, 2-3.
- Ilie M, Hofman V, Long-Mira E, et al. "Sentinel" circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLOS One 2014;9:e111597. [Crossref] [PubMed]
- Vansteenkiste J, De Ruysscher D, Eberhardt WE, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi89-98. [Crossref] [PubMed]
- Hou JM, Krebs M, Ward T, et al. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol 2011;178:989-96. [Crossref] [PubMed]
- Okumura Y, Tanaka F, Yoneda K, et al. Circulating tumor cells in pulmonary venous blood of primary lung cancer patients. Ann Thorac Surg 2009;87:1669-75. [Crossref] [PubMed]
- Yu N, Zhou J, Cui F, et al. Circulating tumor cells in lung cancer: detection methods and clinical applications. Lung 2015;193:157-71. [Crossref] [PubMed]
- Elsayes KM, Kielar AZ, Chernyak V, et al. LI-RADS: a conceptual and historical review from its beginning to its recent integration into AASLD clinical practice guidance. J Hepatocell Carcinoma 2019;6:49-69. [Crossref] [PubMed]
- Hosokawa S, Toyooka S, Fujiwara Y, et al. Comprehensive analysis of EGFR signaling pathways in Japanese patients with non-small cell lung cancer. Lung Cancer 2009;66:107-13. [Crossref] [PubMed]
- Kubo T, Yamamoto H, Lockwood WW, et al. MET gene amplification or EGFR mutation activate MET in lung cancers untreated with EGFR tyrosine kinase inhibitors. Int J Cancer 2009;124:1778-84. [Crossref] [PubMed]
- Ladanyi M, Pao W. Lung adenocarcinoma: guiding EGFR-targeted therapy and beyond. Mod Pathol 2008;21:S16-22. [Crossref] [PubMed]
- Sordella R, Bell DW, Haber DA, et al. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science 2004;305:1163-7. [Crossref] [PubMed]
- Bünger S, Zimmermann M, Habermann JK. Diversity of assessing circulating tumor cells (CTCs) emphasizes need for standardization: a CTC Guide to design and report trials. Cancer Metastasis Rev 2015;34:527-45. [Crossref] [PubMed]
- Onstenk W, Gratama JW, Foekens JA, et al. Towards a personalized breast cancer treatment approach guided by circulating tumor cell (CTC) characteristics. Cancer Treat Rev 2013;39:691-700. [Crossref] [PubMed]
- Wang FB, Yang XQ, Yang S, et al. A higher number of circulating tumor cells (CTC) in peripheral blood indicates poor prognosis in prostate cancer patients--a meta-analysis. Asian Pac J Cancer Prev 2011;12:2629-35. [PubMed]


