
Breast radiotherapy in elderly women: myths, controversies, and current techniques in the adjuvant setting
Introduction
Breast cancer (BC) is the most common type of cancer in women in developed countries (1). The probability of developing BC increases with age. The majority of cases occur in women over age 60, with women over age 70 and 80, respectively, accounting for 30% and 12% of diagnoses (2,3). BC-related deaths have decreased drastically since the 1990s, although the main beneficiaries of this reduction are women under age 75, with cancer-specific mortality decreasing by 2.5% per year in women <75 years since the 1990s, but only by 1.1% per annum in older women (4). In Europe, the overall mortality rate decreased by 13% from the 1990–1994 period to the 2000–2004 period, with a significantly greater reduction in mortality among women under age 65 versus older women (17% vs. 6%, respectively) (5).
Adjuvant whole breast radiation therapy (WBRT) is one of the mainstays of treatment for BC, improving both local control (LC) and overall survival (OS) (6), as shown in the meta-analysis conducted by the Early Breast Cancer Trialists Collaborative Group (7). The value of adjuvant WBRT in young women or women with high-risk disease (regardless of age) is unquestioned, and it is considered a standard treatment option in clinical practice (8). However, in recent years, the use of adjuvant WBRT in older patients with low-risk disease has declined, especially since clinical guidelines included the option to obviate adjuvant RT based on reported findings from several studies suggesting that RT does not appear to improve OS (8-10). However, as we discuss in this review, the evidence to support the omission of adjuvant RT in older women is based on studies with important limitations.
In this context, a critical evaluation of the available scientific evidence regarding the value of adjuvant RT in the treatment of older women with BC will improve treatment selection and increase our understanding of the various factors—epidemiological, biological, social, and economic—that could influence the management of these patients, and this is what we are going to try to clarify in this review.
What does the term “elderly” mean and how does it influence the management of patients with BC?
There is a noteworthy lack of consensus regarding the definition of the term “elderly”. As early as 1995, the United Nations Committee on Economic Social and Cultural Rights of Older Persons (11) rejected the use of this term due to its lack of specificity and because it was assumed to refer to frail patients with precarious health (12-14). Although this term is commonly used to refer to people over age 65, this cut-off point is not based on scientific evidence, but rather borrowed (in all likelihood) from the field of socioeconomics since this is the age at which people in industrialized countries generally stop working and begin to collect retirement benefits (15). However, it may be inappropriate to apply this term to all people over age 65 given the long mean life expectancy in Western countries (>80 years) (16,17) and considering that the health status of women in this age group is highly variable (18).
Rather than using an arbitrary age to define “elderly”, it would be more reasonable to use objective criteria to classify patients according to their “biological” rather than their chronological age. However, the instruments most commonly used in routine clinical practice to assess health status (e.g., the Karnofsky index, ECOG, performance status) have important limitations with regard to their capacity to assess the main domains of interest in elderly patients. Moreover, those tools cannot identify changes that could potentially be reversed through early interventions. The International Society of Geriatric Oncology (SIOG), in conjunction with the European Society of Breast Cancer Specialists (EUSOMA), has published recommendations for the management of elderly patients with BC. According to those recommendations, age should not be considered an impediment to the use of RT, but an objective geriatric assessment—such as the comprehensive geriatric assessment (CGA)—should be performed prior to making the therapeutic decision (19). The CGA is important because it provides valuable data to help select the most appropriate therapeutic approach, after careful consideration of the risks and benefits of the intervention, based on the patient’s life expectancy and baseline health status (20,21).
Myth 1: older women with BC have a better prognosis
This is a myth, as studies show that women over age 75 diagnosed with BC actually have worse survival outcomes than younger patients (22). Although this finding appears to be inconsistent with the fact that a higher proportion of tumours in this patient population present biological characteristics that are, a priori, suggestive of good prognosis (i.e., higher expression of estrogen and progesterone receptors; less peritumoral vascular invasion; lower rates of HER2/Neu overexpression; a lower proliferation index; a higher proportion with normal p53 expression; and fewer deleterious mutations) (23-25). However, the higher mortality rate among older women whose disease is, at least theoretically, more benign can be explained by the factors discussed below.
Are these tumours as indolent as they appear?
At diagnosis, elderly women with BC are more likely to present with nodal and distant metastases, and/or to present molecular subtypes that are more aggressive than expected (26,27). Jenkins et al. evaluated 2,150 patients diagnosed with BC. After using the PAM 50® platform to reclassify patients, they found that the prevalence of luminal B, triple negative, and HER2+ cancer (28%, 13% and 13%, respectively) was higher than expected among patients over age 70 (28). Furthermore, the condition of the host also plays a role, with some studies showing that age-related alterations of the immune system disrupts mutation detection and repair mechanism, thus making the elderly more susceptible to developing more aggressive cancers, even in subtypes with an ostensibly better prognosis (29,30).
Late diagnosis
Late diagnosis among elderly patients can primarily be attributed to two main factors. First, the interruption of routine screening mammography and preventive medicine in older patients. In most countries with publicly-funded screening and prevention programs, routine screening mammographies are usually phased out around age 70 (31,32). The use of this somewhat arbitrary cut-off point is partly related to clinical trials conducted to evaluate the effectiveness of screening programs, which generally exclude patients in this age group, even though the findings from observational studies and computer models have shown that there may be a survival benefit for screening older women who have a long life expectancy (33-35). Nevertheless, this topic is controversial and no international consensus has yet been reached (36-38). The second main factor to explain late diagnosis in this patient population is the delay between the time the patient notices a suspicious breast lesion and subsequent medical evaluation of the lesion (39). This delay is especially common in the most frail or dependent patients, and also influenced by sociodemographic and economic factors (40,41).
Undertreatment
The final—but not least important—factor that may explain the higher mortality rate in older women is undertreatment. Studies show that close to 50% of older patients diagnosed with BC—especially those over age 70—receive suboptimal treatment that deviates from the recommendations of clinical guidelines (40,42). As a result, both prognosis and survival are worse in these patients (43). While the main reasons for undertreatment are not entirely clear, it is likely multifactorial. One explanation may be the presence of comorbidities, which can negatively impact the patient’s capacity to tolerate the indicated treatment. It is also possible that the treating physician believes that the indicated treatment is unlikely to provide a clinical or survival benefit due to the patient’s baseline health status. In other cases, the factors associated with undertreatment are social, such as difficulties in the patient’s ability to travel autonomously to the treatment centre, care dependency, or due to the specific preferences of the patient and/or family members responsible for providing care. In other cases, medical paternalism may play a role in treatment selection. Some physicians may avoid prescribing morbidity-inducing treatments, underestimate life expectancy, and/or question the patient’s ability to tolerate treatment. Advanced age is independently associated not only with less adherence to the recommendations of clinical guidelines, but also with a lower probability of receiving BCS, a greater use of hormonal therapy, less use of adjuvant chemotherapy, and a lower probability of receiving radiotherapy (RT), even in patients in good general condition (44,45).
Myth 2: there is sufficient evidence to guide treatment selection
Older women, especially those in their 70s, are underrepresented in clinical trials (46) due to strict inclusion criteria, a failure to inform them about this option, or due to a medical or family concern about possible side effects (47-49). For this reason, level 1 evidence in this population is limited, and the evidence that is available is subject to debate. An article published in 2011 by the EORTC recommended that clinical trials be carried out in older patients (50). That publication even provided specific recommendations about how such a study should be designed, emphasizing the need to include endpoints related to quality of life (QoL), functional status and independence (in addition to the usual measures of efficacy). They also suggested the use of age 70 as the cut-off point for “old age” for study design purposes, including only patients who meet this age criterium. To conduct such a study, it is essential to use geriatric assessment tools such as the CGA to adequately stratify patients into comparable, homogeneous subgroups (50).
Controversy: can adjuvant WBRT be safely omitted after breast-conserving surgery (BCS)?
Most of the clinical trials performed to evaluate the role of adjuvant WBRT have excluded patients older than age 70. In a meta-analysis, Clarke et al. demonstrated that adjuvant WBRT decreased cancer-specific mortality at 15 years; however, only 9% of the patients included in that meta-analysis were over age 70 (51). The absolute benefit of adjuvant WBRT in patients with early-stage disease decreases with age, which explains why several recent prospective trials have examined the effect of omitting adjuvant RT after BCS in selected patients (Table 1). Those trials assessed a range of endpoints, including OS, local recurrence (LR), and progression-free survival (PFS). The CALGB 9343 (53) and PRIME II (56) trials also included QoL as an endpoint.
Table 1
| Variable | Fyles (52) | CALGB 9343 (53) (Hughes et al.) | ABCSG 8 (54) (Pötter et al.) | BASO II (55) (Blamey et al.) | PRIME II (56) (Klunkler et al.) |
|---|---|---|---|---|---|
| Patients, n | 769 | 636 | 869 | 1,135 (2×2: 406)Ɨ | 1,326 |
| Study type | Multicentric randomized | Multicentric randomized | Multicentric randomized | Multicentric randomized and 2×2 | Multicentric randomized |
| Age, y | ≥50 | ≥70 | ≥50 | ≥50 | ≥65 |
| Tumour size (all pN0) (cm) | <5 | <2 | <3 | <2 | <3 |
| RE/RP | 100% | 100% | 100% | 100% | 100% |
| Menopausal status | 100% | 100% | 100% | 100% | 100% |
| Surgery | BCS | BCS | BCS | WLE | BCS |
| Randomization | Tam + WBRT/Tam | Tam + WBRT/Tam | Tam + WBRT/Tam | No WBRT, no Tam/WBRT alone/Tam alone/Tam + WBRT | Tam + WBRT/Tam |
| N | 386/383 | 317/319 | 414/417 | 95/107/106/98 | 658/668 |
| Follow up | 5 and 8 years | 12.6 years | 5 years | 10 years | 5 years |
| LR | 5 yrs: 0.6%/7.7%*; 8 yrs: 3.5%/17.5% | 2%/10%** | 0.4%/5.1%*** | 15%/6.5%/7.5%/0%**** | 1.3%/4.1%***** |
| DFS | 91% vs. 84%, P=0.004 | 98% vs. 91%, HR 0.18, P<0.01 | 97.9% vs. 93.9%, HR 3.48, P=0.0021 | 83%, HR 1/93%, HR 0.37/93%, HR 0.40/0, HR 0 | 90% vs. 84% |
| OS | 91% vs. 84%, P= 0.004 | 76% vs. 66% | 97.9 vs. 94.5, P=0.18 | 96% | 93% vs. 93% |
Ɨ1,135 randomized to intention-to-treat, 406 in the 2×2 evaluation; *HR 9.3, P<0.001; **P<0.001; ***HR 10.2, P=0.0001; ****P<0.001; *****HR 5.19, P<0.001. BCS, breast-conserving surgery; DFS, disease-free survival; OS, overall survival; LR, local relapse; Tam, tamoxifen; WBRT, whole breast radiation therapy; HR, hazard ratio; RE/RP, estrogen and progesterone status; WLE, wide local excision (specimen margins >0.5 cm, if less, reexcision required).
In the CALGB 9343 study (53), 636 women over age 70 with stage T1N0, hormone receptor-positive (RE+) BC were randomized to receive adjuvant WBRT with tamoxifen or adjuvant tamoxifen alone. At 10 years, LC rates were significantly better in the adjuvant WBRT group (98% vs. 90%). The PRIME II trial (56) included women over age 65 with tumours <3 cm, N0, and RE+ who were randomized to the same treatments as in the CALGB 9343 study. At 5 years, LC was better in the adjuvant WBRT group, with no significant between-group differences in QoL, leading the authors to conclude that the combined use of adjuvant WBRT and tamoxifen after BCS does not negatively impact functional competence. The BASO II trial (55) evaluated 1,135 patients over age 50 with grade 1, stage T1 BC. The study assessed adjuvant treatment using a 2×2 design (with or without adjuvant WBRT and with or without tamoxifen). At 10 years, LC was better in the group that received adjuvant WBRT + tamoxifen, with no local relapses in that group. The 60 month follow-up results of the ABCSG 8 trial (54) reported outcomes from 869 patients (mean age, 66 years) with tumours <3 cm, RE+, grade 1 or 2, and N0 who were randomized after BCS to tamoxifen/anastrozole with or without adjuvant WBRT. In that study, adjuvant WBRT had a significant positive impact on LC. Finally, Fyles et al. (52) evaluated 769 patients over age 50 with T1-T2 tumours who underwent BCS and were randomized to receive adjuvant WBRT or adjuvant tamoxifen; local relapse rates were significantly better in the adjuvant WBRT group at both 5 years (0.6% vs. 7.7%) and at 8 years (3.5% vs. 17.6%) of follow up.
In summary, all of these trials showed that adjuvant WBRT + tamoxifen was significantly superior to adjuvant tamoxifen alone in terms of LC, although this advantage did not translate into an improvement in OS. The finding that combined treatment did not increase OS seemed to imply that adjuvant WBRT could be safely omitted in selected patients. As a result, this approach is now considered an alternative to standard treatment (adjuvant WBRT + tamoxifen) in selected patients, and it is even included in some clinical guidelines (8).
Should we systematically omit adjuvant WBRT in elderly women with a good prognosis?
The findings of the aforementioned studies suggest that adjuvant WBRT can be omitted because it does not—despite its positive influence on LC—appear to improve OS. However, these findings must be interpreted cautiously, in part because it remains unclear whether those results can be extrapolated to the general population—particularly older women (>70 years)—who were underrepresented in most of those trials (except for the PRIME II and CALGB-9343 trials).
The meta-analysis by Matuschek and colleagues published in 2017, which included all of the aforementioned trials, revealed some highly interesting findings (57). First, although the individual studies included in the meta-analysis failed to show a survival benefit in OS for combined treatment with adjuvant WBRT + tamoxifen, this treatment approach significantly lowered the risk of LR (hazard ratio, 6.8), corresponding to an absolute reduction in LR of 3–5% and 9–14% at 5 and 10 years, respectively. This decrease in LR implies an increase in OS at 5- and 10-years of 3% and 7%, respectively. In this regard, the results of the CALGB-9343 trial—the only randomized controlled trial with a 10-year follow-up—show no recognizable plateau on the survival curves, raising the possibility that these curves will continue to diverge after year 10 in that trial, and possibly in the other clinical trials (53).
The retrospective study published by Herskovic et al. in 2018 presented some very interesting findings. Those authors evaluated the impact of omitting adjuvant WBRT in a real-world sample (outside of the controlled conditions of clinical trials) of women over age 65 with low-risk BC. The study retrospectively evaluated 61,395 women from the National Cancer Database who were diagnosed with BC during the years 2006–2013. At 48.7 months of follow-up, the OS rate in patients who received adjuvant WBRT + tamoxifen was significantly higher than in the patients who received adjuvant tamoxifen alone (93% vs. 83.6%, P<0.001), with survival curves that began to separate at month 24. Despite the limitations inherent to the retrospective study design, the findings of that study suggest that large-scale population studies are the only type of study capable of detecting significant differences in OS because prospective trials with short follow-ups do not have sufficient statistical power to detect such differences (58).
The clinical trials discussed above that have compared adjuvant WBRT plus tamoxifen to adjuvant tamoxifen alone conclude that the two treatments are essentially equivalent in terms of OS. However, it is difficult to translate these results to real world settings given that 20% to 50% of patients stop taking tamoxifen due to poor tolerance (59,60), which implies that a significant proportion of these patients (who may not have received adjuvant WBRT) will not receive the full prescribed treatment of tamoxifen. As a result, these patients are likely to have a higher rate of LR, with a greater risk of cancer-specific mortality. Indeed, the study carried out by Killander et al. in Sweden confirmed this effect. Those authors compared BCS + adjuvant WBRT to BCS alone, finding 15-year LR rates, respectively, of 11.5% vs. 23.9% (P<0.001), with a trend towards worse OS in the group that did not receive adjuvant WBRT (71.1% vs. 68.4%, P<0.68) (61).
Given the findings described above, it is clear that we must proceed with extreme caution when choosing to omit RT in postmenopausal patients, even those with low-risk disease. Although it may be reasonable to consider omitting RT in patients with a life expectancy less than 5 years, determined objectively according to a validated CGA. This cautious approach is important to avoid introducing selection bias and to ensure that patients with a long life expectancy are not exposed to the unnecessary risk of developing locally recurrent disease caused by suboptimal treatment, which could potentially have a negative impact on survival. Therefore, the decision to omit adjuvant RT should only be taken after careful consideration of the risks and benefits of doing so. In this assessment, it is crucial to consider the implications of early interruption of hormonal treatment (due to poor tolerance), and to ensure that strict oncological controls will be followed.
Controversy: rethinking toxicity
Recent studies have shown that elderly patients generally tolerate RT as well as younger patients (62-64), even when interventional techniques such as brachytherapy are used (65,66). These findings can likely be extrapolated to the treatment of BC, although few prospective studies have specifically evaluated this question (67). Despite the evidence support the use of adjuvant RT described in the preceding paragraphs, one reason given for obviating RT in older women is the potential for heart and lung toxicity, which could negatively impact QoL (68-70). However, the findings from the CALGB-9343 trial, which used validated instruments to assess QoL, showed that there were no differences in QoL between patients who received adjuvant WBRT+ tamoxifen versus those who received tamoxifen alone (53). The PRIME II study did not find any significant differences in QoL at 60 months post-treatment, although there were differences between groups in logistical concerns (transportation or accommodations) during the RT treatment phase (56). In terms of lung toxicity, radiation pneumonitis has been associated with the several different variables, as follows: the size of the lung volume irradiated within the tangential fields; irradiation of the supraclavicular and internal mammary lymph nodes; prior exposure to chemotherapy or tamoxifen; and smoking habit. Nonetheless, the incidence of symptomatic pneumonitis in patients treated with RT for BC remains negligible (71,72).
How does RT affect the heart in left BC?
In 2013, Darby et al. published a high-impact case-control study that correlated the mean heart dose (MHD) with the probability of an ischemic cardiac event, concluding that for each 1 Gy increase in MHD, the relative risk increased by 7%, and no dose level was considered safe (73). Despite the methodological quality of that study, it had several important limitations, mainly attributable to its retrospective design. The patient cohort was obtained from historical records of patients treated from 1958 through the year 2001, thus most of these patients were treated prior to the development of three-dimensional radiotherapy (3D-RT). In addition, the groups were not balanced in terms of comorbidities, the baseline cardiac risk was unknown in many of the patients, and the MHDs were estimated from a random selection of 20 treatment plans because the actual dosimetric values were unavailable. However, this prediction model was subsequently evaluated by van den Bogaard et al. in a 910 patient cohort treated with 3D-RT, with a follow-up of 9 years. The results of that study validated the model, showing that the accumulated incidence of acute cardiac events increased by 16.5% per Gy of MHD and that the best predictor of risk was the volume of the left ventricle receiving 5 Gy (74). Despite the limitations of those studies and the uncertainties surrounding the specific mechanisms of cardiac damage (75), it is inexcusable not to do everything possible to minimize the MHD using advanced technology (76), especially in a patient population that often presents comorbidities that may increase the negative impact of RT on their overall cardiac risk (77).
Beyond cobalt and conventional fractionation
Until the 1990s, the main radiotherapeutic treatment in patients treated with BCS was adjuvant WBRT using conventional fractionation of 45–50 Gy (daily sessions of 1.8–2 Gy), with/without a boost to the tumour bed (78). However, since that time, numerous alternative RT schemes have been explored. Some schemes have sought to reduce the number of sessions by increasing the dose per session while others have sought to decrease the size of the target volume. The advantage of such approaches is that they limit the number of hospital visits needed for RT treatment and they also lower costs without decreasing treatment efficacy and without increasing treatment-related toxicity (79,80). It is worth noting that practically all of the trials conducted to evaluate these different RT regimens have involved patients over age 50, with older women making up a substantial proportion of the patients, and thus the results are applicable to “elderly” patients.
Hypofractionated whole breast radiotherapy (HF-WBRT)
HF-WBRT is similar to adjuvant WBRT but with higher doses per session and fewer sessions (81). The radiobiological basis for this hypofractionated approach is based on the hypothesis that the alpha/beta ratio of the tumour is similar to that of the surrounding healthy tissue, and thus larger fractions would be more effective without causing severe damage to healthy tissues (82). In the RMH/GOG trial, the alpha/beta for BC was calculated as 4 Gy. That study compared two different hypofractionated schemes (39 Gy/13 fraction vs. 42.9 Gy/13 fraction) to conventional fractionation, finding that both hypofractionated schemes were isoeffective (83,84). Several phase III randomized trials have compared the oncological and cosmetic outcomes of HF-WBRT to conventional schemes. The START A, START B and the Canadian study all found that these treatments were therapeutically equivalent in terms of LC and OS outcomes, with a trend towards better acute cosmesis for the hypofractionated regimens, without significant differences in chronic toxicity (85-88) (Table 2) or cardiac toxicity in left BC (89). The results of these studies were reanalyzed in several meta-analyses (90-92), which confirmed the findings. As a result, this hypofractionation schedule is now considered standard and supported by level 1 evidence in patients in whom irradiation of the breast or mastectomy bed is indicated (93-96). Delivering a boost to the tumour bed lowers the LR rate in all patients, but it is not clear whether a boost should be routinely administered given the lack of evidence demonstrating that this would improve OS. Moreover, the use of a boost has been associated with a slight increase in the risk of chronic skin toxicity (97). However, when necessary, the boost can be performed with HF-WBRT techniques that offer integrated boost, without causing a substantial increase in toxicity (98).
Table 2
| Variable | START trial A (85) | START trial B (87) | Canadian study (88) |
|---|---|---|---|
| Patients, n | 2,236 | 2,215 | 1,234 |
| Study type | Multicentric, randomized | Multicentric, randomized | Multicentric, randomized |
| Age, years | |||
| ≤60 | 1,358 (60.7%) | 1,331 (60%) | 646 (52.3%) |
| >60 | 878 (39.3%) | 884 (40%) | 588 (47.7%) |
| Histological type | |||
| Invasive ductal | 1,750 (78.3%) | 1,708 (77.1%) | |
| Invasive lobular | 266 (11.9%) | 254 (11.5%) | Invasive carcinoma |
| Other | 220 (9.9%) | 453 (11.4%) | |
| Tumor size (cm) | |||
| ≤2 | 1,138 (50.9%) | 1,412 (63.8%) | 994 (80.6%) |
| >2 | 1,085 (48.6%) | 795 (35.8%) | 240 (19.4%) |
| Not known | 13 (0.5%) | 8 (0.4%) | |
| Primary surgery | |||
| Breast-conserving (BCS) | 1,900 (85.0%) | 2,038 (92.0%) | BCS alone |
| Mastectomy | 336 (15.0%) | 177 (8.0%) | |
| Randomization | 50 Gy, 25 fxƗ/41.6 Gy, 13 fx/39 Gy, 13 fx | 50 Gy, 25 fx/40 Gy, 15 fx | 50 Gy, 25 fx/42.5 Gy, 16 fx |
| N (randomization) | 749/750/737 | 1,105/1,110 | 612/612 |
| Follow up | 5 and 10 years | 5 and 10 years | 10 years |
| Local relapse (estimated % with event by 10 yrs) | 7.4%/6.3%*/8.8%** | 5.5%/4.3%*** | 6.7%/6.2%**** |
| Normal tissue effects (breast induration, telangiectasia, edema) | Significantly less common in the 39 Gy group vs. the 50 Gy group | Significantly less common in the 40 Gy group vs. the 50 Gy group | 71.3%/69.8%ǂ |
ƗFractions; *HR 0.91, P=0.65; **HR 1.18, P=0.41; ***HR 0.77, P=0.21; ****absolute difference, 0.5 percentage points, 95% CI, −2.5 to 3.5; ǂgood or excellent cosmetic outcomes (absolute difference, 1.5 percentage points; 95% CI, −6.9 to 9.8).
Partial breast irradiation (PBI)
PBI consists of treating the lumpectomy/tumorectomy bed alone, based on the assumption that 95% of LRs occur in the involved quadrant (99,100). However, the reality is that the role of PBI remains undefined due to the short follow-up of the studies that have evaluated this technique. Nevertheless, the role of PBI has evolved in recent years. Whereas it was previously considered an intermediate RT scheme situated between adjuvant WBRT and no RT, it is now considered a therapeutic alternative to adjuvant WBRT in selected low-risk patients, an indication that has been recognized in clinical guidelines. Although PBI was first limited to brachytherapy modalities (101,102), publication of the IMPORT LOW and Barcelona trials has provided sufficient evidence to support the use of external RT for PBI (103,104). Intraoperative radiotherapy (IORT) (105,106), a technique that is administered in a single session (intra- or peri-operatively), merits special mention as an example of a cost-effective technique that provides maximum concentration of local treatment (107,108). The two main trials that have evaluated IORT are the ELIOTT and TARGIT-A trials, with 5 and 3.8 years of follow-up, respectively. Although IORT has not been found to negatively impact OS, both of those clinical trials found higher LR rates in the untreated breast areas compared to the areas that received adjuvant WBRT (4.4% vs. 0.4% and 3.3% vs. 1.3%, respectively). For this reason, IORT should only be indicated with caution outside of clinical trials (109).
Numerous randomized trials have evaluated the oncological and cosmetic results of PBI compared to adjuvant WBRT (104-108,110-113) (Table 3). Meta-analyses of those trials have shown that although LR and primary second tumours were more common in patients treated with PBI, this had no negative impact on OS (114-116). Moreover, the meta-analysis by Vaidya et al. even found a modest but significant benefit in OS for PBI versus adjuvant WBRT (a difference of 1.3%, 95% CI, −2.5% to 0.0%, P=0.05, by the random effects model and 1.0%, 95% CI, −2.3% to 0.3%, P=0.13, by the fixed effects model) (117).
Table 3
| Variable | IMPORT LOW (103) | Barcelona (104) | GEC-ESTRO (112) | TARGIT-A (105) | ELIOT (106) | Hungary (109) | University of Florence (111) | RAPID (111) |
|---|---|---|---|---|---|---|---|---|
| Patients, n | 2,016 | 102 | 1,184 | 3,451 | 1,305 | 258 | 520 | 2,135 |
| Study type | Multicentric, randomized | Multicentric, randomized | Multicentric, randomized | Multicentric, randomized | Single center, randomized | Multicentric, randomized | Multicentric, randomized | Multicentric, randomized |
| Randomization | WBRT/HF-WBRT/PBI | PBI/WBRT | PBI/WBRT | IORT/WBRT | IORT/WBRT | PBI/WBRT | PBI/WBRT | PBI/WBRT |
| N | 674/673/669 | 51/51 | 633/551 | 1,721/1,730 | 651/654 | 128/130 | 260/260 | 1,070/1,065 |
| Dose-fractionation PBI arm | 40 Gy/15 fx | 37.5 Gy/10 fx BID | 32 Gy/8 fx, 30.3 Gy/7 fx (HDR) BID; 50 Gy (PDR) | 20 Gy SD to the surface of the tumor bed | 21 Gy SD prescribed to the 90% depth | 36.4 Gy/7 fx (HDR); 50 Gy/25 fx (electron) | 30 Gy/5 fx (QOD) | 38.5 Gy/10 fx BID |
| Technique | IMRT | 3D-CRT | HDR | IORT | IORT (electron) | HDr/electron | IMRT | 3D-CRT |
| Age distribution | ||||||||
| ≤60 | Mean age: WBRT: 63 y | Mean age: WBRT: 70.1 y; PBI: 67.1 y | 536 Pt (45.3%) | 1,347 Pt (39.1%) | 640 Pt (49.1%) | 152 Pt (58.9%) | 223 Pt (42.8%) | ≤50: 257 Pt (12%) |
| >60 | Reduced WBRT: 63 y; PBI: 62 y | 648 Pt (54.7%) | 2,104 Pt (60.9%) | 665 Pt (51%) | 106 Pt (41.1%) | 297 Pt (57.1%) | >50: 1,878 Pt (88%) | |
| Histology | IDC | IDC | IC/DCIS | IDC | IDC/ILC | IDC | IC/DCIS | IDC/DCIS |
| Tumor size (cm) | ≤3 | ≤3 | ≤3 | ≤3.5 | ≤2.5 | ≤2 | ≤2.5 | ≤3 |
| Nodal status | Negative/pN1 | Negative | Negative/pN1mi/pN1a (by ALND) | N0, N1 | Negative. If positive: WBRT | N0, N1mi | Negative, pN1 | Negative |
| Follow up | 5-year cumulative incidence | 5 years | 5 years | 5 years | 5 years | 5 years | 5 years | 5 and 8 year cumulative rates |
| LR (%) | 1.1/0.2/0.8 | 0 | 1.44/0.92 | 3.3/1.3 | 4.4/0.4 | 4.7/3.4 | 1.5/1.9 | 5 y: 2.3, 8 y: 3.0/5 y: 1.7, 8 y: 2.8 |
| OS (%) | No significant differences | No significant differences | 97.3/95.5. No significant differences | No differences, but significantly fewer non-breast-cancer deaths with TARGIT | 96.8/96.9. No significant differences |
94.6/91.8. No significant differences | 99.4/96.6. No significant differences | – |
BCS, breast conserving surgery; ALND, axillary lymph node dissection; IDC, invasive ductal carcinoma; IC, invasive carcinoma (any type); DCIS, ductal carcinoma in situ; GEC-ESTRO, Groupe Européen de Curiethérapie and European Society for Radiotherapy and Oncology; ASBS, American Society of Breast Surgeons; ASTRO, American Society for Therapeutic Radiology and Oncology; ABS, American Brachytherapy; BID, twice a day (bis in die); HDR, high dose rate interstitial brachytherapy; PDR, pulsed dose rate brachytherapy; SD, single-dose; QOD, every other day (quaque altera die); Pt, patients; WBRT, whole breast radiotherapy; HF-WBRT, hypofractionated whole breast radiotherapy.
Therefore, even though the probability of developing LR is slightly higher in patients who undergo PBI, this approach may be an interesting alternative to adjuvant WBRT in elderly patients with low-risk disease and a long life expectancy who present a high risk of early discontinuation of hormonotherapy. PBI could also be of value in patients who would benefit from fewer RT treatment sessions to minimize the need to travel to the hospital. Although no randomized trials have been conducted to compare PBI to the omission of adjuvant WBRT, the published data suggest that LR rates are lower in patients who receive PBI, which would support the maxim that “some radiotherapy is better than none at all”.
What about the technology?
Administering a homogenous dose distribution to the target volume is crucial to avoid producing “hot spots” that may cause local toxicity. Likewise, it is essential to minimize the dose to the organs at risk (OAR) (118,119). To achieve these objectives, we must not only select the most appropriate technique for each case, but also develop strategies to minimize the risks present throughout the entire treatment process.
In recent years, several studies have compared the dosimetric results of different external RT techniques for both adjuvant WBRT and PBI, without identifying any clearly superior approach (Figure 1). Moreover, the studies that have compared 3D-WBRT, inverse IMRT, field-in-field-RT, tomotherapy and/or VMAT for WBRT and PBI have reported conflicting results (120,121). The contradictory results of these dosimetric comparisons are probably due to the limited number of patients in those studies and because the results cannot always be extrapolated to all real-life patients due to phenotypic differences between women. Therefore, we must select the most appropriate approach based on each patient’s anatomy and functional status (122,123). Nevertheless, it seems clear that techniques such as inverse IMRT, VMAT, and tomotherapy are all capable of achieving adequate dose conformity with better cardiac protection in patients who require irradiation of the internal mammary or supraclavicular nodal areas, even though this requires the administration of lower doses over larger lung volumes or to the contralateral breast (124,125); even so, there is no evidence that these low doses increase the risk of second tumours (126,127). However, the positive impact of these treatment modalities is not as relevant to patients treated with PBI or those who receive WBRT without regional nodal irradiation, especially if the treatment is performed using specific positioning or other techniques to protect the OARs, as we discuss below.
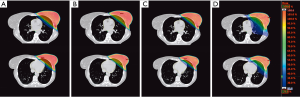
In patients with pendulous breasts the prone position allows for good dosimetric homogeneity, with lower doses to the OARs, particularly the lung (128). Most dosimetric studies have found that prone positioning decreases the MHD compared to supine positioning, although not in all patients (129,130). In some women, the position of the heart is influenced by gravity and can “fall” towards the rib cage, thus increasing the MHD and the dose to the left ventricle (131). In addition, this position is not suitable for all patients since it can be uncomfortable and it is difficult to maintain in patients who have limited mobility (132).
Another approach to limiting the radiation dose to OARs is forced breathing or the deep inspiration breath-hold (DIBH) technique (Figure 2), which has been shown to provide the best cardiac protection of available methods (133-135). During inspiration, the lung expands and the diaphragm flattens, moving the heart away from the rib cage. The radiation is administered at this point, when the heart is at its most distant point from the target, thus reducing the MHD. The DIBH technique even may provide better dosimetric results with EBRT than with brachytherapy (136). There are several different modalities for this technique (137), including voluntary breath-hold in which the patient is instructed when to inspire and when to hold the breath during treatment. This implies a need for prior training and the active involvement of the patient during treatment. This technique may be difficult to perform in patients who have difficulties following complex visual or auditory commands (138). Another alternative breath-hold approach is the use of active breathing control (ABC) devices, which are similar to the CPAP (continuous positive airway pressure) ventilator. ABC devices are used to monitor and control respiratory flow, interrupting breathing at the moment the radiation is administered (139). This technique provides better dosimetric results and greater reproducibility, but the device can be bothersome for elderly patients (140). Therefore, selection of the most appropriate procedures must be individualized and adapted to suit the functional characteristics and comorbidities of elderly patients.

A look ahead to the future
What is the next step?
New fractionation schedules and protocols for adjuvant RT are needed to further improve treatment tolerance and adherence and to reduce side effects (141,142). Cost-effectiveness is also an important consideration. The use of extreme hypofractionation schedules (>5 Gy per session) for both PBI and WBRT appears to be both feasible and efficacious with minimal toxicity in frail patients or those who find it difficult to travel to the clinic on a daily basis (143-145). Although some evidence to support this approach has already been published, we are still awaiting the long-term results of prospective randomized trials—including the FAST trial (146) and NCT01803958 (147)—to confirm the safety and efficacy of extreme hypofractionated regimens.
The surgical resection of tumours in elderly patients with BC, even those with early-stage disease, is becoming increasingly less common. Consequently, there is an important need to develop non-invasive, low-toxicity treatments for patients with inoperable tumours or those who refuse surgery. In fact, these are exactly the types of patients in whom radical RT may play a role given the poor results—in terms of both LC and OS—achieved with tamoxifen alone. The technical feasibility of radical treatment with SBRT or proton therapy has already been investigated in several studies (148-150), with prospective trials currently being planned (151).
Given the wide heterogeneity among elderly patients in terms of health status and life expectancy, the true challenge for the future is to match individual patients to the most appropriate RT protocol—which may even involve the omission of RT in highly selected cases—and to optimize health resources in an era of population ageing. In this regard, it is crucial to use objective measures to estimate life expectancy. Similarly, clinical trials are needed to determine the optimal treatment approach in the elderly. Despite the growing body of evidence, many unanswered questions remain. Although the numerous randomized and prospective clinical trials that have evaluated the omission of adjuvant WBRT appear to provide high quality evidence, these studies—as we have seen—have limited statistical power and relatively short follow-up periods. Studies with much longer follow-up times are needed to detect significant differences in OS, but it is worth underscoring that large scale retrospective population studies have already detected early differences in OS. Although such studies have important limitations and potential biases due to their retrospective design, the large number of patients in those studies may compensate for any design-related drawbacks; moreover, such studies provide real-world clinical evidence, outside of the highly controlled conditions of clinical trials. Indeed, for older women with a limited life expectancy, the findings of these population studies may be more relevant than those of clinical trials. Logically, the limited life expectancy of these patients further reduces the statistical power of the randomized trials, thus making it even more difficult to demonstrate the impact of any intervention on OS, even though the failure to treat these patients in real life could negatively impact both survival and QoL (152,153).
We believe that Big Data and Real World Data (RWD) could play an important role in overcoming the challenges described above. First, however, we must establish a precise definition of these terms, which remain unclear in the field of medicine (154). Ghani et al. (155) define big data as large datasets that were not limited in size and scope during the design phase, and which have not been collected to answer a specific hypothesis or question. The idea is that these data are collected without any initial hypothesis, but rather to create a large dataset for subsequent analysis to identify associations between the data, which may generate new models. For our purposes, it would perhaps be more interesting to analyse RWD to create “Real World Evidence” (156). Since such data are based on population registries and observational studies in which there is usually an initial hypothesis, the method used to analyse these data is deductive, seeking to identify causal relationships (157).
As we have discussed, adjuvant WBRT is often omitted based on evidence suggesting that it does not improve OS. This treatment approach (i.e., the omission of WBRT) has been integrated into routine clinical practice based on results from conventional clinical trials with limited follow-up. However, when treatment approaches such as this are analysed using data from real-world populations obtained through population-based registries, it becomes possible to determine the true impact of the treatment—adjuvant WBRT plus tamoxifen may improve OS compared to adjuvant tamoxifen alone. Thus, the wider use of RWD would allow us to confirm—or refute—the efficacy of an intervention in non-ideal (i.e., real world) conditions. However, studies based on RWD should not be considered true substitutes for clinical trials. This is especially true considering that we still do not know how to accurately interpret the results obtained from such datasets given the heterogeneous sources of data and/or the variability in quality, complexity and integrity of the data included in those datasets. For this reason, any analysis of RWD must be done cautiously (158-161).
Conclusions
The optimal treatment of older women with BC is challenging. Moreover, there is no clear consensus regarding the definition of the term “elderly”. Clinical trials targeted specifically at this population are needed to clarify the many questions surrounding the optimal treatment of these patients. In the relatively near future, it seems likely that information technology and Big Data will help to improve treatment selection. However, based on the current evidence, there are no patient subgroups in which RT can be safely omitted. The available evidence shows that the risk of recurrence is higher in patients who do not receive RT, which could negatively impact OS. It is necessary to incorporate new techniques and further subclassify patients to facilitate treatment adherence, minimize toxicity, optimize costs, and preserve QoL.
Acknowledgments
Bradley Londres—Biomedical writer & editor; Member, Mediterranean Editors & Translators (MET). Francisco Marcos Jiménez, MD, PhD—Department of Radiation Oncology, Hospital Universitario Quironsalud Madrid; Department of Radiation Oncology Hospital Quironsalud La Luz; Universidad Europea de Madrid. Luis Leonardo Guerrero, MD—Department of Radiation Oncology, Hospital Quironsalud La Luz; Yolanda Molina—Radiophysics Department, Hospital Universitario Quironsalud Madrid; Miguel Ángel Infante—Radiophysics Department, Hospital Quironsalud La Luz.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Vincent Vinh-Hung and Nam P Nguyen) for the series “Radiotherapy for Breast Cancer in Advanced Age” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.09). The series “Radiotherapy for Breast Cancer in Advanced Age” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Yancik R, Ries LAG. Cancer in Older Persons: An International Issue in an Aging World. Semin Oncol 2004;31:128-36. [Crossref] [PubMed]
- Glaser R, Marinopoulos S, Dimitrakakis C. Breast cancer treatment in women over the age of 80: A tailored approach. Maturitas 2018;110:29-32. [Crossref] [PubMed]
- Smith BD, Jiang J, McLaughlin SS, et al. Improvement in Breast Cancer Outcomes Over Time: Are Older Women Missing Out? J Clin Oncol 2011;29:4647-53. [Crossref] [PubMed]
- La Vecchia C, Bertuccio P, Bosetti C, et al. Cancer mortality in Europe, 2000-2004, and an overview of trends since 1975. Ann Oncol 2010;21:1323-60. [Crossref] [PubMed]
- Van de Steene J, Soete G, Storme G. Adjuvant radiotherapy for breast cancer significantly improves overall survival: the missing link. Radiother Oncol 2000;55:263-72. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet 2011;378:1707-16. [Crossref] [PubMed]
- Guidelines NCCN. Cited 2018;2019:13.
- Rutter CE, Lester-Coll NH, Mancini BR, et al. The evolving role of adjuvant radiotherapy for elderly women with early-stage breast cancer. Cancer 2015;121:2331-40. [Crossref] [PubMed]
- McCormick B, Ottesen RA, Hughes ME, et al. Impact of guideline changes on use or omission of radiation in the elderly with early breast cancer: practice patterns at National Comprehensive Cancer Network institutions. J Am Coll Surg 2014;219:796-802. [Crossref] [PubMed]
- UN Economic and Social Council. General Comment No. 6: The Economic, Social and Cultural Rights of Older Persons. UN Comm Econ Soc Cult Rights 1995. Available online: https://www.refworld.org/docid/4538838f11.html
- Avers D, Brown M, Chui KK, et al. Editor’s message: Use of the term “elderly”. J Geriatr Phys Ther 2011;34:153-4. [Crossref] [PubMed]
- Theresa M. Nemmers. The Influence of Ageism and Ageist Stereotypes on the Elderly. Physical & Occupational Therapy In Geriatrics 2015;22:11-20.
- Lawler M, Selby P, Aapro MS, et al. Ageism in cancer care. BMJ 2014;348:g1614. [Crossref] [PubMed]
- Orimo H, Ito H, Suzuki T, et al. Reviewing the definition of “elderly”. Geriatrics & Gerontology International 2016;6:149-58. [Crossref]
- WHO. Global Health Observatory (GHO): Data Repository. GHO 2018. Available online: https://www.who.int/gho/en/
- Eurostat. Mortality and life expectancy statistics. Available online: https://ec.europa.eu/eurostat/statisticsexplained/index.php?title=Mortality_and_life_expectancy_statistics
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;10:117-71.
- Biganzoli L, Wildiers H, Oakman C, et al. Management of elderly patients with breast cancer: Updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol 2012;13:e148-60. [Crossref] [PubMed]
- Girre V, Falcou MC, Gisselbrecht M, et al. Does a geriatric oncology consultation modify the cancer treatment plan for elderly patients? J Gerontol A Biol Sci Med Sci 2008;63:724-30. [Crossref] [PubMed]
- Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Crit Rev Oncol Hematol 2008;65:156-63. [Crossref] [PubMed]
- Schairer C, Carroll L, Mink PJ, et al. Probabilities of Death From Breast Cancer and Other Causes Among Female Breast Cancer Patients. JNCI J Natl Cancer Inst 2004;96:1311-21. [Crossref] [PubMed]
- Pierga JY, Girre V, Laurence V, et al. Characteristics and outcome of 1755 operable breast cancers in women over 70 years of age. Breast 2004;13:369-75. [Crossref] [PubMed]
- Gennari R, Curigliano G, Rotmensz N, et al. Breast carcinoma in elderly women: Features of disease presentation, choice of local and systemic treatments compared with younger postmenopausal patients. Cancer 2004;101:1302-10. [Crossref] [PubMed]
- Laird-Fick HS, Gardiner JC, Tokala H, et al. HER2 status in elderly women with breast cancer. J Geriatr Oncol 2013;4:362-7. [Crossref] [PubMed]
- Wildiers H, Van Calster B, Van De Poll-Franse LV, et al. Relationship between age and axillary lymph node involvement in women with breast cancer. J Clin Oncol 2009;27:2931-7. [Crossref] [PubMed]
- Freyer G, Braud AC, Chaibi P, et al. Dealing with metastatic breast cancer in elderly women: Results from a French study on a large cohort carried out by the “observatory on elderly patients”. Ann Oncol 2006;17:211-6. [Crossref] [PubMed]
- Jenkins EO, Deal AM, Anders CK, et al. Breast cancer intrinsic subtypes by PAM50 in older women. J Clin Oncol 2012;30:1524.
- Wu Y, Wei J, Chen X, et al. Comprehensive transcriptome profiling in elderly cancer patients reveals aging-altered immune cells and immune checkpoints. Int J Cancer 2019;144:1657-63. [Crossref] [PubMed]
- Provinciali M, Pierpaoli E, Malavolta M, et al. Breast Cancer and Immunosenescence. In: Fulop T, Franceschi C, Hirokawa K, et al. editors. Handbook of Immunosenescence: Basic Understanding and Clinical Implications. Cham: Springer International Publishing, 2017:1-31.
- Mandelblatt JS, Silliman R. Hanging in the Balance: Making Decisions About the Benefits and Harms of Breast Cancer Screening Among the Oldest Old Without a Safety Net of Scientific Evidence. J Clin Oncol 2009;27:487-90. [Crossref] [PubMed]
- Badgwell BD, Giordano SH, Duan ZZ, et al. Mammography Before Diagnosis Among Women Age 80 Years and Older With Breast Cancer. J Clin Oncol 2008;26:2482-8. [Crossref] [PubMed]
- Oeffinger KC, Fontham ETH, Etzioni R, et al. Breast Cancer Screening for Women at Average Risk: 2015 Guideline Update From the American Cancer Society 2015 Breast Cancer Screening Recommendations for Women at Average Risk 2015 Breast Cancer Screening Recommendations for Women at Average Risk. JAMA 2015;314:1599-614. [Crossref] [PubMed]
- Siu AL. Force on behalf of the USPST. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement Screening for Breast Cancer. Ann Intern Med 2016;164:279-96. [Crossref] [PubMed]
- Bevers TB, Helvie M, Bonaccio E, et al. Breast Cancer Screening and Diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:1362-89. [Crossref] [PubMed]
- Beau AB, Andersen PK, Vejborg I, et al. Limitations in the Effect of Screening on Breast Cancer Mortality. J Clin Oncol 2018;36:2988-94. [Crossref] [PubMed]
- Welch HG, Prorok PC, O’Malley AJ, et al. Breast-Cancer Tumor Size, Overdiagnosis, and Mammography Screening Effectiveness. N Engl J Med 2016;375:1438-47. [Crossref] [PubMed]
- Qaseem A, Lin JS, Mustafa RA, et al. Screening for Breast Cancer in Average-Risk Women: A Guidance Statement from the American College of Physicians. Ann Intern Med 2019;170:547-60. [Crossref] [PubMed]
- Arndt V, Stürmer T, Stegmaier C, et al. Patient delay and stage of diagnosis among breast cancer patients in Germany - a population based study. Br J Cancer 2002;86:1034. [Crossref] [PubMed]
- Spazzapan S, Crivellari D, Bedard P, et al. Therapeutic management of breast cancer in the elderly. Expert Opin Pharmacother 2011;12:945-60. [Crossref] [PubMed]
- DeSantis CE, Ma J, Goding Sauer A, et al. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin 2017;67:439-48. [Crossref] [PubMed]
- Lavelle K, Todd C, Moran A, et al. Non-standard management of breast cancer increases with age in the UK: a population based cohort of women > or =65 years. Br J Cancer 2007;96:1197. [Crossref] [PubMed]
- Bouchardy C, Rapiti E, Fioretta G, et al. Undertreatment Strongly Decreases Prognosis of Breast Cancer in Elderly Women. J Clin Oncol 2003;21:3580-7. [Crossref] [PubMed]
- Hancke K, Denkinger MD, König J, et al. Standard treatment of female patients with breast cancer decreases substantially for women aged 70 years and older: A German clinical cohort study. Ann Oncol 2010;21:748-53. [Crossref] [PubMed]
- Barthélémy P, Heitz D, Mathelin C, et al. Adjuvant chemotherapy in elderly patients with early breast cancer. Impact of age and comprehensive geriatric assessment on tumor board proposals. Crit Rev Oncol Hematol 2011;79:196-204. [Crossref] [PubMed]
- Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 1999;341:2061-7. [Crossref] [PubMed]
- Siminoff LA, Zhang A, Colabianchi N, et al. Factors that predict the referral of breast cancer patients onto clinical trials by their surgeons and medical oncologists J Clin Oncol 2000;18:1203-11. [Crossref] [PubMed]
- Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol 2005;23:3112-24. [Crossref] [PubMed]
- Jones JM, Nyhof-Young J, Moric J, et al. Identifying motivations and barriers to patient participation in clinical trials. J Cancer Educ 2006;21:237-42. [Crossref] [PubMed]
- Pallis AG, Ring A, Fortpied C, et al. EORTC workshop on clinical trial methodology in older individuals with a diagnosis of solid tumors. Ann Oncol 2011;22:1922-6. [Crossref] [PubMed]
- Clarke M, Collins R, Darby S, et al. EBCTCG. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106. [Crossref] [PubMed]
- Fyles AW, McCready DR, Manchul LA, et al. Tamoxifen with or without Breast Irradiation in Women 50 Years of Age or Older with Early Breast Cancer. N Engl J Med 2004;351:963-70. [Crossref] [PubMed]
- Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: Long-term follow-up of CALGB 9343. J Clin Oncol 2013;31:2382-7. [Crossref] [PubMed]
- Pötter R, Gnant M, Kwasny W, et al. Lumpectomy Plus Tamoxifen or Anastrozole With or Without Whole Breast Irradiation in Women With Favorable Early Breast Cancer. Int J Radiat Oncol Biol Phys 2007;68:334-40. [Crossref] [PubMed]
- Blamey RW, Bates T, Chetty U, et al. Radiotherapy or tamoxifen after conserving surgery for breast cancers of excellent prognosis: British Association of Surgical Oncology (BASO) II trial. Eur J Cancer 2013;49:2294-302. [Crossref] [PubMed]
- Kunkler IH, Williams LJ, Jack WJL, et al. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): A randomised controlled trial. Lancet Oncol 2015;16:266-73. [Crossref] [PubMed]
- Matuschek C, Bölke E, Haussmann J, et al. The benefit of adjuvant radiotherapy after breast conserving surgery in older patients with low risk breast cancer- a meta-analysis of randomized trials. Radiat Oncol 2017;12:60. [Crossref] [PubMed]
- Herskovic AC, Wu X, Christos PJ, et al. Omission of Adjuvant Radiotherapy in the Elderly Breast Cancer Patient: Missed Opportunity? Clin Breast Cancer 2018;18:418-31. [Crossref] [PubMed]
- Barron TI, Connolly RM, Bennett K, et al. Early discontinuation of tamoxifen: A lesson for oncologists. Cancer 2007;109:832-9. [Crossref] [PubMed]
- Kemp A, Preen DB, Saunders C, et al. Early discontinuation of endocrine therapy for breast cancer: who is at risk in clinical practice? Springerplus 2014;3:282. [Crossref] [PubMed]
- Killander F, Karlsson P, Anderson H, et al. No breast cancer subgroup can be spared postoperative radiotherapy after breast-conserving surgery. Fifteen-year results from the Swedish Breast Cancer Group randomised trial, SweBCG 91 RT. Eur J Cancer 2016;67:57-65. [Crossref] [PubMed]
- Momm F, Becker G, Bartelt S, et al. The elderly, fragile tumor patient: Radiotherapy as an effective and most feasible treatment modality. J Pain Symptom Manage 2004;27:3-4. [Crossref] [PubMed]
- Schrijvers D. ESMO handbook of cancer in the senior patient 2010.
- Horiot JC. Radiation Therapy and the Geriatric Oncology Patient. J Clin Oncol 2007;25:1930-5. [Crossref] [PubMed]
- Kauer-Dorner D, Berger D. The Role of Brachytherapy in the Treatment of Breast Cancer. Breast Care (Basel) 2018;13:157-61. [Crossref] [PubMed]
- Lancellotta V, Kovács G, Tagliaferri L, et al. Age Is Not a Limiting Factor in Interventional Radiotherapy (Brachytherapy) for Patients with Localized Cancer. Biomed Res Int 2018;2018:2178469. [Crossref] [PubMed]
- Arraras JI, Manterola A, Asin G, et al. Quality of life in elderly patients with localized breast cancer treated with radiotherapy. A prospective study. Breast 2016;26:46-53. [Crossref] [PubMed]
- Gokula K, Earnest A, Wong LC. Meta-analysis of incidence of early lung toxicity in 3-dimensional conformal irradiation of breast carcinomas. Radiat Oncol 2013;8:268. [Crossref] [PubMed]
- Murofushi KN, Oguchi M, Gosho M, et al. Radiation-induced bronchiolitis obliterans organizing pneumonia (BOOP) syndrome in breast cancer patients is associated with age. Radiat Oncol 2015;10:103. [Crossref] [PubMed]
- McGale P, Darby SC, Hall P, et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol 2011;100:167-75. [Crossref] [PubMed]
- Fragkandrea I, Kouloulias V, Mavridis P, et al. Radiation induced pneumonitis following whole breast radiotherapy treatment in early breast cancer patients treated with breast conserving surgery: A single institution study. Hippokratia 2013;17:233-8. [PubMed]
- Agrawal S. Clinical relevance of radiation pneumonitis in breast cancers. South Asian J Cancer 2013;2:19-20. [Crossref] [PubMed]
- Darby SC, Ewertz M, McGale P, et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. N Engl J Med 2013;368:987-98. [Crossref] [PubMed]
- van den Bogaard VAB, Ta BDP, van der Schaaf A, et al. Validation and Modification of a Prediction Model for Acute Cardiac Events in Patients with Breast Cancer Treated with Radiotherapy Based on Three-Dimensional Dose Distributions to Cardiac Substructures. J Clin Oncol 2017;35:1171-8. [Crossref] [PubMed]
- Merzenich H, Bartkowiak D, Schmidberger H, et al. 3D conformal radiotherapy is not associated with the long-term cardiac mortality in breast cancer patients: a retrospective cohort study in Germany (PASSOS-Heart Study). Breast Cancer Res Treat 2017;161:143-52. [Crossref] [PubMed]
- Piroth MD, Baumann R, Budach W, et al. Heart toxicity from breast cancer radiotherapy. Strahlenther Onkol 2019;195:1-12. [Crossref] [PubMed]
- Nagel G, Wedding U, Hoyer H, et al. The impact of comorbidity on the survival of postmenopausal women with breast cancer. J Cancer Res Clin Oncol 2004;130:664-70. [Crossref] [PubMed]
- Litière S, Werutsky G, Fentiman IS, et al. Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol 2012;13:412-9. [Crossref] [PubMed]
- Morrow M, White J, Moughan J, et al. Factors predicting the use of breast-conserving therapy in stage I and II breast carcinoma. J Clin Oncol 2001;19:2254-62. [Crossref] [PubMed]
- Monten C, Lievens Y. Adjuvant breast radiotherapy: How to trade-off cost and effectiveness? Radiother Oncol 2018;126:132-8. [Crossref] [PubMed]
- Yarnold J, Bentzen SM, Coles C, et al. Hypofractionated whole-breast radiotherapy for women with early breast cancer: Myths and realities. Int J Radiat Oncol Biol Phys 2011;79:1-9. [Crossref] [PubMed]
- Fisher CM, Rabinovitch R. Frontiers in radiotherapy for early-stage invasive breast cancer. J Clin Oncol 2014;32:2894-901. [Crossref] [PubMed]
- Yarnold J, Ashton A, Bliss J, et al. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: Long-term results of a randomised trial. Radiother Oncol 2005;75:9-17. [Crossref] [PubMed]
- Owen JR, Ashton A, Bliss JM, et al. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. Lancet Oncol 2006;7:467-71. [Crossref] [PubMed]
- START Trialists' Group. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol 2008;9:331-41. [Crossref] [PubMed]
- Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 2013;14:1086-94. [Crossref] [PubMed]
- START Trialists' Group. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet 2008;371:1098-107. [Crossref] [PubMed]
- Whelan TJ, Pignol JP, Levine MN, et al. Long-Term Results of Hypofractionated Radiation Therapy for Breast Cancer. N Engl J Med 2010;362:513-20. [Crossref] [PubMed]
- Martin S, Prise KM, Hill MA. Pushing the frontiers of radiobiology: A special feature in memory of Sir Oliver Scott and Professor Jack Fowler. Br J Radiol 2018;92:20189005. [Crossref]
- Budach W, Bölke E, Matuschek C. Hypofractionated Radiotherapy as Adjuvant Treatment in Early Breast Cancer. A Review and Meta-Analysis of Randomized Controlled Trials. Breast Care 2015;10:240-5. [Crossref] [PubMed]
- Valle LF, Agarwal S, Bickel KE, et al. Hypofractionated whole breast radiotherapy in breast conservation for early-stage breast cancer: a systematic review and meta-analysis of randomized trials. Breast Cancer Res Treat 2017;162:409-17. [Crossref] [PubMed]
- Hickey BE, James ML, Lehman M, et al. Hypofractionated radiation therapy for early breast cancer. Cochrane Database Syst Rev 2016;7:CD003860. [PubMed]
- Bloomfield DJ. Development of Postoperative Radiotherapy for Breast Cancer: UK Consensus Statements — a Model of Patient, Clinical and Commissioner Engagement? Clin Oncol (R Coll Radiol) 2017;29:639-41. [Crossref] [PubMed]
- Harnett A. Fewer fractions of adjuvant external beam radiotherapy for early breast cancer are safe and effective and can now be the standard of care. Why the UK’s NICE accepts fewer fractions as the standard of care for adjuvant radiotherapy in early breast cancer. Breast 2010;19:159-62. [Crossref] [PubMed]
- Smith BD, Bellon JR, Blitzblau R, et al. Radiation therapy for the whole breast: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol 2018;8:145-52. [Crossref] [PubMed]
- Wang SL, Fang H, Song YW, et al. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol 2019;20:352-60. [Crossref] [PubMed]
- Bartelink H, Maingon P, Poortmans P, et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol 2015;16:47-56. [Crossref] [PubMed]
- Alford SL, Prassas GN, Vogelesang CR, et al. Adjuvant breast radiotherapy using a simultaneous integrated boost: Clinical and dosimetric perspectives. J Med Imaging Radiat Oncol 2013;57:222-9. [Crossref] [PubMed]
- Liljegren G, Holmberg L, Adami HO, et al. Sector resection with or without postoperative radiotherapy for stage I breast cancer: five-year results of a randomized trial. J Natl Cancer Inst 1994;86:717-22. [Crossref] [PubMed]
- Vicini FA, Kestin LL, Goldstein NS. Defining the clinical target volume for patients with early-stage breast cancer treated with lumpectomy and accelerated partial breast irradiation: A pathologic analysis. Int J Radiat Oncol Biol Phys 2004;60:722-30. [Crossref] [PubMed]
- Shah C, Vicini F, Shaitelman SF, et al. The American Brachytherapy Society consensus statement for accelerated partial-breast irradiation. Brachytherapy 2018;17:154-70. [Crossref] [PubMed]
- Skowronek J, Wawrzyniak-Hojczyk M, Ambrochowicz K. Brachytherapy in accelerated partial breast irradiation (APBI) - Review of treatment methods. J Contemp Brachytherapy 2012;4:152-64. [Crossref] [PubMed]
- Coles CE, Griffin CL, Kirby AM, et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet 2017;390:1048-60. [Crossref] [PubMed]
- Rodríguez N, Sanz X, Dengra J, et al. Five-Year Outcomes, Cosmesis, and Toxicity With 3-Dimensional Conformal External Beam Radiation Therapy to Deliver Accelerated Partial Breast Irradiation. Int J Radiat Oncol Biol Phys 2013;87:1051-7. [Crossref] [PubMed]
- Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer:5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014;383:603-13. [Crossref] [PubMed]
- Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol 2013;14:1269-77. [Crossref] [PubMed]
- Esposito E, Douek M. Update on intraoperative radiotherapy: New challenges and issues. Ecancermedicalscience 2018;12:793. [Crossref] [PubMed]
- Patel R, Ivanov O, Voigt J. Lifetime cost-effectiveness analysis of intraoperative radiation therapy versus external beam radiation therapy for early stage breast cancer. Cost Eff Resour Alloc 2017;15:22. [Crossref] [PubMed]
- Tom MC, Joshi N, Vicini F, et al. The American Brachytherapy Society consensus statement on intraoperative radiation therapy. Brachytherapy ;18:242-57. [Crossref] [PubMed]
- Polgár C, Ott OJ, Hildebrandt G, et al. Late side-effects and cosmetic results of accelerated partial breast irradiation with interstitial brachytherapy versus whole-breast irradiation after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: 5-year resul. Lancet Oncol 2017;18:259-68. [Crossref] [PubMed]
- Strnad V, Ott OJ, Hildebrandt G, et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: a randomised, phase 3, non-inferiority trial. Lancet 2016;387:229-38. [Crossref] [PubMed]
- Livi L, Meattini I, Marrazzo L, et al. Accelerated partial breast irradiation using intensity-modulated radiotherapy versus whole breast irradiation: 5-year survival analysis of a phase 3 randomised controlled trial. Eur J Cancer 2015;51:451-63. [Crossref] [PubMed]
- Whelan T, Julian J, Levine M, et al. Abstract GS4-03: RAPID: A randomized trial of accelerated partial breast irradiation using 3-dimensional conformal radiotherapy (3D-CRT). Cancer Res 2019;79:GS4-03.
- Korzets Y, Fyles A, Shepshelovich D, et al. Toxicity and clinical outcomes of partial breast irradiation compared to whole breast irradiation for early-stage breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Marta GN, Macedo CR, Carvalho H de A, et al. Accelerated partial irradiation for breast cancer: Systematic review and meta-analysis of 8653 women in eight randomized trials. Radiother Oncol 2015;114:42-9. [Crossref] [PubMed]
- Hickey BE, Lehman M, Francis DP, et al. Partial breast irradiation for early breast cancer. Cochrane Database Syst Rev 2016;CD007077. [PubMed]
- Vaidya JS, Bulsara M, Wenz F, et al. Reduced Mortality With Partial-Breast Irradiation for Early Breast Cancer: A Meta-Analysis of Randomized Trials. Int J Radiat Oncol Biol Phys 2016;96:259-65. [Crossref] [PubMed]
- Chen MF, Chen WC, Lai CH, et al. Predictive factors of radiation-induced skin toxicity in breast cancer patients. BMC Cancer 2010;10:508. [Crossref] [PubMed]
- Balaji K, Subramanian B, Yadav P, et al. Radiation therapy for breast cancer: Literature review. Med Dosim 2016;41:253-7. [Crossref] [PubMed]
- Jin GH, Chen LX, Deng XW, et al. A comparative dosimetric study for treating left-sided breast cancer for small breast size using five different radiotherapy techniques: Conventional tangential field, filed-in-filed, Tangential-IMRT, Multi-beam IMRT and VMAT. Radiat Oncol 2013;8:89. [Crossref] [PubMed]
- Haciislamoglu E, Colak F, Canyilmaz E, et al. Dosimetric comparison of left-sided whole-breast irradiation with 3DCRT, forward-planned IMRT, inverse-planned IMRT, helical tomotherapy, and volumetric arc therapy. Phys Med 2015;31:360-7. [Crossref] [PubMed]
- Meattini I, Guenzi M, Fozza A, et al. Overview on cardiac, pulmonary and cutaneous toxicity in patients treated with adjuvant radiotherapy for breast cancer. Breast Cancer 2017;24:52-62. [Crossref] [PubMed]
- Ratosa I, Jenko A, Oblak I. Breast size impact on adjuvant radiotherapy adverse effects and dose parameters in treatment planning. Radiol Oncol 2018;52:233-44. [Crossref] [PubMed]
- Schubert LK, Gondi V, Sengbusch E, et al. Dosimetric comparison of left-sided whole breast irradiation with 3DCRT, forward-planned IMRT, inverse-planned IMRT, helical tomotherapy, and topotherapy. Radiother Oncol 2011;100:241-6. [Crossref] [PubMed]
- Popescu CC, Olivotto IA, Beckham WA, et al. Volumetric Modulated Arc Therapy Improves Dosimetry and Reduces Treatment Time Compared to Conventional Intensity-Modulated Radiotherapy for Locoregional Radiotherapy of Left-Sided Breast Cancer and Internal Mammary Nodes. Int J Radiat Oncol Biol Phys 2010;76:287-95. [Crossref] [PubMed]
- Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys 2006;65:1-7. [Crossref] [PubMed]
- Filippi AR, Vanoni V, Meduri B, et al. Intensity Modulated Radiation Therapy and Second Cancer Risk in Adults. Int J Radiat Oncol Biol Phys 2018;100:17-20. [Crossref] [PubMed]
- Formenti SC, DeWyngaert JK, Jozsef G, et al. Prone vs. supine positioning for breast cancer radiotherapy. JAMA 2012;308:861-3. [Crossref] [PubMed]
- Griem KL, Fetherston P, Kuznetsova M, et al. Three-dimensional photon dosimetry: A comparison of treatment of the intact breast in the supine and prone position. Int J Radiat Oncol Biol Phys 2003;57:891-9. [Crossref] [PubMed]
- Lymberis SC, De Wyngaert JK, Parhar P, et al. Prospective assessment of optimal individual position (prone versus supine) for breast radiotherapy: Volumetric and dosimetric correlations in 100 patients. Int J Radiat Oncol Biol Phys 2012;84:902-9. [Crossref] [PubMed]
- Chino JP, Marks LB. Prone Positioning Causes the Heart To Be Displaced Anteriorly Within the Thorax: Implications for Breast Cancer Treatment. Int J Radiat Oncol Biol Phys 2008;70:916-20. [Crossref] [PubMed]
- Boute B, Veldeman L, Speleers B, et al. The relation between patient discomfort and uncompensated forces of a patient support device for breast and regional lymph node radiotherapy. Appl Ergon 2018;72:48-57. [Crossref] [PubMed]
- Boda-Heggemann J, Knopf AC, Simeonova-Chergou A, et al. Deep Inspiration Breath Hold - Based Radiation Therapy: A Clinical Review Int J Radiat Oncol Biol Phys 2016;94:478-92. [Crossref] [PubMed]
- Bergom C, Currey A, Desai N, et al. Deep Inspiration Breath Hold: Techniques and Advantages for Cardiac Sparing During Breast Cancer Irradiation. Front Oncol 2018;8:87. [Crossref] [PubMed]
- Yeung R, Conroy L, Long K, et al. Cardiac dose reduction with deep inspiration breath hold for left-sided breast cancer radiotherapy patients with and without regional nodal irradiation. Radiat Oncol 2015;10:200. [Crossref] [PubMed]
- Holliday EB, Kirsner SM, Thames HD, et al. Lower mean heart dose with deep inspiration breath hold-whole breast irradiation compared with brachytherapy-based accelerated partial breast irradiation for women with left-sided tumors. Pract Radiat Oncol 2017;7:80-5. [Crossref] [PubMed]
- Bartlett FR, Colgan RM, Carr K, et al. The UK HeartSpare Study: Randomised evaluation of voluntary deep-inspiratory breath-hold in women undergoing breast radiotherapy. Radiother Oncol 2013;108:242-7. [Crossref] [PubMed]
- Latty D, Stuart KE, Wang W, et al. Review of deep inspiration breath-hold techniques for the treatment of breast cancer. J Med Radiat Sci 2015;62:74-81. [Crossref] [PubMed]
- Wong JW, Sharpe MB, Jaffray DA, et al. The use of active breathing control (ABC) to reduce margin for breathing motion. Int J Radiat Oncol Biol Phys 1999;44:911-9. [Crossref] [PubMed]
- Eldredge-Hindy HB, Duffy D, Yamoah K, et al. Modeled risk of ischemic heart disease following left breast irradiation with deep inspiration breath hold. Pract Radiat Oncol 2015;5:162-8. [Crossref] [PubMed]
- Thompson MK, Poortmans P, Chalmers AJ, et al. Practice-changing radiation therapy trials for the treatment of cancer: where are we 150 years after the birth of Marie Curie? Br J Cancer 2018;119:389-407. [Crossref] [PubMed]
- Sen S, Wang SY, Soulos PR, et al. Examining the cost-effectiveness of radiation therapy among older women with favorable-risk breast cancer. J Natl Cancer Inst 2014;106:dju008. [Crossref] [PubMed]
- Sanz J, Zhao M, Rodríguez N, et al. Once-Weekly Hypofractionated Radiotherapy for Breast Cancer in Elderly Patients: Efficacy and Tolerance in 486 Patients. Biomed Res Int 2018;2018:8321871. [Crossref] [PubMed]
- Brunt AM, Wheatley D, Yarnold J, et al. Acute skin toxicity associated with a 1-week schedule of whole breast radiotherapy compared with a standard 3-week regimen delivered in the UK FAST-Forward Trial. Radiother Oncol 2016;120:114-8. [Crossref] [PubMed]
- Ortholan C, Hannoun-Lévi JM, Ferrero JM, et al. Long-term results of adjuvant hypofractionated radiotherapy for breast cancer in elderly patients. Int J Radiat Oncol Biol Phys 2005;61:154-62. [Crossref] [PubMed]
- FAST Trialists group. First results of the randomised UK FAST Trial of radiotherapy hypofractionation for treatment of early breast cancer (CRUKE/04/015). Radiother Oncol 2011;100:93-100. [Crossref] [PubMed]
- NCT01803958. Breast Cancer With Low Risk Of Local Recurrence: Partial and Accelerated Radiation With Three-Dimensional Conformal Radiotherapy (3DCRT) Vs. Standard Radiotherapy After Conserving Surgery (Phase III Study). Available online: https://clinicaltrials.gov/ct2/show/NCT01803958
- Lischalk JW, Chen H, Repka MC, et al. Definitive hypofractionated radiation therapy for early stage breast cancer: Dosimetric feasibility of stereotactic ablative radiotherapy and proton beam therapy for intact breast tumors. Adv Radiat Oncol 2018;3:447-457. [Crossref] [PubMed]
- Shibamoto Y, Murai T, Suzuki K, et al. Definitive Radiotherapy With SBRT or IMRT Boost for Breast Cancer: Excellent Local Control and Cosmetic Outcome. Technol Cancer Res Treat 2018;17:1533033818799355. [Crossref] [PubMed]
- Barry A, Fyles A. Establishing the Role of Stereotactic Ablative Body Radiotherapy in Early-Stage Breast Cancer. Int J Breast Cancer 2018;2734820. [PubMed]
- NCT03585621. A Phase I-II Study of Stereotactic Body Radiation Therapy for Breast Cancer (SBRT Breast). Available online: https://clinicaltrials.gov/ct2/show/NCT03585621
- Smith BD, Gross CP, Smith GL, et al. Effectiveness of radiation therapy for older women with early breast cancer. J Natl Cancer Inst 2006;98:681-90. [Crossref] [PubMed]
- Smith GL, Smith BD. Radiation treatment in older patients: A framework for clinical decision making. J Clin Oncol 2014;32:2669-78. [Crossref] [PubMed]
- Raghupathi W, Raghupathi V. Big data analytics in healthcare: promise and potential. Health Inf Sci Syst 2014;2:3. [Crossref] [PubMed]
- Ghani KR, Zheng K, Wei JT, et al. Harnessing Big Data for Health Care and Research: Are Urologists Ready? Eur Urol 2014;66:975-7. [Crossref] [PubMed]
- Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-World Evidence — What Is It and What Can It Tell Us? N Engl J Med 2016;375:2293-7. [Crossref] [PubMed]
- Mendoza JB, Forns JR, Gutiérrez LQ, et al. Oportunidades y Retos de los Macrodatos (Big Data) en la toma de decisiones sanitarias. Fundación Gaspar Casal 2018.
- Haider AH, Bilimoria KY, Kibbe MR. A Checklist to Elevate the Science of Surgical Database Research. JAMA Surg 2018;153:505-7. [Crossref] [PubMed]
- Kruse CS, Goswamy R, Raval Y, et al. Challenges and Opportunities of Big Data in Health Care: A Systematic Review. JMIR Med Inform 2016;4:e38. [Crossref] [PubMed]
- Islam MS, Hasan M, Wang X, et al. A Systematic Review on Healthcare Analytics: Application and Theoretical Perspective of Data Mining. Healthcare (Basel) 2018;6:54. [Crossref] [PubMed]
- Booth CM, Karim S, Mackillop WJ. Real-world data: towards achieving the achievable in cancer care. Nat Rev Clin Oncol 2019;16:312-25. [Crossref] [PubMed]


