Survival benefit of radiotherapy in metastatic esophageal cancer: a population-based study
Introduction
Esophageal cancer (EC) is one of the most fatal malignant tumors globally, with a 5-year survival rate of 15–25% (1-3). With the improvement in diagnostic techniques, there are more than 50% patients detected at metastatic stage with incurable metastatic disease at diagnosis. For metastatic EC with distant metastasis, systematic chemotherapy is recommended as the standard therapy, however, the overall survival (OS) is still poor. External beam radiotherapy has been performed in the management for EC as definitive, preoperative, postoperative, or palliative therapy combining with chemotherapy (4-7). For patients with metastatic EC, radiotherapy is used as a palliative treatment modality to relieve symptoms such as dysphagia and chest pain (8,9). However, the effect of radiotherapy on survival of patients with metastatic EC is unclear.
The purpose of this study was to assess the impact of radiotherapy on the OS of metastatic EC based on the data available in the Surveillance, Epidemiology, and End Results (SEER) database, attempting to provide a novel concept for the change of traditional treatment modality to metastatic EC.
Methods
Patients
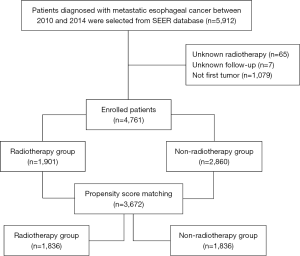
The SEER database, one of the largest databases of oncology patients in the United States, includes cancer incidence, treatment, and survival information for approximately 30% of the US population. SEER*Stat software version 8.3.5 was used and SEER data between 1973 and 2014 [“Incidence-SEER 18 Regs Custom data (with additional treatment fields), Nov 2016 sub (1973–2014 varying)”] was chosen for this study. Adult patients diagnosed with EC who had metastatic diseases between 2010 and 2014 (n=5,912) were enrolled. Patients treated with beam radiation (combined with or without other type of radiotherapy) were included in the cohort. Patients with unknown radiotherapy data were excluded (n=65). Patients for whom the presence of follow-up was unknown were not included (n=7). In addition, patients who were presented with “N/A not first tumor” were excluded (within SEER database) (n=1,079). All authors did not have access to information that could identify individual participants (Figure 1).
Statistical analysis
The enrolled patients were divided into two groups, radiotherapy group and non-radiotherapy group, and were longitudinally classified by age, sex, insurance, histological type, differentiation, metastatic sites (the bone, brain, liver and lung), chemotherapy code. Absolute numbers and incidence proportions were calculated.
All statistical analyses were performed using the SPSS statistical software (version 22.0; IBM Corporation). Propensity score matching model was performed to reduce the bias of patients’ selection and obtain the balanced population of radiotherapy and non-radiotherapy group. The standardized differences for matched variables were less than 0.1. Chi-square test was used to identify the differences of two groups. Kaplan-Meier method was used to obtain survival information. Log-rank test and Cox regression analyses were implemented to evaluate covariates for OS and esophageal cause-specific survival (CSS). A value of P<0.05 were considered statistically significant for all analyses.
Results
A total of 4,761 patients were finally enrolled in this study, including 1,901 with radiotherapy and 2,870 without. The baseline features of the 4,761 eligible patients are provided in Table 1. The majority of the patients were 60 to 79 years old (55.1%), male (84.1%), insured (92.3%), white (84.9%), adenocarcinoma (64.5%), poorly differentiated (47.9%). As for metastatic sites (to the brain, bone, lung, and liver), 3178 (66.8%) of patients had 1 or 2 metastatic sites, 202 patients (4.2%) had 3 or 4 sites. There were 993 (20.9%) patients who had no metastasis in any site of the liver, brain, bone and lung.
Table 1
| Characteristics | Before propensity score matching, n (%) | After propensity score matching, n (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All patients (n=4,761) | Radiotherapy | P | All patients (n=3,672) | Radiotherapy | P | ||||||
| Yes (n=1,901) | No (n=2,860) | Yes (n=1,836) | No (n=1,836) | ||||||||
| Age, year | <0.001 | 0.229 | |||||||||
| 20–39 | 70 (1.5) | 19 (1.0) | 51 (1.8) | 50 (1.4) | 18 (1.0) | 32 (1.7) | |||||
| 40–59 | 1,559 (32.7) | 670 (35.2) | 889 (31.1) | 1,257 (34.2) | 636 (34.6) | 621 (33.8) | |||||
| 60–79 | 2,622 (55.1) | 1,051 (55.3) | 1,571 (54.9) | 2,037 (55.5) | 1,022 (55.7) | 1,015 (55.3) | |||||
| ≥80 | 510 (10.7) | 161 (8.5) | 349 (12.2) | 328 (8.9) | 160 (8.7) | 168 (9.2) | |||||
| Sex | 0.868 | 0.383 | |||||||||
| Male | 4,002 (84.1) | 1,600 (84.2) | 2,402 (84.0) | 3,113 (84.8) | 1,547 (84.3) | 1,566 (85.3) | |||||
| Female | 759 (15.9) | 301 (15.8) | 458 (16.0) | 559 (15.2) | 189 (15.7) | 270 (14.7) | |||||
| Insurance code | 0.003 | 0.093 | |||||||||
| Insured | 4,396 (92.3) | 1,783 (93.8) | 2,613 (91.4) | 3,421 (93.2) | 1,723 (93.8) | 1,698 (92.5) | |||||
| Uninsured | 235 (4.9) | 82 (4.3) | 153 (5.3) | 160 (4.4) | 77 (4.2) | 83 (4.5) | |||||
| Unknown | 130 (2.7) | 36 (1.9) | 94 (3.3) | 91 (2.5) | 36 (2.0) | 55 (3.0) | |||||
| Race | <0.001 | 0.868 | |||||||||
| White | 4,043 (84.9) | 1,563 (82.2) | 2,480 (86.7) | 3,089 (84.1) | 1,543 (84.0) | 1,546 (84.2) | |||||
| Black | 465 (9.8) | 212 (11.2) | 253 (8.8) | 363 (9.9) | 180 (9.8) | 183 (10.0) | |||||
| Other | 237 (5.0) | 120 (6.3) | 117 (4.1) | 207 (5.6) | 107 (5.8) | 100 (5.4) | |||||
| Unknown | 16 (0.3) | 6 (0.3) | 10 (0.3) | 13 (0.4) | 6 (0.3) | 7 (0.4) | |||||
| Histological type | <0.001 | 0.002 | |||||||||
| SCC | 1,118 (23.5) | 547 (28.8) | 571 (20.0) | 913 (24.9) | 492 (26.8) | 421 (22.9) | |||||
| ADC | 3,072 (64.5) | 1,174 (61.8) | 1,898 (66.4) | 2,370 (64.5) | 1,164 (63.4) | 1,206 (65.7) | |||||
| Other | 330 (6.9) | 134 (7.0) | 196 (6.9) | 243 (6.6) | 134 (7.3) | 109 (5.9) | |||||
| Unknown | 241 (5.1) | 46 (2.4) | 195 (6.8) | 146 (4.0) | 46 (2.5) | 100 (5.4) | |||||
| Differentiation | <0.001 | 0.147 | |||||||||
| Poorly differentiated | 2,282 (47.9) | 929 (48.9) | 1,353 (47.3) | 1,801 (49.0) | 901 (49.1) | 900 (49.0) | |||||
| Moderately differentiated | 1,261 (26.5) | 542 (28.5) | 719 (25.1) | 961 (26.2) | 518 (28.2) | 443 (24.1) | |||||
| Well differentiated | 102 (2.1) | 48 (2.5) | 54 (1.9) | 84 (2.3) | 46 (2.5) | 38 (2.1) | |||||
| Undifferentiated | 72 (1.5) | 32 (1.7) | 40 (1.4) | 53 (1.4) | 32 (1.7) | 21 (1.1) | |||||
| Unknown | 944 (19.8) | 350 (18.4) | 694 (24.3) | 773 (21.1) | 339 (18.5) | 434 (23.6) | |||||
| Bone metastasis | <0.001 | <0.001 | |||||||||
| Yes | 1,123 (23.6) | 564 (29.7) | 559 (19.5) | 884 (24.1) | 550 (30.0) | 334 (18.2) | |||||
| No | 3,437 (72.2) | 1,278 (67.2) | 2,159 (75.5) | 2,659 (72.4) | 1,227 (66.8) | 1,432 (78.0) | |||||
| Unknown | 201 (4.2) | 59 (3.1) | 142 (5.0) | 129 (3.5) | 59 (3.2) | 70 (3.8) | |||||
| Brain metastasis | <0.001 | <0.001 | |||||||||
| Yes | 256 (5.4) | 181 (9.5) | 75 (2.6) | 226 (6.2) | 181 (9.9) | 45 (2.5) | |||||
| No | 4,269 (89.7) | 1,652 (86.9) | 2,617 (91.5) | 3,291 (89.6) | 1,587 (86.4) | 1,704 (92.8) | |||||
| Unknown | 236 (5.0) | 68 (3.6) | 168 (5.9) | 155 (4.2) | 68 (3.7) | 87 (4.7) | |||||
| Liver metastasis | <0.001 | <0.001 | |||||||||
| Yes | 2,270 (47.7) | 675 (35.5) | 1,595 (55.8) | 1,652 (45.0) | 666 (36.3) | 986 (53.7) | |||||
| No | 2,321 (48.8) | 1,170 (61.5) | 1,151 (40.2) | 1,906 (51.9) | 1,115 (60.7) | 791 (43.1) | |||||
| Unknown | 170 (3.6) | 56 (3.0) | 114 (4.0) | 114 (3.1) | 55 (3.0) | 59 (3.2) | |||||
| Lung metastasis | 0.001 | 0.291 | |||||||||
| Yes | 1,375 (28.9) | 526 (27.7) | 849 (29.7) | 1,037 (28.2) | 510 (27.8) | 527 (28.7) | |||||
| No | 3,132 (65.8) | 1,297 (68.2) | 1,835 (64.2) | 2,470 (67.3) | 1,249 (68.0) | 1,221 (66.5) | |||||
| Unknown | 254 (5.3) | 78 (4.1) | 176 (6.2) | 165 (4.5) | 77 (4.2) | 88 (4.8) | |||||
| Metastatic sites to the brain, bone, lung, and liver | <0.001 | 0.084 | |||||||||
| 0 | 993 (20.9) | 487 (25.6) | 506 (17.7) | 828 (22.5) | 456 (24.8) | 372 (20.3) | |||||
| 1–2 | 3,178 (66.8) | 1,196 (62.9) | 1,982 (69.3) | 2,449 (66.7) | 1,163 (63.3) | 1,286 (70.0) | |||||
| 3–4 | 202 (4.2) | 104 (5.5) | 98 (3.4) | 142 (3.9) | 104 (5.7) | 38 (2.1) | |||||
| Unknown | 388 (8.1) | 114 (6.0) | 274 (9.6) | 253 (6.9) | 113 (6.2) | 140 (7.6) | |||||
| Chemotherapy | <0.001 | <0.001 | |||||||||
| Yes | 2,880 (60.5) | 1,403 (73.8) | 1,477 (51.6) | 2,494 (67.9) | 1,338 (72.9) | 1,156 (63.0) | |||||
| No/unknown | 1,881 (39.5) | 498 (26.2) | 1,383 (48.4) | 1,178 (32.1) | 498 (27.1) | 680 (37.0) | |||||
SCC, squamous carcinoma; ADC, adenocarcinoma.
The median OS (mOS) and CSS (mCSS) for the entire cohort were 4.9 and 5.0 months, and the radiotherapy group was 7.0 and 6.9 months, while 3.0 and 4.0 months in the non-radiotherapy group, respectively (P<0.001) (Figure 2A,B). Univariate and multivariable Cox regression demonstrated that radiotherapy was significantly associated with longer mOS and mCSS (Tables 2,3). In addition, metastatic disease to 1–2 sites (P<0.001) or 3–4 metastatic sites (P<0.001) of the brain, bone, lung and liver, or greater than 80 years (P=0.002), uninsured status (P<0.001), and non-chemotherapy treatment (P<0.001) were significantly associated with poorer OS, while age 20 to 39 years (P=0.002), 40 to 59 years (P=0.002), 60 to 79 years (P=0.003), female (P<0.001), ADC (P=0.035), moderately differentiated (P<0.001) and well differentiated tumor (P=0.040) were significantly associated with better OS. Moreover, metastatic disease to 1–2 sites (P<0.001) or 3–4 sites (P<0.001), greater than 80 years old (P=0.016), uninsured status (P<0.001), non-chemotherapy treatment (P<0.001) were significantly associated with decreased CSS. Factors that were statistically associated with longer CSS in multivariable Cox regression analysis were female (P<0.001) and moderately differentiated tumor (P<0.001).
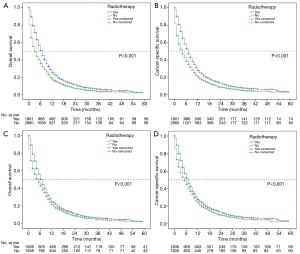
Table 2
| Characteristics | Before propensity score matching | After propensity score matching | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OS | CSS | OS | CSS | |||||||||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |||||||
| Age, y | ||||||||||||||||||
| ≥80 | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||||||||
| 60–79 | 0.665 | 0.601–0.735 | <0.001 | 0.688 | 0.620–0.765 | <0.001 | 0.714 | 0.629–0.809 | 0.001 | 0.753 | 0.660–0.860 | <0.001 | ||||||
| 40–59 | 0.604 | 0.543–0.673 | <0.001 | 0.628 | 0.562–0.703 | <0.001 | 0.644 | 0.564–0.735 | <0.001 | 0.686 | 0.597–0.788 | <0.001 | ||||||
| 20–39 | 0.496 | 0.371–0.663 | <0.001 | 0.531 | 0.396–0.712 | <0.001 | 0.570 | 0.405–0.803 | <0.001 | 0.617 | 0.435–0.875 | <0.001 | ||||||
| Sex | ||||||||||||||||||
| Male | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||||||||
| Female | 0.915 | 0.839–0.997 | 0.043 | 0.990 | 0.823–0.984 | 0.021 | 0.882 | 0.797–0.977 | 0.016 | 0.867 | 0.780–0.963 | 0.008 | ||||||
| Insurance code | ||||||||||||||||||
| Insured | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||||||||
| Uninsured | 1.376 | 1.198–1.581 | <0.001 | 1.386 | 1.202–1.598 | <0.001 | 1.446 | 1.224–1.708 | <0.001 | 1.444 | 1.217–1.714 | <0.001 | ||||||
| Unknown | 1.175 | 0.975–1.418 | 0.091 | 1.132 | 0.931–1.378 | 0.214 | 1.173 | 0.937–1.469 | 0.165 | 1.160 | 0.919–1.464 | 0.211 | ||||||
| Race | ||||||||||||||||||
| White | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||||||||
| Black | 1.127 | 1.017–1.248 | 0.023 | 1.142 | 1.028–1.268 | 0.013 | 1.143 | 1.017–1.284 | 0.025 | 1.151 | 1.021–1.297 | 0.021 | ||||||
| Other | 0.869 | 0.752–1.004 | 0.056 | 0.842 | 0.724–0.979 | 0.025 | 0.917 | 0.785–1.071 | 0.273 | 0.900 | 0.767–1.057 | 0.200 | ||||||
| Unknown | 0.994 | 0.577–1.714 | 0.984 | 0.970 | 0.550–1.709 | 0.915 | 0.940 | 0.505–1.749 | 0.844 | 0.892 | 0.464–1.717 | 0.733 | ||||||
| Histological type | ||||||||||||||||||
| SCC | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||||||||
| ADC | 0.881 | 0.818–0.949 | 0.001 | 0.880 | 0.815–0.950 | 0.001 | 0.865 | 0.787–0.929 | <0.001 | 0.853 | 0.783–0.929 | <0.001 | ||||||
| Other | 1.059 | 0.928–1.209 | 0.396 | 1.070 | 0.934–1.225 | 0.330 | 1.081 | 0.927–1.261 | 0.318 | 1.083 | 0.926–1.268 | 0.318 | ||||||
| Unknown | 1.576 | 1.364–1.821 | <0.001 | 1.511 | 1.299–1.757 | <0.001 | 1.512 | 1.264–1.810 | <0.001 | 1.459 | 1.210–1.759 | <0.001 | ||||||
| Differentiation | ||||||||||||||||||
| Poor | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||||||||
| Moderate | 0.792 | 0.734–0.855 | <0.001 | 0.798 | 0.738–0.862 | <0.001 | 0.741 | 0.679–0.808 | <0.001 | 0.753 | 0.689–0.823 | <0.001 | ||||||
| Well | 0.802 | 0.647–0.995 | 0.045 | 0.810 | 0.650–1.009 | 0.061 | 0.802 | 0.632–1.018 | 0.069 | 0.816 | 0.639–1.040 | 0.101 | ||||||
| Undifferentiated | 1.030 | 0.800–1.326 | 0.820 | 1.054 | 0.815–1.364 | 0.686 | 1.010 | 0.753–1.354 | 0.947 | 1.048 | 0.779–1.410 | 0.759 | ||||||
| Unknown | 0.964 | 0.891–1.044 | 0.373 | 0.958 | 0.882–1.040 | 0.303 | 0.880 | 0.802–0.966 | 0.007 | 0.877 | 0.796–0.965 | 0.007 | ||||||
| Bone metastasis | ||||||||||||||||||
| No | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||||||||
| Yes | 1.391 | 1.293–1.495 | <0.001 | 1.399 | 1.29801.507 | <0.001 | 1.436 | 1.332–1.560 | <0.001 | 1.143 | 1.326–1.571 | <0.001 | ||||||
| Unknown | 1.108 | 0.950–1.292 | 0.191 | 1.069 | 0.911–1.255 | 0.413 | 1.045 | 0.861–1.267 | 0.658 | 1.033 | 0.846–1.262 | 0.748 | ||||||
| Brain metastasis | ||||||||||||||||||
| No | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||||||||
| Yes | 1.255 | 1.097–1.435 | 0.001 | 1.255 | 1.093–1.441 | 0.001 | 1.282 | 1.109–1.482 | 0.001 | 1.287 | 1.109–1.493 | 0.001 | ||||||
| Unknown | 1.060 | 0.920–1.221 | 0.421 | 1.024 | 0.883–1.188 | 0.751 | 0.987 | 0.827–1.178 | 0.888 | 0.978 | 0.815–1.174 | 0.813 | ||||||
| Liver metastasis | ||||||||||||||||||
| No | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||||||||
| Yes | 1.246 | 1.170–1.327 | <0.001 | 1.265 | 1.185–1.350 | <0.001 | 1.264 | 1.176–1.359 | <0.001 | 1.282 | 1.190–1.381 | <0.001 | ||||||
| Unknown | 1.109 | 0.938–1.313 | 0.226 | 1.058 | 0.886–1.264 | 0.530 | 0.988 | 0.803–1.215 | 0.907 | 0.939 | 0.754–1.168 | 0.570 | ||||||
| Lung metastasis | ||||||||||||||||||
| No | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||||||||
| Yes | 1.279 | 1.194–1.370 | <0.001 | 1.274 | 1.187–1.368 | <0.001 | 1.295 | 1.196–1.402 | <0.001 | 1.294 | 1.193–1.404 | <0.001 | ||||||
| Unknown | 1.090 | 0.948–1.253 | 0.228 | 1.086 | 0.941–1.253 | 0.261 | 0.985 | 0.827–1.173 | 0.865 | 0.994 | 0.831–1.189 | 0.951 | ||||||
| Metastatic sites to the brain, bone, lung, and liver | ||||||||||||||||||
| 0 | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||||||||
| 1–2 | 1.458 | 1.346–1.579 | <0.001 | 1.473 | 1.357–1.600 | <0.001 | 1.499 | 1.371–1.639 | <0.001 | 1,157 | 1.384–1.664 | <0.001 | ||||||
| 3–4 | 2.268 | 1.932–2.664 | <0.001 | 2.290 | 1.941–2.701 | <0.001 | 2.253 | 1.945–2.847 | <0.001 | 2.377 | 1.954–2.892 | <0.001 | ||||||
| Unknown | 1.556 | 1.378–1.780 | <0.001 | 1.563 | 1.370–1.784 | <0.001 | 1.435 | 1.229–1.676 | <0.001 | 1.452 | 1.238–1.704 | <0.001 | ||||||
| Radiotherapy | ||||||||||||||||||
| Yes | 1.000 | 1.000 | 1.000 | |||||||||||||||
| No | 1.407 | 1.320–1.499 | <0.001 | 1.405 | 1.316–1.500 | <0.001 | 1.231 | 1.146–1.322 | <0.001 | 1.293 | 1.146–1.327 | <0.001 | ||||||
| Chemotherapy | ||||||||||||||||||
| Yes | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||||||||
| No/unknown | 3.367 | 3.153–3.596 | <0.001 | 3.341 | 3.123–3.575 | <0.001 | 3.331 | 3.083–3.600 | <0.001 | 3.314 | 3.060–3.590 | <0.001 | ||||||
OS, overall survival; CSS, cancer-specific survival.
Table 3
| Characteristics | Before propensity score matching | After propensity score matching | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OS | CSS | OS | CSS | |||||||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |||||
| Age, y | ||||||||||||||||
| ≥80 | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||||||
| 60–79 | 0.853 | 0.769–0.947 | 0.003 | 0.875 | 0.785–0.975 | 0.016 | 0.867 | 0.762–0.986 | 0.030 | 0.915 | 0.799–1.048 | 0.198 | ||||
| 40–59 | 0.837 | 0.749–0.937 | 0.002 | 0.859 | 0.765–0.966 | 0.011 | 0.835 | 0.727–0.958 | 0.010 | 0.889 | 0.769–1.027 | 0.111 | ||||
| 20–39 | 0.623 | 0.465–0.835 | 0.002 | 0.661 | 0.491–0.890 | 0.006 | 0.653 | 0.462–0.923 | 0.016 | 0.915 | 0.799–1.048 | 0.198 | ||||
| Sex | ||||||||||||||||
| Male | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||||||
| Female | 0.854 | 0.782–0.934 | <0.001 | 0.840 | 0.766–0.920 | <0.001 | 0.828 | 0.746–0.920 | <0.001 | 0.815 | 0.731–0.908 | <0.001 | ||||
| Insurance code | ||||||||||||||||
| Insured | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||||||
| Uninsured | 1.343 | 1.166–1.547 | <0.001 | 1.332 | 1.151–1.540 | <0.001 | 1.404 | 1.184–1.663 | <0.001 | 1.387 | 1.165–1.652 | <0.001 | ||||
| Unknown | 0.881 | 0.727–1.068 | 0.197 | 0.854 | 0.698–1.045 | 0.126 | 0.958 | 0.758–1.210 | 0.718 | 0.953 | 0.749–1.213 | 0.696 | ||||
| Race | ||||||||||||||||
| White | 1.000 | 1.000 | 1.000 | |||||||||||||
| Black | 1.073 | 0.959–1.202 | 0.220 | 1.083 | 0.965–1.217 | 0.175 | 1.052 | 0.925–1.197 | 0.439 | 1.051 | 0.921–1.199 | 0.461 | ||||
| Other | 0.894 | 0.771–1.036 | 0.137 | 0.865 | 0.741–1.009 | 0.064 | 0.897 | 0.765–1.051 | 0.177 | 0.879 | 0.746–1.036 | 0.124 | ||||
| Unknown | 0.881 | 0.507–1.534 | 0.655 | 0.858 | 0.482–1.527 | 0.602 | 0.804 | 0.424–1.523 | 0.503 | 0.750 | 0.382–1.470 | 0.402 | ||||
| Histological type | ||||||||||||||||
| SCC | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||||||
| ADC | 0.920 | 0.852–0.994 | 0.035 | 0.925 | 0.848–1.009 | 0.078 | 0.933 | 0.848–1.026 | 0.151 | 0.924 | 0.838–1.018 | 0.110 | ||||
| Other | 1.019 | 0.889–1.169 | 0.787 | 1.048 | 0.908–1.210 | 0.521 | 1.130 | 0.959–1.331 | 0.144 | 1.132 | 0.957–1.340 | 0.149 | ||||
| Unknown | 1.258 | 1.081–1.464 | 0.003 | 1.221 | 1.038–1.435 | 0.016 | 1.330 | 1.101–1.607 | 0.003 | 1.285 | 1.055–1.564 | 0.013 | ||||
| Differentiation | ||||||||||||||||
| Poor | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||||||
| Moderate | 0.767 | 0.710–0.828 | <0.001 | 0.772 | 0.713–0.835 | <0.001 | 0.766 | 0.701–0.837 | <0.001 | 0.778 | 0.711–0.853 | <0.001 | ||||
| Well | 0.798 | 0.643–0.990 | 0.040 | 0.804 | 0.645–1.003 | 0.053 | 0.785 | 0.618–0.997 | 0.047 | 0.795 | 0.622–1.105 | 0.065 | ||||
| Undifferentiated | 0.855 | 0.660–1.109 | 0.238 | 0.888 | 0.681–1.158 | 0.380 | 0.809 | 0.594–1.102 | 0.179 | 0.845 | 0.618–1.155 | 0.290 | ||||
| Unknown | 0.798 | 0.735–0.867 | <0.001 | 0.800 | 0.735–0.871 | <0.001 | 0.772 | 0.702–0.849 | <0.001 | 0.771 | 0.699–0.851 | <0.001 | ||||
| Metastatic sites to the brain, bone, lung, and liver | ||||||||||||||||
| 0 | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||||||
| 1–2 | 1.357 | 1.251–1.471 | <0.001 | 1.363 | 1.253–1.482 | <0.001 | 1.414 | 1.291–1.548 | <0.001 | 1.427 | 1.300–1.567 | <0.001 | ||||
| 3–4 | 2.365 | 2.012–2.781 | <0.001 | 2.379 | 2.014–2.810 | <0.001 | 2.460 | 2.029–2.981 | <0.001 | 2.477 | 2.032–3.019 | <0.001 | ||||
| Unknown | 1.290 | 1.132–1.471 | <0.001 | 1.291 | 1.127–1.478 | <0.001 | 1.153 | 0.982–1.353 | 0.082 | 1.162 | 0.986–1.370 | 0.074 | ||||
| Radiotherapy | ||||||||||||||||
| Yes | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||||||
| No | 1.229 | 1.151–1.313 | <0.001 | 1.227 | 1.147–1.313 | <0.001 | 1.218 | 1.132–1.310 | <0.001 | 1.219 | 1.131–1.314 | <0.001 | ||||
| Chemotherapy | ||||||||||||||||
| Yes | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||||||
| No/unknown | 3.211 | 2.995–3.442 | <0.001 | 3.204 | 2.982–3.441 | <0.001 | 3.195 | 2.944–3.467 | <0.001 | 3.204 | 2.946–3.485 | <0.001 | ||||
OS, overall survival; CSS, cancer-specific survival.
After propensity score matching, 3,672 of 4,761 patients were included (1,836 for each of radiotherapy or non-radiotherapy group) (Table 1) and also found that radiotherapy improved OS and CSS (P<0.001) (Figure 2C,D). Radiotherapy showed significant 2-year survival benefits in patients with age older than 80 years (2-year OS, P=0.048; CSS, P=0.018), male (2-year OS, P=0.020; CSS, P=0.011), white race (2-year OS, P=0.038; CSS, P=0.006), squamous carcinoma (2-year OS, P=0.002; CSS, P<0.001), poor differentiation (2-year OS, P=0.002; CSS, P<0.001), brain (2-year OS, P<0.001; CSS, P<0.001) metastasis, other sites (sites except for the brain, bone, lung, and liver, 2-year OS, P<0.001; CSS, P<0.001), however, there were no statistically significant survival differences in patients with bone and liver metastasis, 1–2 or 3–4 metastatic sites (Table 4). Interestingly, no survival difference was found between chemotherapy alone and radiotherapy combined with chemotherapy (2-year OS, P=0.177; CSS, P=0.080). Cox regression demonstrated that radiotherapy was an independent prognostic factor which was significantly associated with longer mOS and mCSS in matched patients (Tables 2,3). In addition, metastatic disease to 1–2 sites (P<0.001) or 3–4 metastatic sites (P<0.001) of the brain, bone, lung and liver, age greater than 80 years (P≤0.030), uninsured status (P<0.001), male (P<0.001), poor differentiation (P≤0.047), non-chemotherapy treatment (P<0.001) were associated with poorer OS. While metastatic disease to 1–2 sites (P <0.001) or 3–4 sites (P<0.001), uninsured status (P<0.001), male (P<0.001), poor differentiation (P<0.001), non-chemotherapy treatment (P<0.001) were significantly associated with decreased CSS.
Table 4
| Characteristics | 2-year OS | 2-year CSS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RT (%) | Non-RT (%) | Z | P | RT (%) | Non-RT (%) | Z | P | ||
| RT | 11.6 | 8.8 | 2.325 | 0.020 | 13.4 | 9.9 | 2.602 | 0.009 | |
| Age | |||||||||
| 20–39 | 8.4 | 15.6 | 0.65 | 0.517 | 9.3 | 15.6 | −0.54 | 1.410 | |
| 40–59 | 12.3 | 10.6 | 0.83 | 0.407 | 13.6 | 11.9 | 0.75 | 0.452 | |
| 60–79 | 11.5 | 8.4 | 1.90 | 0.057 | 13.2 | 9.3 | 2.29 | 0.022 | |
| ≥80 | 9.1 | 2.9 | 1.98 | 0.048 | 13.8 | 4.2 | 2.37 | 0.018 | |
| Sex | |||||||||
| Male | 10.9 | 8.1 | 2.33 | 0.020 | 12.5 | 9.1 | 2.53 | 0.011 | |
| Female | 15.1 | 13.6 | 0.43 | 0.665 | 17.7 | 15.4 | 0.59 | 0.554 | |
| Insurance code | |||||||||
| Insured | 11.7 | 9.2 | 2.08 | 0.038 | 13.4 | 10.3 | 2.30 | 0.021 | |
| Uninsured | 8.2 | 0.0 | 2.28 | 0.023 | 10.2 | 0.0 | 2.43 | 0.015 | |
| Unknown | 15.3 | 7.5 | 1.00 | 0.320 | 16.7 | 8.1 | 1.01 | 0.312 | |
| Race | |||||||||
| White | 12.0 | 8.7 | 2.45 | 0.014 | 13.6 | 9.7 | 2.74 | 0.006 | |
| Black | 7.0 | 8.9 | 0.60 | 0.551 | 8.8 | 9.4 | 0.17 | 0.865 | |
| Other | 13.8 | 10.3 | 0.65 | 0.515 | 17.0 | 12.5 | 0.73 | 0.465 | |
| Unknown | 20.0 | 0.0 | 1.12 | 0.264 | 40.0 | 0.0 | 1.826 | 0.068 | |
| Histological type | |||||||||
| SCC | 12.1 | 5.6 | 3.04 | 0.002 | 13.8 | 6.0 | 3.51 | 0.000 | |
| ADC | 12.1 | 10.6 | 0.96 | 0.335 | 13.8 | 11.7 | 1.24 | 0.216 | |
| Other | 7.1 | 6.0 | 0.28 | 0.782 | 9.4 | 7.2 | 0.48 | 0.633 | |
| Unknown | 4.9 | 3.0 | 0.51 | 0.609 | 8.7 | 4.8 | 0.76 | 0.448 | |
| Differentiation | |||||||||
| Poor | 12.1 | 5.6 | 3.04 | 0.002 | 10.8 | 7.1 | 3.51 | 0.000 | |
| Moderate | 12.1 | 10.6 | 0.96 | 0.335 | 18.2 | 12.3 | 2.14 | 0.032 | |
| Well | 7.1 | 6.0 | 0.28 | 0.782 | 16.4 | 8.2 | 1.03 | 0.301 | |
| Undifferentiated | 4.9 | 3.0 | 0.51 | 0.609 | 0.0 | 15.1 | 1.61 | 0.108 | |
| Unknown | 12.1 | 5.6 | 3.04 | 0.002 | 13.6 | 13.1 | 0.16 | 0.870 | |
| Bone metastasis | |||||||||
| Yes | 4.2 | 4.6 | 0.26 | 0.798 | 5.1 | 5.5 | 0.20 | 0.841 | |
| No | 14.6 | 9.7 | 0.87 | 0.384 | 16.5 | 10.8 | 3.48 | 0.001 | |
| Unknown | 14.9 | 9.4 | 0.82 | 0.411 | 21.9 | 9.8 | 1.65 | 0.099 | |
| Brain metastasis | |||||||||
| Yes | 8.1 | 0.0 | 3.52 | 0.000 | 10.7 | 0.0 | 3.82 | 0.000 | |
| No | 11.6 | 9.0 | 2.16 | 0.031 | 13.2 | 10.1 | 2.30 | 0.021 | |
| Unknown | 18.9 | 9.5 | 1.45 | 0.148 | 23.1 | 10.0 | 1.89 | 0.059 | |
| Liver metastasis | |||||||||
| Yes | 7.0 | 8.7 | 1.04 | 0.296 | 8.4 | 9.7 | 0.71 | 0.481 | |
| No | 13.7 | 8.6 | 3.01 | 0.003 | 15.3 | 9.6 | 3.10 | 0.002 | |
| Unknown | 20.7 | 11.9 | 1.16 | 0.244 | 30.0 | 12.8 | 2.07 | 0.038 | |
| Lung metastasis | |||||||||
| Yes | 8.3 | 7.0 | 0.66 | 0.511 | 9.7 | 8.3 | 2.27 | 0.023 | |
| No | 12.4 | 9.4 | 2.02 | 0.044 | 14.1 | 10.4 | 1.78 | 0.076 | |
| Unknown | 20.1 | 12.3 | 1.20 | 0.229 | 24.6 | 12.7 | 1.78 | 0.076 | |
| Metastatic sites to the brain, bone, lung, and liver | |||||||||
| 0 | 19.5 | 9.0 | 3.80 | 0.000 | 21.2 | 9.9 | 3.80 | 0.000 | |
| 1–2 | 8.6 | 9.1 | 0.37 | 0.710 | 10.1 | 10.3 | 0.81 | 0.420 | |
| 3–4 | 2.6 | 0.0 | 1.44 | 0.149 | 3.8 | 0.0 | 1.52 | 0.129 | |
| Unknown | 17.2 | 7.7 | 2.00 | 0.045 | 21.1 | 8.2 | 2.51 | 0.012 | |
| Chemotherapy | |||||||||
| Yes | 14.8 | 12.6 | 1.35 | 0.177 | 16.8 | 13.7 | 1.75 | 0.080 | |
| No/unknown | 2.8 | 2.2 | 0.55 | 0.579 | 3.5 | 2.7 | 0.59 | 0.556 | |
OS, overall survival; CSS, cancer-specific survival.
Discussion
This is the first large population-based study evaluating the effect of radiotherapy in the management of metastatic EC based on the SEER database, revealing that radiotherapy was an independent prognostic factor associated with survival benefits of patients with metastatic EC. EC is one of the leading cause of cancer deaths worldwide with poor prognosis (3,10,11). In general, radiotherapy plays an important role in the treatment of local EC. A study reported a 5-year OS rate of 21% in 101 patients with locally EC receiving radiotherapy alone (12). Then chemoradiotherapy became the preferred treatment and had been shown to improve the quality of life and prolong survival for patients with local metastatic or unresectable EC. Systemic therapy is the standard treatment for metastatic disease, but symptoms caused by metastasis disease often require multidisciplinary management including radiotherapy. However, the value of radiotherapy in the treatment of metastatic EC has not yet been fully evaluated before. This present study can complement the treatment recommended in the current guidelines.
Although the research from Wu et al. (13) found that combining surgery with radiotherapy could improve survival in metastatic EC, it based on older populations, older methods of radiotherapy, and did not analyze the clinical benefits of chemotherapy combined with radiation therapy. A phase II study compared concurrent chemoradiation therapy (CCRT) with chemotherapy alone in stage IV esophageal squamous cell carcinoma, demonstrating that CCRT significantly prolonged median progression-free survival (mPFS, 9.3 vs. 4.7 months, P=0.021) and mOS (18.3 vs. 10.2 months, P=0.001) (14). This study challenged the status of standard treatment modality for metastatic EC treated with chemotherapy alone. The results showed that metastatic EC patients had good tolerance to concurrent chemoradiotherapy, and both OS and PFS were higher than chemotherapy alone. The possible mechanisms for radiotherapy to prolong the survival of metastatic disease may be improve local control rate under the premise of effective systemic therapy, produce certain cytokines that further inhibit the proliferation and metastasis of tumor cells. However, the phase II study was presented only in an abstract format, and the value of radiotherapy in the treatment of metastatic EC has not yet been fully evaluated. Thus, it was initially confirmed that the survival benefits of radiotherapy in metastatic EC based on this retrospective and propensity-matched study, which laid the foundation for the following clinical studies.
However, the addition of radiotherapy to chemotherapy showed no survival benefits, which was significantly inconsistent with the study mentioned above. The reason for the discrepancy between the two conclusions may be that the specific combination modalities of chemotherapy and radiotherapy such as concurrent or sequential therapy were unknown in this study which might influence survival, and that chemotherapy plays a leading role in the treatment of metastatic EC rather than radiotherapy.
The liver is the most common metastatic site of metastatic EC (47.7% of all enrolled patients), there are only few case reports that have reported the local treatment of hepatic metastasis with good clinical efficacy (15,16), while this study showed no survival benefits in matched patients with liver metastasis when radiotherapy was applied. As for patients with more than 3 metastatic sites, radiotherapy did not improve survival, indicating the limitations of local treatment for patients with multiple metastases.
The majority of studies found that the prognosis of patients with ADC is much better than the patients with SCC (17,18), however, these studies included all clinical stages of EC, but there was no significant prognostic difference between ADC and SCC in matched patients with advanced stage, and patients of both two types showed survival benefits from radiotherapy. Besides, the multivariable Cox regression analysis demonstrated that age, sex, insurance status, differentiation, chemotherapy were also important prognostic factors for metastatic EC.
There were few limitations in this study. Firstly, The SEER database does not provide information about the sites (both primary and metastatic sites) and dose of radiation therapy. Thus, there were no data of treatment response of radiotherapy, which might affect the results of analysis. Secondly, information relating to comorbidities and performance status was not available in the SEER database, which may influence the treatment approach, resulting in selective bias. Thirdly, the impact of chemotherapy regimens was unknown as no data was available in the SEER database. Last, the combination modalities of radiation and chemotherapy had not been shown in the SEER database, which is worthy of further investigations to maximize the survival benefits of radiotherapy in metastatic EC.
Conclusions
This large population-based study demonstrated that radiotherapy could improve the survival of patients with metastatic EC, which provides a line of evidence to guide the current treatment. Further randomized studies are warranted to assess the value of radiotherapy in the management of metastatic EC.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.06.15). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Data were retrieved from SEER database. The study was approved by the Ethics Committee of Renmin Hospital of Wuhan University. Informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Domper Arnal MJ, Ferrandez Arenas A, Lanas Arbeloa A. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol 2015;21:7933-43. [Crossref] [PubMed]
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400-12. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7. [Crossref] [PubMed]
- Li QQ, Liu MZ, Hu YH, et al. Definitive concomitant chemoradiotherapy with docetaxel and cisplatin in squamous esophageal carcinoma. Dis Esophagus 2010;23:253-9. [Crossref] [PubMed]
- Kleinberg L, Forastiere AA. Chemoradiation in the management of esophageal cancer. J Clin Oncol 2007;25:4110-7. [Crossref] [PubMed]
- Conroy T, Galais MP, Raoul JL, et al. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol 2014;15:305-14. [Crossref] [PubMed]
- Shridhar R, Almhanna K, Meredith KL, et al. Radiation therapy and esophageal cancer. Cancer Control 2013;20:97-110. [Crossref] [PubMed]
- Chun SG, Skinner HD, Minsky BD. Radiation Therapy for Locally Advanced Esophageal Cancer. Surg Oncol Clin N Am 2017;26:257-76. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Torre LA, Siegel RL, Ward EM, et al. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev 2016;25:16-27. [Crossref] [PubMed]
- Sykes AJ, Burt PA, Slevin NJ, et al. Radical radiotherapy for carcinoma of the oesophagus: an effective alternative to surgery. Radiother Oncol 1998;48:15-21. [Crossref] [PubMed]
- Wu SG, Xie WH, Zhang ZQ, et al. Surgery Combined with Radiotherapy Improved Survival in Metastatic Esophageal Cancer in a Surveillance Epidemiology and End Results Population-based Study. Sci Rep 2016;6:28280. [Crossref] [PubMed]
- Li T, Lv J, Li F, et al. Prospective Randomized Phase 2 Study of Concurrent Chemoradiation Therapy (CCRT) Versus Chemotherapy Alone in Stage IV Esophageal Squamous Cell Carcinoma (ESCC). Int J Radiat Oncol Biol Phys 2016;96:S1. [Crossref]
- Huddy JR, Thomas RL, Worthington TR, et al. Liver metastases from esophageal carcinoma: is there a role for surgical resection? Dis Esophagus 2015;28:483-7. [Crossref] [PubMed]
- Muroi H, Nakajima M, Satomura H, et al. Effectiveness of proton beam therapy on liver metastases of esophageal cancer: report of a case. Int Surg 2015;100:180-4. [Crossref] [PubMed]
- Gertler R, Stein HJ, Langer R, et al. Long-term outcome of 2920 patients with cancers of the esophagus and esophagogastric junction: evaluation of the New Union Internationale Contre le Cancer/American Joint Cancer Committee staging system. Ann Surg 2011;253:689-98. [Crossref] [PubMed]
- Siewert JR, Ott K. Are squamous and adenocarcinomas of the esophagus the same disease? Semin Radiat Oncol 2007;17:38-44. [Crossref] [PubMed]