
The effect of metabolic risk factors on cancer mortality among blacks and whites
Introduction
A black-white disparity in total cancer death has been pervasive in the US, despite the general decline in cancer mortality rates due to reduced tobacco smoking, more widespread cancer screening and testing, and improved therapies (1). The reasons for black-white differences in cancer outcomes and survivorship are multifactorial. Factors from individual (2,3), clinical (4,5) and environmental (6-8) levels contribute to this disparity but were not able to provide a complete explanation. Previous studies found racial differences in cancer outcomes remain even after adjusting for stage, age, sex, socioeconomic status and access to health care (9-12). One factor that might contribute to the racial differences in cancer death but that has not fully evaluated is metabolic syndrome (MS). MS is defined as a cluster of at least three of the following risk factors: central obesity, glucose intolerance, dyslipidemia and hypertension (13). There is a growing interest in MS because of its high prevalence among US adults (14) and its link with risk of many chronic conditions including various cancers (15-18). MS and its individual components may be also related to increased cancer mortality (19-22). One perspective study found MS was associated with an increased risk of all-cause cancer mortality in men (20). Another population-based study in the US also reported a significant association between MS and total and lung cancer mortality. Specifically, systolic blood pressure (BP) and serum glucose were positively associated with an increased risk of death from total cancer (19). However, these studies exploring MS and cancer outcomes often aggregated racial groups, thereby masking possible differences in association by race (19-22).
In comparison to whites, blacks demonstrated a higher risk of dying from chronic conditions such as cardiovascular diseases and diabetes as a result of MS (23-25). However, limited data has shown MS is associated with higher cancer mortality in blacks compared to whites (8). There may be variations in risk of cancer mortality related to MS and its individual risk components. Previous studies found that while hypertension was independently associated with cancer death in black female breast cancer patients (26), diabetes was documented to increase risk for cancer death only among white cancer patients (27,28). Another study also reported that abnormal glucose tolerance was positively associated with significant cancer mortality in a nearly all white sample (29). However, no data has demonstrated the same association in black cancer patients. To improve race-specific cancer care, especially chronic disease risk management, more research is warranted to examine if MS and what specific metabolic risk factors contribute to cancer death in two racial groups separately.
Objective
Using a nationally representative sample, this study aimed to examine the association between MS and its individual components and total cancer mortality in whites vs. blacks.
Methods
Data: National Health and Nutrition Examination Survey III (NHANES III) [1988–1994] assessed the health status of a nationally representative sample of the civilian noninstitutionalized US population. The study consists of interviews, physical examinations and data from blood sample analyses. A full description of survey design and methodology can be found elsewhere (30). The NHANES III mortality follow-up study was conducted and linked with the National Death Index. The linked study provides mortality follow-up from the date of baseline NHANES III [1988–1994] through December 31, 2006 (30). Of 19,620 participants aged 20 years or older, we excluded 230 individuals with pregnancy status, 574 of non-white or non-black racial/ethnic background and 795 without demographic information. Another 2,993 were excluded due to missing measurements on MS components. A total of 15,803 individuals (11,081 whites and 4,722 blacks) were included in our analysis. The National Center for Health Statistics Institutional Review Board approved the survey protocols, and informed consent was obtained from all subjects. The present study was not reviewed by the Institutional Review Board of the University of Missouri because the data analyzed are de-identified and publicly accessible.
Variables: we used death from any cancer cause in the NHANES III mortality follow-up study as the main outcome of interest in the analysis. We used the National Heart, Lung and Blood Institute criteria to evaluate MS and individual metabolic factors (13). MS was defined as the presence of at least three of five risk factors: reduced high density lipoprotein (HDL) cholesterol (<50 mg/dL), elevated triglycerides (TG) (≥150 mg/dL), impaired fasting blood glucose (≥100 mg/dL), elevated waist circumference (≥88 cm for women and ≥102 cm for men) and elevated BP (≥130 mmHg systolic BP or≥85 mmHg diastolic BP) (31). We also created a composite variable with presence of ≤1, 2, 3 or ≥4 individual MS components.
We chose covariates based on their relevance to MS and cancer death (32). We kept variables if they altered parameter estimates by 10% or more and/or had P value (<0.025) for their regression coefficients (33). Covariates included gender, age (20–29, 30–39, 40–49, 50–59, 60–69 or ≥70), poverty-to-income ratio (≤1-under poverty line, >1 to <3, or ≥3), insurance status (yes vs. no), and smoking status (non-smoker, former smoker or non-smoker).
Analysis: we firstly described baseline characteristics of participants by race. Chi-square tests were used for the descriptive analysis. Secondly, we used separate models to evaluate interactions between race and MS as well as between race and MS components. If interactions were statistically significant (i.e., P<0.05), we then used Cox proportional hazards regression models to estimate hazard ratio (HR) and 95% confidence intervals (CIs) of cancer death for individual metabolic risk factors and MS by race. We performed linear trend tests for the four categories of MS. Considering the complexity of the sampling design, survey-related commands (e.g., Proc Surveyphreg) were employed to adjust for the complex survey design effect. In particular, the Primary Sampling Unit (PSU), stratum and design weight for each observation was taken into account in the analysis (30).
Results
The distribution of age, sex, insurance and number of MS components were comparable between blacks and whites (Table 1). However, there were marked differences between blacks and whites regarding poverty to income ratio, smoking status, and the individual MS components. In comparison to whites, higher percentages of blacks (9% vs. 27%) lived under the poverty line and were current smokers (28% vs. 34%). Relative to whites, blacks also had significantly higher prevalence of central obesity (40% vs. 45%), BP (10% vs. 18%) and impaired fasting blood glucose (28% vs. 34%); but lower prevalence of reduced HDL (36% vs. 27%) and elevated TG (36% vs. 27%) (Table 1).
Table 1
| Characteristics | Non-Hispanic White [n=11,081, % (SE)]a | Non-Hispanic Black [n=4,722, % (SE)] |
|---|---|---|
| Age | ||
| ≥20 to <30 | 20.90 (0.94) | 26.25 (0.87) |
| ≥30 to <40 | 23.81 (0.86) | 26.76 (0.80) |
| ≥40 to <50 | 20.62 (0.79) | 20.57 (0.87) |
| ≥50 to <60 | 12.79 (0.47) | 10.77 (0.60) |
| ≥60 to <70 | 11.64 (0.55) | 8.71 (0.61) |
| ≥70 | 10.22 (0.69) | 6.93 (0.60) |
| Gender | ||
| Male | 48.84 (0.43) | 44.90 (0.91) |
| Female | 51.15 (0.43) | 55.09 (0.94) |
| Poverty to income ratio | ||
| ≤1 (under poverty) | 9.42 (0.70) | 26.52 (1.73) |
| >1 to <3 | 38.32 (1.15) | 43.67 (1.29) |
| ≥3 | 52.27 (1.36) | 29.80 (1.37) |
| Smoking | ||
| Never | 44.08 (0.91) | 50.37 (1.09) |
| Former | 27.52 (0.63) | 16.10 (0.68) |
| Current | 28.41 (0.92) | 33.52 (1.04) |
| Insurance | ||
| Yes | 88.27 (0.75) | 84.49 (1.48) |
| No | 11.73 (0.75) | 15.51 (1.48) |
| MS | ||
| Yes (≥3 components) | 23.11 (0.82) | 21.84 (0.58) |
| Central obesity | 39.64 (0.82) | 44.66 (1.09) |
| High blood pressure | 10.33 (0.45) | 17.77 (0.75) |
| Low HDL | 36.01 (1.21) | 26.86 (0.87) |
| High triglycerides | 36.03 (1.14) | 26.84 (0.81) |
| Impaired fasting glucose | 28.30 (1.45) | 33.53 (0.84) |
| Number of MS components | ||
| ≤1 | 54.71 (1.33) | 53.78 (0.70) |
| 2 | 22.18 (0.81) | 24.38 (0.69) |
| 3 | 15.16 (0.51) | 14.55 (0.45) |
| ≥4 | 7.95 (0.47) | 7.29 (0.40) |
a, percentages in the table are weighted. MS, Metabolic syndrome; HDL, high density lipoprotein.
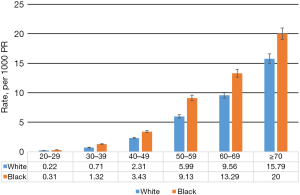
The mean follow-up period was 16.7 years for all participants in this study. Of 15,740 eligible participants, 1,129 (7.2%) died from cancer. Figure 1 shows blacks had significantly higher cancer mortality across all age groups (P<0.05).
We found significant interactions between race and MS as well as between race and MS components in models (all P<0.05). We then performed race-stratified analysis for the association between MS, its components and cancer mortality (Table 2). After adjusting for all covariates, MS was not statistically significantly associated with risk of cancer death for either racial group. With respect to MS components in whites, the risk of cancer death increased 29% with central obesity, 26% with low HDL and 45% with impaired fasting glucose. In adjusted HR models, high BP was significantly associated with a 41% increased risk for cancer death in blacks (Table 2). Among whites, after covariate adjustment in the model, the risk for cancer death increased with the presence of 2, 3 and ≥4 components of MS, and trend test in the HR was statistically significant (Table 2).
Table 2
| MS and MS components | Non-Hispanic White | Non-Hispanic Black | |||
|---|---|---|---|---|---|
| Crude model, HR (95% CI) | Adjusted model, HR (95% CI) | Crude model, HR (95% CI) | Adjusted model, HR (95% CI) | ||
| MS (≥3 factors)1 | |||||
| No | Ref. | Ref. | Ref. | Ref. | |
| Yes | 2.22 (1.82–2.71)** | 1.19 (0.99–1.44) | 1.61 (1.24–2.11)** | 0.88 (0.67–1.16) | |
| Central obesity | |||||
| No | Ref. | Ref. | Ref. | Ref. | |
| Yes | 2.26 (1.83–2.81)** | 1.29 (1.05–1.59)* | 1.18 (0.94–1.49) | 0.83 (0.62–1.04) | |
| High blood pressure | |||||
| No | Ref. | Ref. | Ref. | Ref. | |
| Yes | 1.55 (1.22–1.97)* | 1.15 (0.91-7.91) | 2.15 (1.68–2.75)** | 1.41 (1.10–1.80)* | |
| Low HDL | |||||
| No | Ref. | Ref. | Ref. | Ref. | |
| Yes | 1.36 (1.12–1.63)* | 1.26 (1.04–1.52)* | 0.94 (0.70–1.24) | 0.91 (0.69–1.20) | |
| High triglycerides | |||||
| No | Ref. | Ref. | Ref. | Ref. | |
| Yes | 1.57 (1.28–1.93)** | 1.02 (0.84–1.23) | 1.47 (1.11–1.94)** | 0.91 (0.70–1.19) | |
| Impaired fasting glucose | |||||
| No | Ref. | Ref. | Ref. | Ref. | |
| Yes | 2.40 (1.91–3.02)** | 1.45 (1.19–1.76)* | 1.66 (1.32–2.08)** | 1.03 (0.80–1.32) | |
| Number of MS | |||||
| ≤1 | Ref. | Ref. | Ref. | Ref. | |
| 2 | 2.00 (1.58–2.55)** | 1.36 (1.07–1.73)* | 1.30 (0.91–1.85) | 1.01 (0.70–1.46) | |
| 3 | 2.57 (2.03–3.27)** | 1.35 (1.05–1.72)* | 1.61 (1.09–2.40)* | 1.02 (0.68–1.51) | |
| ≥4 | 3.38 (2.35–4.86)** | 1.60 (1.13–2.27)* | 2.09 (1.36–3.21)* | 1.00 (0.65–1.53) | |
| P for trend–MS categories | <0.0001 | 0.01 | <0.0001 | 0.91 | |
**, P<0.005; *, P<0.05. 1, adjusted model included age, gender, income, insurance status, and smoking status; sampling weight has been taken into account in Cox-proportional hazards regression. HDL, high density lipoprotein; MS, metabolic syndrome; HR, hazard ratios.
The impact of demographic variables on cancer death was identified between blacks and whites (results not shown in tables or figures). Older age and current/former smoking status were significant risk factors for cancer death in both racial groups. Women were less likely to die from cancer than men; however, the adjusted model did not show statistical significance in whites. Higher income groups were less likely to die from cancer in both groups too, but only in whites the association reached to statistical significance (results not shown in tables and figures).
Discussion
Our study showed that the adverse effect of metabolic risk factors on cancer death varied between blacks vs. whites. Firstly, we found among MS components, elevated BP was a significant risk factor to cancer mortality in blacks. Compared to blacks who have normal BP, those with elevated BP had a more than 40% higher risk of cancer death. In comparison to whites, hypertension is more common, develops in younger age groups, and is associated with significantly higher cardiovascular and all-cause mortality in blacks (34,35). Observational research on racial difference in cancer death attributable to hypertension is limited and findings have been mixed (19,20,26,36,37). Without stratifying racial group, one study found elevated systolic BP was significantly associated with a higher risk of total mortality in a US cohort (19), while another one did not find a significant relationship in a men-only cohort (20). In a Korean population-based study (36) and in another study combining seven cohorts from three European countries (37), researchers found elevated BP was significantly associated with increased total cancer death. Specifying racial groups, one study found a null association of hypertension and total cancer mortality in whites and a marginal significant association in blacks (38). Another one found a significantly higher risk of breast cancer death in black women than in white women (26).
Several biological studies have proposed possible mechanisms of hypertension and malignancy. One study pointed out that increased expression of inositol triphosphate and cytosolic calcium is related to the pathogenesis of hypertension and in the early stages of cell proliferation that are activated by endogenous oncogenes (39). Another study showed aberrant carcinogen binding to deoxyribonucleic acid in lymphocytes of hypertensive patients (40). Cell death via apoptosis can also influence the growth of vascular smooth muscle cells, and related aberrations have also been identified in hypertension (41). Epidemiological data also showed a significant association between hypertension and total cancer mortality without specifying race (37,42). Our findings documented the heightened risk for cancer mortality due to hypertension in blacks. Cancer prevention and control program should address the excess risk of cancer death due to high BP in blacks. Further research is needed to clarify reasons for racial disparities in hypertension-related cancer death (23).
We also found in whites, compared to individuals with normal fasting glucose level, those with impaired level had a 45% higher risk of total cancer death while in blacks, the association did not reach to statistical significance. Even though mortality specifically due to diabetes has been reported higher in blacks than in whites (38), few studies have examined whether the impaired fasting glucose level causes discrepancies in overall cancer death between blacks and white. One study evaluating a group of individuals 45 years of age and older found impaired fasting glucose significantly increased risk for cancer death in blacks but not in whites (38) while two breast cancer studies found diabetes was associated with a significantly increased hazard for breast cancer-specific death in white patients only (27,28). Researchers also found diabetes was associated with a later state of cancer diagnosis and more aggressive tumor grade for white women only, which in part, may account for the survival reduction in white women (28).
It has been documented that impaired fasting glucose status was associated with a high risk of cancer death in European, Japanese and US cohorts (29,43,44). A previous study suggested that oxidative stress and accumulated advanced glycation end-products induced by hyperglycemia at the cellular level may influence cancer development and progression (45). Insulin resistance may promote cancer cell proliferation and may possibly be related to worse cancer prognosis as well (46,47). Further research should investigate whether these biological mechanisms varied by race.
Race-specific findings on impaired fasting glucose level and cancer death in our study should also be interpreted with caution. It is possible that other risk factors contributing to prediabetes or diabetes, such as insulin resistance and obesity are high among blacks in the absence of prediabetes or diabetes, thus attenuating the risk of death toward the null by making blacks with and without prediabetes or diabetes similar.
We acknowledge that the large sample size poses a potential danger in detecting statistically significant, yet clinically unimportant differences when focusing on HR. It is also possible that using all-cancer death as an outcome overestimates the association between hypertension and impaired fasting glucose and cancer mortality in our study. Additional studies with perspective design are warranted to examine the race-specific association between hypertension and impaired fasting glucose and site-specific cancer death.
MS risk components such as low HDL and central obesity were also significantly associated with cancer death in whites but not in blacks. There are inconsistent associations between lipids and cancer risk and outcomes across epidemiological studies (48); and few studies examined the particular relationship between low HDL and cancer outcomes (48). Central obesity was previously associated with risk of cancer death in several race-combined studies or studies that mostly included white participants (49,50). The sparse and inconclusive findings warrant additional research attention to race-specific analysis of low HDL or central obesity and cancer death.
Limitations
The study has several limitations. Firstly, all metabolic risk factors in this study were only measured once at baseline. Therefore, we were not able to examine any time-varying effects of these factors on cancer mortality by race group. Secondly, we could not analyze the association between metabolic risk factors and each type of cancer mortality because the number of site-specific cancer death was very small in NHANES III. Of all sites of cancer, lung cancer contributed to the largest number of deaths (n=201) but the relationship between MS and its mortality was not significant as reported previously (19). Thirdly, because this is an observational study, it is possible that unmeasured (e.g., other treatments for MS or cancer) confounders could have distorted the results. However, we have tested and adjusted a series of confounders in the analysis from available information in NHANES III. Furthermore, the observed associations may not be causal in our study. However, the strength of the association, the dose-response manner and biological plausibility lend strong support to the relationship between metabolic risk factors and cancer death.
Strengths
The major strength of this study is that the analysis was based on a large prospective cohort. This study covers a nationally representative sample of the two major racial groups. All five MS components were objectively measured with validated assessment tools or experimental methods. This precludes recall bias that is often seen in questionnaire-based exposure measurement.
Conclusions
In summary, we found effect of metabolic risk factors on total cancer mortality differed by race. High BP was significantly associated with total cancer death in blacks while in whites, central obesity, low HDL and impaired fasting glucose in particular were positively associated with cancer death. The results highlight the importance of early detection and management of metabolic risks especially hypertension and prediabetes/diabetes to prevent cancer death in blacks and whites, respectively. Active monitoring of BP throughout the period of cancer treatment is recommended for cancer patients especially for black cancer patients with elevated BP (51). Awareness and management of the glycemic status should also be part of patients’ cancer care plans particularly for white cancer patients with prediabetes/diabetes (52). Integrating cancer-care and co-management of metabolic risk conditions is set to play an increasing role in modern health services (51-53). With respects to research, further exploration of lifestyle modifications for MS in clinical trials and observational studies that include blacks and whites in cancer prognosis and survival are warranted. Further studies need to investigate whether overall MS, or individual metabolic risk factors, is associated with death from certain types of cancer, and whether the association varies by race.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hui-Yi Lin, Tung-Sung Tseng) for the series “Population Science in Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.06.25). The series “Population Science in Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The National Center for Health Statistics Institutional Review Board approved the survey protocols, and informed consent was obtained from all subjects.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Satcher D, Fryer GE Jr, McCann J, et al. What if we were equal? A comparison of the black-white mortality gap in 1960 and 2000. Health Aff (Millwood) 2005;24:459-64. [Crossref] [PubMed]
- Siddharth S, Sharma D. Racial Disparity and Triple-Negative Breast Cancer in African-American Women: A Multifaceted Affair between Obesity, Biology, and Socioeconomic Determinants. Cancers (Basel) 2018;10: [Crossref] [PubMed]
- Smith CJ, Minas TZ, Ambs S. Analysis of Tumor Biology to Advance Cancer Health Disparity Research. Am J Pathol 2018;188:304-16. [Crossref] [PubMed]
- Hyslop T, Weinberg DS, Schulz S, et al. Occult tumor burden contributes to racial disparities in stage-specific colorectal cancer outcomes. Cancer 2012;118:2532-40. [Crossref] [PubMed]
- Le H, Ziogas A, Lipkin SM, et al. Effects of socioeconomic status and treatment disparities in colorectal cancer survival. Cancer Epidemiol Biomarkers Prev 2008;17:1950-62. [Crossref] [PubMed]
- Hossain F, Danos D, Prakash O, et al. Neighborhood Social Determinants of Triple Negative Breast Cancer. Front Public Health 2019;7:18. [Crossref] [PubMed]
- Sharma S, O'Keefe SJ. Environmental influences on the high mortality from colorectal cancer in African Americans. Postgrad Med J 2007;83:583-9. [Crossref] [PubMed]
- Krieger N, Chen JT, Waterman PD, et al. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter?: the Public Health Disparities Geocoding Project. Am J Epidemiol 2002;156:471-82. [Crossref] [PubMed]
- Copson E, Maishman T, Gerty S, et al. Ethnicity and outcome of young breast cancer patients in the United Kingdom: the POSH study. Br J Cancer 2014;110:230-41. [Crossref] [PubMed]
- Govindarajan R, Shah RV, Erkman LG, et al. Racial differences in the outcome of patients with colorectal carcinoma. Cancer 2003;97:493-8. [Crossref] [PubMed]
- McGee SA, Durham DD, Tse CK, et al. Determinants of breast cancer treatment delay differ for African American and White women. Cancer Epidemiol Biomarkers Prev 2013;22:1227-38. [Crossref] [PubMed]
- Kim S, Dolecek TA, Davis FG. Racial differences in stage at diagnosis and survival from epithelial ovarian cancer: a fundamental cause of disease approach. Soc Sci Med 2010;71:274-81. [Crossref] [PubMed]
- Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640-5. [Crossref] [PubMed]
- Aguilar M, Bhuket T, Torres S, et al. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA 2015;313:1973-4. [Crossref] [PubMed]
- Ballantyne CM, Hoogeveen RC, McNeill AM, et al. Metabolic syndrome risk for cardiovascular disease and diabetes in the ARIC study. Int J Obes (Lond) 2008;32:S21-4. [Crossref] [PubMed]
- Braun S, Bitton-Worms K, LeRoith D. The link between the metabolic syndrome and cancer. Int J Biol Sci 2011;7:1003-15. [Crossref] [PubMed]
- Lorenzo C, Okoloise M, Williams K, et al. The metabolic syndrome as predictor of type 2 diabetes: the San Antonio heart study. Diabetes Care 2003;26:3153-9. [Crossref] [PubMed]
- Esposito K, Chiodini P, Colao A, et al. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care 2012;35:2402-11. [Crossref] [PubMed]
- Gathirua-Mwangi WG, Monahan PO, Murage MJ, et al. Metabolic syndrome and total cancer mortality in the Third National Health and Nutrition Examination Survey. Cancer Causes Control 2017;28:127-36. [Crossref] [PubMed]
- Jaggers JR, Sui X, Hooker SP, et al. Metabolic syndrome and risk of cancer mortality in men. Eur J Cancer 2009;45:1831-8. [Crossref] [PubMed]
- Matthews CE, Sui X, LaMonte MJ, et al. Metabolic syndrome and risk of death from cancers of the digestive system. Metabolism 2010;59:1231-9. [Crossref] [PubMed]
- Ni J, Zhu T, Zhao L, et al. Metabolic syndrome is an independent prognostic factor for endometrial adenocarcinoma. Clin Transl Oncol 2015;17:835-9. [Crossref] [PubMed]
- Lackland DT. Racial differences in hypertension: implications for high blood pressure management. Am J Med Sci 2014;348:135-8. [Crossref] [PubMed]
- Mensah GA, Mokdad AH, Ford ES, et al. State of disparities in cardiovascular health in the United States. Circulation 2005;111:1233-41. [Crossref] [PubMed]
- Rosenstock S, Whitman S, West JF, et al. Racial disparities in diabetes mortality in the 50 most populous US cities. J Urban Health 2014;91:873-85. [Crossref] [PubMed]
- Braithwaite D, Tammemagi CM, Moore DH, et al. Hypertension is an independent predictor of survival disparity between African-American and white breast cancer patients. Int J Cancer 2009;124:1213-9. [Crossref] [PubMed]
- Wu AH, Kurian AW, Kwan ML, et al. Diabetes and other comorbidities in breast cancer survival by race/ethnicity: the California Breast Cancer Survivorship Consortium (CBCSC). Cancer Epidemiol Biomarkers Prev 2015;24:361-8. [Crossref] [PubMed]
- Santorelli ML, Hirshfield KM, Steinberg MB, et al. Racial differences in the effects of comorbidity on breast cancer-specific survival. Cancer Causes Control 2017;28:809-17. [Crossref] [PubMed]
- Saydah SH, Loria CM, Eberhardt MS, et al. Abnormal glucose tolerance and the risk of cancer death in the United States. Am J Epidemiol 2003;157:1092-100. [Crossref] [PubMed]
- National health and nutritoin examination survey data [database on the Internet] 2016. Accessed: 2017.
- Grundy SM, Brewer HB Jr, Cleeman JI, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004;109:433-8. [Crossref] [PubMed]
- Park YW, Zhu S, Palaniappan L, et al. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med 2003;163:427-36. [Crossref] [PubMed]
- Bursac Z, Gauss CH, Williams DK, et al. Purposeful selection of variables in logistic regression. Source Code Biol Med 2008;3:17. [Crossref] [PubMed]
- Clark LT, El-Atat F. Metabolic syndrome in African Americans: implications for preventing coronary heart disease. Clin Cardiol 2007;30:161-4. [Crossref] [PubMed]
- Kung HC, Xu J. Hypertension-related Mortality in the United States, 2000-2013. NCHS Data Brief 2015;1-8. [PubMed]
- Lee JS, Cho SI, Park HS. Metabolic syndrome and cancer-related mortality among Korean men and women. Ann Oncol 2010;21:640-5. [Crossref] [PubMed]
- Stocks T, Van Hemelrijck M, Manjer J, et al. Blood pressure and risk of cancer incidence and mortality in the Metabolic Syndrome and Cancer Project. Hypertension 2012;59:802-10. [Crossref] [PubMed]
- Akinyemiju T, Moore JX, Judd S, et al. Metabolic dysregulation and cancer mortality in a national cohort of blacks and whites. BMC Cancer 2017;17:856. [Crossref] [PubMed]
- Meyer P. Increased intracellular calcium: from hypertension to cancer. J Hypertens Suppl 1987;5:S3-4. [Crossref] [PubMed]
- Largent JA, McEligot AJ, Ziogas A, et al. Hypertension, diuretics and breast cancer risk. J Hum Hypertens 2006;20:727-32. [Crossref] [PubMed]
- Hamet P. Cancer and hypertension. An unresolved issue. Hypertension 1996;28:321-4. [Crossref] [PubMed]
- Grossman E, Messerli FH, Boyko V, et al. Is there an association between hypertension and cancer mortality? Am J Med 2002;112:479-86. [Crossref] [PubMed]
- Zhou XH, Qiao Q, Zethelius B, et al. Diabetes, prediabetes and cancer mortality. Diabetologia 2010;53:1867-76. [Crossref] [PubMed]
- Hirakawa Y, Ninomiya T, Mukai N, et al. Association between glucose tolerance level and cancer death in a general Japanese population: the Hisayama Study. Am J Epidemiol 2012;176:856-64. [Crossref] [PubMed]
- Abe R, Yamagishi S. AGE-RAGE system and carcinogenesis. Curr Pharm Des 2008;14:940-5. [Crossref] [PubMed]
- Rajpathak SN, Gunter MJ, Wylie-Rosett J, et al. The role of insulin-like growth factor-I and its binding proteins in glucose homeostasis and type 2 diabetes. Diabetes Metab Res Rev 2009;25:3-12. [Crossref] [PubMed]
- Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 2008;8:915-28. [Crossref] [PubMed]
- Radisauskas R, Kuzmickiene I, Milinaviciene E, et al. Hypertension, serum lipids and cancer risk: A review of epidemiological evidence. Medicina (Kaunas) 2016;52:89-98. [Crossref] [PubMed]
- Hidayat K, Du X, Chen G, et al. Abdominal Obesity and Lung Cancer Risk: Systematic Review and Meta-Analysis of Prospective Studies. Nutrients 2016;8. [PubMed]
- Leitzmann MF, Moore SC, Koster A, et al. Waist circumference as compared with body-mass index in predicting mortality from specific causes. PLoS One 2011;6:e18582. [Crossref] [PubMed]
- Mouhayar E, Salahudeen A. Hypertension in cancer patients. Tex Heart Inst J 2011;38:263-5. [PubMed]
- Hershey DS. Importance of Glycemic Control in Cancer Patients with Diabetes: Treatment through End of Life. Asia Pac J Oncol Nurs 2017;4:313-8. [Crossref] [PubMed]
- Sarfati D, Koczwara B, Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer J Clin 2016;66:337-50. [Crossref] [PubMed]


