Pooled analysis of the clinical benefit of cyclooxygenase-2 inhibitors combined with chemotherapy in advanced non-small cell lung cancer
Introduction
Lung cancer, one of the most common malignant tumors, has the highest mortality rate among all human cancers (1,2). The incidence rate of lung cancer is also the highest among all human tumors, with around 1.8 million new cases each year (2), most of which are advanced non-small cell lung cancer (NSCLC) (3,4). Classical chemotherapy exerts its antitumor activity by causing damage and inducing apoptosis in rapidly dividing cells and has been a cornerstone of standard cancer treatment for several decades (5). The rationale for classical chemotherapy is to kill the malignant cells and reduce the tumor size (6). Because the success rate of lung cancer treatment has reached a plateau in recent years, new treatment strategies are urgently required to improve clinical efficacy in patients with advanced NSCLC (7).
Cyclooxygenase-2 (COX-2) inhibitors, an enzyme expressed in the inflammatory and neoplastic tissues, has been closely associated with tumor development including apoptosis, angiogenesis, and tumor invasiveness (8). In particular, COX-2 is involved in the conversion of arachidonic acid into prostaglandin and other bioactive lipids (1). Apart from being associated with inflammation, COX-2 induces large amounts of prostaglandin E2 in the tumor tissues (9-12) and is a key factor in tumorigenesis (11,13-18). COX-2 inhibitors, such as celecoxib, rofecoxib, and apricoxib have been used in the management of advanced NSCLC.
On the basis of previous studies (8,19-21), we hypothesized that protocols using COX-2 inhibitors in addition to chemotherapy would provide important benefits for the management of NSCLC. A study by Edelman et al. (22) found that patients with moderate to high COX-2 protein levels did not have better overall survival (OS) than those showing low expression of COX-2. Several clinical trials found superior effects of chemotherapy plus COX-2 inhibitors as compared to chemotherapy alone in NSCLC patients (23,24). We performed an overlapping meta-analysis of the literature to evaluate the efficacy and safety of COX-2 inhibitors in conjunction with chemotherapy in patients with advanced NSCLC.
Methods
Literature search
Until September 10, 2018, we searched the literature to identify the published meta-analyses and systematic reviews in PubMed, Embase, and the Cochrane Database of Systematic Reviews. The following search terms were used: “non-small cell lung cancer”, “non-small cell lung carcinoma”, “cyclooxygenase-2 inhibitors”, “COX-2 inhibitors”, and “chemotherapy”. The study types were limited to meta-analyses and systematic reviews. The abstracts that were found from these searches were reviewed by two reviewers (ZM Liao and Y Fu). We obtained the full texts of the studies that met our inclusion criteria. The cited studies from the selected meta-analyses were also reviewed to ensure that no studies were missed.
Eligibility criteria
The inclusion criteria were (I) meta-analyses that assessed the efficacy and safety of chemotherapy and COX-2 for NSCLC treatment, (II) the most complete or the most recent meta-analysis that included the same results from the same patients by the same author, and (III) meta-analyses written in Chinese or English language. The exclusion criteria were (I) studies that did not involve the use of chemotherapy, (II) studies without clinical outcomes of interest, and (III) systematic reviews that did not perform meta-analysis or synthesize data.
Data extraction
The data extracted from each study included primary author, year of publication, search date of the last studies, number of included studies including randomized controlled trials, publication language, publication status, databases, inclusion of the primary studies, treatment outcomes, and adverse reactions. The outcome measures of the overlapping meta-analysis included 1-year survival rate (1-year SR), progression-free survival (PFS), OS, quality of life (QOL), overall response rate (ORR), and toxicities.
Quality assessment
The Quality of Reporting of Meta-analyses (QUOROM) system (25) is a tool for assessing the methodological quality of meta-analyses. The 18-category QUOROM checklist generates an overall score according to the quality of the reporting and methodology of a meta-analysis. One point was awarded for each of the 18 possible categories if the study met over half of the standards for that category. The Oxman-Guyatt score was also used to grade the methodology of each meta-analysis (26). Finally, studies were recorded in some case if the study recorded bases within the reviewed literature. Any disagreement regarding the methodological quality was resolved by discussion with an author (W Zheng).
Heterogeneity assessment
Heterogeneity describes between-study variability, which can be related to clinical and methodological differences between the studies. In this meta-analysis, heterogeneity between the comparable studies was tested with the use of the I2 statistics (27), which describes the percentage of total variation across the studies that is attributable to heterogeneity rather than chance. In the I2 statistic, a value of <25% is considered to reflect low heterogeneity; 50−75% is moderate heterogeneity; and >75% is high heterogeneity.
Application of the Jadad decision algorithm
The Jadad decision algorithm (28) is a common tool for investigating the origins of inconsistencies among systematic reviews, such as those concerning quality evaluation, extraction, data synthesis, and statistical analysis. This algorithm has been widely employed to offer critical recommendations about treatment among meta-analyses with conflicting conclusions. The algorithm was independently performed by three authors, who reached a consensus on the optimal evidence from the included meta-analyses.
Results
Literature search
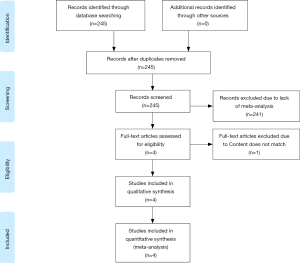
Our initial article search identified 245 studies, of which four (8,19-21) were included based on our study selection algorithm (Figure 1). Four studies were published from 2014 (8) to 2018 (19); All the studies reported conflicts of interest and declared that they had no competing interests. The number of primary studies included in each meta-analysis ranged from four (8) to nine (19) (Table 1), and the studies that met our criteria reported on sample sizes of 922 (8) to 1,794 patients (19). Our study selection algorithm is shown in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (29) diagram (Figure 1).
Table 1
| First author | Date of publication | Date of last literature search | No. of included studies | No. of included RCTs |
|---|---|---|---|---|
| Chen (8) | June 12, 2014 | January 31, 2014 | 4 | 4 |
| Hou (20) | August 29, 2015 | March 2015 | 6 | 6 |
| Zhou (21) | March 23, 2016 | July 2015 | 9 | 9 |
| Dai (19) | February 05, 2018 | March 26, 2017 | 6 | 6 |
RCT, randomized controlled trial.
Search methodology
Each included meta-analysis was searched in the medical databases PubMed and Embase. Marked differences were found among the other databases (Table 2), with four studies (8,19-21) using the Cochrane Library database and only one study (20) using both the China National Knowledge Infrastructure (CNKI) and the China Biomedicine Database on Disc (CBMdisc). Only the study by Dai et al. (19) was registered in ClinicalTrials.gov.
Table 2
| Author | Publication language | Publication status | Search databases | |||||
|---|---|---|---|---|---|---|---|---|
| PubMed/Medline | Embase | Cochrane database | CNKI | CBMdisc | Others | |||
| Chen (8) | + | + | + | + | + | − | − | − |
| Hou (20) | + | + | + | + | + | + | + | − |
| Zhou (21) | + | + | + | + | − | − | − | − |
| Dai (19) | + | + | + | + | + | − | − | + |
+, indicates that the item was reported; −, indicates that the item was not reported; Others, Dai et al. used ClinicalTrials.gov to search literature. NR, not reported; CNKI, China National Knowledge Infrastructure; CBMdisc, China Biomedicine Database on Disc.
The four meta-analyses included 14 primary studies (Table 3). Chen et al. (8) included four primary studies (7,30,31,35) on NSCLC, Hou et al. (20) included six primary studies (7,30,32,34-36), Zhou et al. (21) included nine primary studies (7,22,30,31,33,35,37-39, and Dai et al. (19) included six primary studies (7,30,33,35,38,40).
Table 3
| Primary studies | Year | Chen (8) | Hou (20) | Zhou (21) | Dai (19) |
|---|---|---|---|---|---|
| Lilenbaum (30) | 2006 | + | + | + | + |
| De Ruysscher (31) | 2007 | + | − | + | − |
| Zhou (32) | 2007 | − | + | − | − |
| Gridelli (33) | 2007 | − | − | + | + |
| Edelman (22) | 2008 | − | − | + | − |
| Xiong (34) | 2008 | − | + | − | − |
| Groen (7) | 2011 | + | + | + | + |
| Koch (35) | 2011 | + | + | + | + |
| Liu (36) | 2012 | − | + | − | − |
| Gitlitz (37) | 2014 | − | − | + | − |
| Edelman (38) | 2015 | − | − | + | + |
| Reckamp (39) | 2015 | − | − | + | − |
| Edelman (40) | 2017 | − | − | − | + |
+, indicates that the meta-analysis of colume includes the original study of row; −, indicates that the meta-analysis of colume did not include the original study of row.
Outcome measures
Some discrepancies were found among the outcomes evaluated by each meta-analysis (Table 4). The different outcomes, namely, ORR, PFS, OS, 1-year SR, and toxicities (Table 5) were reported in all the four studies (8,19-21), however, only Zhou et al. (21) and Chen et al. (8) reported QOL (Table 4). All the three studies (8,20,21) reported survival indices, such as OS and PFS, but Hou et al. (20) did not perform any statistical analysis.
Table 4
| Author | ORR (RR) | CB (OR/RR) | PFS (Mo/HR) | OS (Mo/HR) | CR | PR | 1-year SR (OR/RR) | QoL | Oxman-Guyatt Score | QUOROM Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen (8) | + | − | + | + | − | − | +# | + | 5 | 16 |
| Hou (20) | + | + | +* | +* | + | + | + | − | 4 | 13 |
| Zhou (21) | + | − | + | + | − | − | + | + | 5 | 14 |
| Dai (19) | + | − | + | + | − | − | + | − | 5 | 16 |
#, Chen et al. used 1-year mortality to show the survival rate; *, Hou et al. reported progression-free survival; +, indicates that the meta-analysis of row includes the outcome of colume; −, indicates that the meta-analysis of row did not include the outcome of colume. ORR, overall response rate; CB, clinical benefit; PFS, progression-free survival; OS, overall survival; CR, complete release or complete response; PR, partial release or complete response; QoL, quality of life; 1-year SR, 1-year survival rate; OR, odds ratio; RR, relative risk; Mo, month.
Table 5
| Adverse event | Chen (8) | Hou (20) | Zhou (21) | Dai (19) |
|---|---|---|---|---|
| Hematological | ||||
| Leukopenia | − | + | + | + |
| Thrombocytopenia | − | + | − | + |
| Anemia | + | + | − | + |
| Low Hemoglobin | − | − | + | − |
| Neutropenia | − | − | + | − |
| Non-hematological | ||||
| Nausea/vomiting | + | + | + | + |
| Diarrhea | + | + | + | + |
| Asthenia | − | + | − | + |
| Fatigue | − | − | + | − |
| Dyspnea | − | − | + | − |
| Gastric ulcer | − | + | - | − |
| Cardiotoxicity | + | + | + | + |
| Allergy | − | − | + | − |
| Skin rash | + | − | − | − |
| Hepatotoxicity | + | − | − | − |
| Thrombosis/embolism | − | − | + | − |
| Neurotoxicity | − | + | − | − |
+, indicates that the meta-analysis of colume includes the adverse event of row; −, indicates that the meta-analysis of colume did not include the adverse event of row.
Study results
All four studies (8,19-21) concluded that significant improvements in ORR and OS were achieved with chemotherapy plus COX-2 inhibitors. However, some adverse reactions were caused by COX-2 inhibitors. All studies indicated an increased ORR from chemotherapy plus COX-2 inhibitors over chemotherapy alone. In assessing the treatment line, we found a significant effect on ORR when COX-2 inhibitors were used as the first-line treatment, but no obvious effect was found with their use as the second-line treatment. All studies estimated the 1-year SR, which showed no improvement in patients receiving chemotherapy plus celecoxib. All four studies included the common toxicities of COX-2 inhibitors (Table 5), such as hematological events (leukopenia, thrombocytopenia, and anemia), gastrointestinal events (diarrhea, and nausea/vomiting), cardiotoxicity, and other adverse events. It was found that hematological toxicities related to chemotherapy were increased because of the COX-2 inhibitors.
Study quality and validity
QUOROM scores ranging from 0 to 18, were calculated for each study (Table 4), with two studies (8,21) scoring 16 and two studies (20,21) scoring <15 [13 for Hou et al. (20) and 14 for Zhou et al. (21)]. The mean score was 15, and the median score was 16. The Oxman-Guyatt scores ranged from 4 (20) to 5 (19). The mean score was 4.75, and the median score was 5. Three (8,19,21) of the four studies had Oxman-Guyatt scores of 5.
Heterogeneity assessment
Heterogeneity analyses were reported by all the four studies (8,19-21). Three (8,19,21) of the four studies performed a sensitivity or subgroup analysis to assess the influences, such as treatment line, ORR, and type of COX-2 inhibitors (Table 6). Only one meta-analysis (20) did not perform any subgroup analysis.
Table 6
| Items of subgroup or sensitivity analysis | Chen (8) | Hou (20) | Zhou (21) | Dai (19) |
|---|---|---|---|---|
| Statistical heterogeneity analysis | + | + | + | + |
| Subgroup or sensitivity analysis | ||||
| Primary study quality | + | + | + | + |
| Publication bias of primary study | + | + | + | + |
| 1-year survival rate | +# | − | + | + |
| Overall response rate | + | 0 | + | + |
| Progression-free survival | 0 | 0 | 0 | + |
| Overall survival | 0 | 0 | 0 | + |
| Clinical benefit | − | 0 | − | − |
| Toxicities | 0 | 0 | 0 | 0 |
+/−, indicates that formal sensitivity or subgroup analysis was/was not performed; 0, indicates that descriptive data were performed or discussed, but no analysis was performed; #, Chen et al. used 1-year mortality to show the 1-year survival rate.
Application of the Jadad decision algorithm
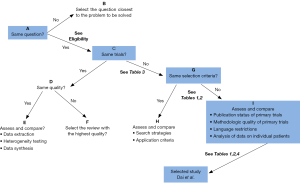
To determine which meta-analysis provided the optimal current evidence, the two lead authors independently used the Jadad decision algorithm (28) and concluded that two (8,19) of the four included studies indicated the highest level of evidence. Dai’s study (19) showed that chemotherapy plus COX-2 inhibitors increased the ORR in advanced NSCLC, especially when combined with the standard treatment. Figure 2, a flow diagram of the Jadad decision algorithm, shows all outcomes of the included meta-analyses.
Discussion
The major purpose of this overlapping meta-analysis was to establish the safety and efficacy of the use of COX-2 inhibitors with chemotherapy. Previous studies have shown that these treatments can increase the ORR and survival indices in NSCLC. However, their use has also been associated with the increased risk of toxicity and shortened OS and PFS. Several studies have investigated this conflict (7,30,31,35), and therefore, this overlapping meta-analysis was conducted to explore the reason for the discordance among the previous meta-analyses and to identify which studies offered the optimal evidence on the treatment of advanced NSCLC. The hypothesis that chemotherapy plus celecoxib could provide more benefits than chemotherapy alone in advanced NSCLC was confirmed.
This review used several tools (QUOROM and Oxman-Guyatt scores, and the Jadad algorithm) to assess the quality of the four meta-analyses (8,19-21). Three (8,19,21) included meta-analyses had Oxman-Guyatt scores of 5 with QUOROM scores of at least 13 (20). One study with no major flaws in its methodology (26) had an Oxman-Guyatt score of 4 (20). Our conclusions are mainly dependent on Dai’s meta-analysis (19), which has the highest QUOROM and Oxman-Guyatt quality assessments among the four included meta-analyses (8,19-21), and provides practical recommendations. Dai et al. (19) found that COX-2 inhibitors showed no impact on survival indices (1-year SR) but improved the ORR in advanced NSCLC when used as the first-line chemotherapy. In contrast, patients with advanced NSCLC who received COX-2 inhibitors as a second-line treatment showed no significant difference.
This study of overlapping meta-analyses also found that celecoxib is likely to lead to a higher incidence rate of hematological toxicities, whereas rofecoxib may not avoid the risk of cardiovascular events. However, Dai et al. (19) analyzed the clinical benefits and indicated that the addition of COX-2 inhibitors to chemotherapy regimens resulted in no significant difference. The study by Chen et al. (8), which had a QUOROM score of 16 and an Oxman-Guyatt score of 5, found a modest activity for celecoxib against advanced cancers and indicated that a better outcome was obtained if celecoxib was combined early with chemotherapy. The study by Dai et al. (19), which also had a QUOROM score of 16 and an Oxman-Guyatt score of 5, found that the type of COX-2 inhibitor used was a deciding factor; and a further subgroup analysis indicated that rofecoxib combined with chemotherapy as the first-line treatment markedly improved ORR in NSCLC patients. Because celecoxib may increase the risk of cardiovascular events in patients with a medical history of heart disease (8,19), clinicians and decision-makers must consider the cardiovascular toxicities caused by COX-2 inhibitors. In contrast to the study by Dai et al. (19), Chen et al. (8) concluded that QOL outcomes were not significantly different between the celecoxib and the control groups. These two studies (8,19) were also identified by the Jadad algorithm as having the highest levels of evidence. The remaining studies (20,21) presented conclusions that were similar to those of the two higher-quality assessments.
The advantage of this overlapping meta-analysis lies in the use of a series of validated independent quality assessment tools to fully assess each study. Additionally, this overlapping meta-analysis is a comprehensive study on researches evaluating the clinical benefits of COX-2 inhibitors combined with chemotherapy in advanced NSCLC. However, there are several limitations in the number of included meta-analyses, including reporting bias (41) and limitations in the trial type. This study was limited to randomized controlled trials and included published and unpublished data, but all our included meta-analyses were from China. Sufficient individual data, such as age, gender, nationality, dosage of COX-2 inhibitors, and follow-up periods, were not reported. Only two meta-analyses (8,19) described most of these detailed data. Furthermore, the primary studies lacked descriptions of treatment allocation concealment (42) and blinding methods (41) and lacked a good number of trials. At the same time, we found that the included meta-analyses did not compare chemotherapy plus COX-2 inhibitors to chemotherapy alone solely for the treatment of NSCLC. For example, Chen et al. (8) added other cancer types, including colorectal, prostate, breast, and ovarian cancers, and other treatment patterns, including hormonal therapy and radiotherapy.
Conclusions
Based on the best available evidence, the use of chemotherapy combined with COX-2 inhibitors (most often celecoxib) had a more significant impact on advanced NSCLC than chemotherapy alone. However, because of the associated adverse reactions of the drugs, we must carefully consider the appropriateness of administering these drugs in patients with a medical history of heart disease. Chemotherapy plus celecoxib had better efficacy as a first-line treatment than as a second-line treatment.
Acknowledgments
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li W, Yue W, Wang H, et al. Cyclooxygenase-2 is associated with malignant phenotypes in human lung cancer. Oncol Lett 2016;12:3836-44. [Crossref] [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Sher T, Dy GK, Adjei AA. Small cell lung cancer. Mayo Clin Proc 2008;83:355-67. [Crossref] [PubMed]
- Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol 2007;25:5570-7. [Crossref] [PubMed]
- Lasalvia-Prisco E, Goldschmidt P, Galmarini F, et al. Addition of an induction regimen of antiangiogenesis and antitumor immunity to standard chemotherapy improves survival in advanced malignancies. Med Oncol 2012;29:3626-33. [Crossref] [PubMed]
- Zitvogel L, Apetoh L, Ghiringhelli F, et al. The anticancer immune response: indispensable for therapeutic success? J Clin Invest 2008;118:1991-2001. [Crossref] [PubMed]
- Groen HJ, Sietsma H, Vincent A, et al. Randomized, placebo-controlled phase III study of docetaxel plus carboplatin with celecoxib and cyclooxygenase-2 expression as a biomarker for patients with advanced non-small-cell lung cancer: the NVALT-4 study. J Clin Oncol 2011;29:4320-6. [Crossref] [PubMed]
- Chen J, Shen P, Zhang XC, et al. Efficacy and safety profile of celecoxib for treating advanced cancers: a meta-analysis of 11 randomized clinical trials. Clin Ther 2014;36:1253-63. [Crossref] [PubMed]
- Hold GL, El-Omar EM. Genetic aspects of inflammation and cancer. Biochem J 2008;410:225-35. [Crossref] [PubMed]
- Kokawa A, Kondo H, Gotoda T, et al. Increased expression of cyclooxygenase-2 in human pancreatic neoplasms and potential for chemoprevention by cyclooxygenase inhibitors. Cancer 2001;91:333-8. [Crossref] [PubMed]
- Denkert C, Kobel M, Berger S, et al. Expression of cyclooxygenase 2 in human malignant melanoma. Cancer Res 2001;61:303-8. [PubMed]
- Masferrer JL, Leahy KM, Koki AT, et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res 2000;60:1306-11. [PubMed]
- Sahin M, Sahin E, Gumuslu S. Cyclooxygenase-2 in cancer and angiogenesis. Angiology 2009;60:242-53. [Crossref] [PubMed]
- Van Dyke AL, Cote ML, Prysak GM, et al. COX-2/EGFR expression and survival among women with adenocarcinoma of the lung. Carcinogenesis 2008;29:1781-7. [Crossref] [PubMed]
- Fidler MJ, Argiris A, Patel JD, et al. The potential predictive value of cyclooxygenase-2 expression and increased risk of gastrointestinal hemorrhage in advanced non-small cell lung cancer patients treated with erlotinib and celecoxib. Clin Cancer Res 2008;14:2088-94. [Crossref] [PubMed]
- Chen YJ, Wang LS, Wang PH, et al. High cyclooxygenase-2 expression in cervical adenocarcinomas. Gynecol Oncol 2003;88:379-85. [Crossref] [PubMed]
- Khuri FR, Wu H, Lee JJ, et al. Cyclooxygenase-2 overexpression is a marker of poor prognosis in stage I non-small cell lung cancer. Clin Cancer Res 2001;7:861-7. [PubMed]
- Gupta S, Srivastava M, Ahmad N, et al. Over-expression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate 2000;42:73-8. [Crossref] [PubMed]
- Dai P, Li J, Ma XP, et al. Efficacy and safety of COX-2 inhibitors for advanced non-small-cell lung cancer with chemotherapy: a meta-analysis. Onco Targets Ther 2018;11:721-30. [Crossref] [PubMed]
- Hou LC, Huang F, Xu HB. Does celecoxib improve the efficacy of chemotherapy for advanced non-small cell lung cancer? Br J Clin Pharmacol 2016;81:23-32. [Crossref] [PubMed]
- Zhou YY, Hu ZG, Zeng FJ, et al. Clinical Profile of Cyclooxygenase-2 Inhibitors in Treating Non-Small Cell Lung Cancer: A Meta-Analysis of Nine Randomized Clinical Trials. PLoS One 2016;11:e0151939. [Crossref] [PubMed]
- Edelman MJ, Watson D, Wang X, et al. Eicosanoid modulation in advanced lung cancer: cyclooxygenase-2 expression is a positive predictive factor for celecoxib + chemotherapy--Cancer and Leukemia Group B Trial 30203. J Clin Oncol 2008;26:848-55. [Crossref] [PubMed]
- Altorki NK, Keresztes RS, Port JL, et al. Celecoxib, a selective cyclo-oxygenase-2 inhibitor, enhances the response to preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer. J Clin Oncol 2003;21:2645-50. [Crossref] [PubMed]
- Nugent FW, Mertens WC, Graziano S, et al. Docetaxel and cyclooxygenase-2 inhibition with celecoxib for advanced non-small cell lung cancer progressing after platinum-based chemotherapy: a multicenter phase II trial. Lung Cancer 2005;48:267-73. [Crossref] [PubMed]
- Moher D, Cook DJ, Eastwood S, et al. Improving the Quality of Reports of Meta-Analyses of Randomised Controlled Trials: The QUOROM Statement. Onkologie 2000;23:597-602. [PubMed]
- Oxman AD, Guyatt GH. Validation of an index of the quality of review articles. J Clin Epidemiol 1991;44:1271-8. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Bmj 2003;327:557-60. [Crossref] [PubMed]
- Jadad AR, Cook DJ, Browman GP. A guide to interpreting discordant systematic reviews. Cmaj 1997;156:1411-6. [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. [Crossref] [PubMed]
- Lilenbaum R, Socinski MA, Altorki NK, et al. Randomized phase II trial of docetaxel/irinotecan and gemcitabine/irinotecan with or without celecoxib in the second-line treatment of non-small-cell lung cancer. J Clin Oncol 2006;24:4825-32. [Crossref] [PubMed]
- De Ruysscher D, Bussink J, Rodrigus P, et al. Concurrent celecoxib versus placebo in patients with stage II-III non-small cell lung cancer: a randomised phase II trial. Radiother Oncol 2007;84:23-5. [Crossref] [PubMed]
- Zhou SW, Zhou CC, Xu JF, et al. First-line regimen of vinorelbine and cisplatin (NP) combined with cyclooxygenase-2 inhibitor celecoxib in advanced non-small-cell lung cancer. J Thorac Oncol 2007;2:2-327. [Crossref]
- Gridelli C, Gallo C, Ceribelli A, et al. Factorial phase III randomised trial of rofecoxib and prolonged constant infusion of gemcitabine in advanced non-small-cell lung cancer: the GEmcitabine-COxib in NSCLC (GECO) study. Lancet Oncol 2007;8:500-12. [Crossref] [PubMed]
- Xiong JP, Xiang XJ, Zhang L, et al. A phase II study of vinorelbine/cisplatin with or without COX-2 inhibitor in first-line treatment of non-small cell lung cancer. Cancer Res Prev Treat 2008;35:201-3.
- Koch A, Bergman B, Holmberg E, et al. Effect of celecoxib on survival in patients with advanced non-small cell lung cancer: a double blind randomised clinical phase III trial (CYCLUS study) by the Swedish Lung Cancer Study Group. Eur J Cancer 2011;47:1546-55. [Crossref] [PubMed]
- Liu GH, Huang JA. Clinical study if celecoxib combined with chemotherapy in the treatment of patients with advanced lung cancer. Chin J Cancer Prev Treat 2012;9:1661-3.
- Gitlitz BJ, Bernstein E, Santos ES, et al. A randomized, placebo-controlled, multicenter, biomarker-selected, phase 2 study of apricoxib in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Thorac Oncol 2014;9:577-82. [Crossref] [PubMed]
- Edelman MJ, Tan MT, Fidler MJ, et al. Randomized, double-blind, placebo-controlled, multicenter phase II study of the efficacy and safety of apricoxib in combination with either docetaxel or pemetrexed in patients with biomarker-selected non-small-cell lung cancer. J Clin Oncol 2015;33:189-94. [Crossref] [PubMed]
- Reckamp KL, Koczywas M, Cristea MC, et al. Randomized phase 2 trial of erlotinib in combination with high-dose celecoxib or placebo in patients with advanced non-small cell lung cancer. Cancer 2015;121:3298-306. [Crossref] [PubMed]
- Edelman MJ, Wang X, Hodgson L, et al. Phase III Randomized, Placebo-Controlled, Double-Blind Trial of Celecoxib in Addition to Standard Chemotherapy for Advanced Non-Small-Cell Lung Cancer With Cyclooxygenase-2 Overexpression: CALGB 30801 (Alliance). J Clin Oncol 2017;35:2184-92. [Crossref] [PubMed]
- Higgins JP. Cochrane handbook for systematic reviews of interventions, v.5.1. Available online: http://www.cochrane-handbook.org. [Last updated on 2011 Mar 05].
- Pildal J, Hrobjartsson A, Jorgensen KJ, et al. Impact of allocation concealment on conclusions drawn from meta-analyses of randomized trials. Int J Epidemiol 2007;36:847-57. [Crossref] [PubMed]