
Efficacy and safety of CalliSpheres® drug-eluting beads transarterial chemoembolization in patients with secondary liver cancer: a preliminary result from CTILC study
Introduction
The liver is one of the most frequent sites of distant metastasis from various carcinomas due to its numerous supplying arteries, and its involvement has been considered to be a critical prognostic factor affecting patients’ survival and quality of life (1,2). According to previous clinical reports, a significant number of patients with various carcinomas would also experience liver metastasis, including an estimated 50% of patients with colorectal cancer, 40% of patients with gastric cancer, and 25% to 50% of patients with pancreatic cancer. Furthermore, the 5-year survival rate dramatically decreases compared to patients without liver metastasis (3-5).
Despite the widespread use of transarterial chemoembolization (TACE), which contributes to antitumor effects and selective ischemia of a targeted tumor to kill the tumor cells (6,7), the conventional TACE (cTACE) is performed using lipiodol and chemotherapeutic agents. However, there still exist some limitations in containing the systemic toxicity caused by the flow of chemotherapeutics in the circulation system and the incapacity to precisely regulate drug release, thereby rendering cTACE ineffective (8,9). To reduce these drawbacks, the drug-eluting bead TACE (DEB-TACE), a novel drug delivery system using microspheres as embolic agents loaded with chemotherapy drugs, has been introduced into clinical practice, and offers higher intratumoral concentration and lower systemic drug concentrations when compared to cTACE, decreasing systemic adverse drug reactions and liver toxicity (10-13).
Although the benefits of DEB-TACE have been confirmed in patients with primary liver cancer, knowledge is still lacking regarding the efficacy and safety of DEB-TACE in patients with secondary liver cancer (14-17). Therefore, the purpose of this study was to assess the treatment response, short-term overall survival (OS), and safety profiles of DEB-TACE treatment in patients with secondary liver cancer.
Methods
Patients
Fifty-five patients with secondary liver cancer underwent DEB-TACE treatment between 2015/11/12 and 2016/11/04 from a CTILC study. The study was a multi-center, prospective cohort study which was registered on clinicaltrials.gov (registry No. NCT03317483) and consecutively enrolled 367 liver cancer patients from 24 medical centers to investigate the efficacy, safety, and prognostic factors of DEB-TACE treatment in Chinese patients with liver cancer. It provided additional and convincing evidence for the role of DEB-TACE treatment in Chinese patients with liver cancer (18). The inclusion criteria for patients of the CTILC study were as follows: (I) diagnosed as primary HCC, primary ICC, or secondary liver cancer confirmed by pathological findings, clinical features, or radiographic examinations according to the American Association for the Study of the Liver Diseases (AASLD) guidelines; (II) age above 18 years; (III) about to receive DEB-TACE treatment with CalliSpheres® according to clinical needs and patients’ willingness; (IV) able to be followed up regularly; (V) life expectancy above 12 months. The exclusion criteria were as follows: (I) history of liver transplantation; (II) history of hematological malignancies; (III) severe hepatic failure (Child-Pugh score of ≥10) or renal failure; (IV) contraindication for angiography, embolization procedure, or artery puncture; (V) cognitive impairment, or unable to understand the study consents; (VI) women in gestation or lactation period. This study was approved by the Ethics committee of the Zhejiang Cancer Hospital. All the patients or their legal guardian provided the written informed consent. This study was conducted according to the Declaration of Helsinki.
Baseline data collection
The following comprehensive baseline data of secondary liver cancer patients were collected: (I) demographic features including age and gender. (II) Clinical features including multifocal or unifocal tumor distribution (unifocal disease meant the single lesion, and unifocal disease patients could receive resection, while some of them might be unbearable for the surgery resection or unwilling to receive surgery due to other reasons (such as advanced age and severe cirrhosis). Thus, they chose DEB-TACE treatment, tumor location, largest nodule size, portal vein invasion status (the extent of portal vein thrombosis was incomplete occlusion of the portal vein trunk, and DEB-TACE treatment was suitable for patients with incomplete occlusion of portal vein trunk or the compensatory collateral vessel formation between the hepatic artery and the portal vein despite complete obstruction; meanwhile, patients whose portal vein trunk was completely embolized by tumor and had less collateral vessel formation were contraindicated for DEB-TACE treatment), hepatic vein invasion, ECOG performance status, primary cancer, and cycles of DEB-TACE treatment. (III) Blood routine indexes including white blood cell (WBC), red blood cell (RBC), absolute neutrophil (ANC), hemoglobin (Hb), and platelet (PLT) count. (IV) Liver function indexes including albumin (ALB), total protein (TP), total bilirubin (TBIL), total bile acid (TBA), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP). (V) Kidney function indexes including blood creatinine (BCr) and blood urea nitrogen (BUN); (VI) tumor markers including alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), and carbohydrate antigen 199 (CA199). (VII) Treatment history including cTACE history, surgery history, systematic chemotherapy history, radiofrequency ablation history, and targeted therapy history. (VIII) Chemoembolization reagents and combination of ordinary embolization agent.
Treatment
TACE was carried out using a micro-puncture system via placing a 5F vascular introducer (Boston Scientific, USA) through a transfemoral arterial access route. Angiography of the hepatic artery was executed to provide the liver’s vascular anatomy. The CalliSpheres® (Jiangsu Hengrui Medicine Co, Ltd., China) DEB loading was performed as follows: after the injection of CBs to the chemotherapy reagent solution was performed, the mixed solution was shaken up every 5 minutes for 30 minutes in an injector at a temperature of 23–28 °C. After that, adding non-ionic contrast agent into the same injector was performed at the ratio of (1–1.2):1 compared to the mixed solution and placed for 5 minutes at a temperature of 23–28 °C for use. Two mL 100–300 µm CalliSpheres® DEBs (Jiangsu Hengrui Medicine Co, Ltd., China) loaded with 50–80 mg of anthracyclines (doxorubicin) for metastatic lung cancer and breast cancer patients, and 100–200 mg irinotecan for intestinal cancer, gastric cancer, and pancreatic cancer patients, were used and administered until stasis in each patient. After the procedure, patients were admitted and monitored to the hospital overnight.
Efficacy assessment
Treatment response to DEB-TACE was performed at 1 to 3 months after DEB-TACE treatment by computerized tomography (CT) and magnetic resonance imaging (MRI) based on modified response evaluation criteria in solid tumors (mRECIST). The categories were defined as follows: as (I) complete response (CR), the disappearance of any arterial enhancement of targeted tumors; (II) partial response (PR), more than a 30% decrease in the sum of the diameter of targeted tumors (with arterial enhancement), using the baseline sum of diameter of targeted tumor as a reference; (III) stable disease (SD), the decrease in sum of the diameter of the targeted tumor with arterial enhancement) not reaching PR, with its increase less than progressive disease (PD); (IV) PD, more than 20% increase in the sum of diameter of targeted tumor (with arterial enhancement), taking the smallest diameter of the viable lesion as a reference. In addition, the objective response rate (ORR) was defined as the proportion of patients who achieved CR and PR.
Overall survival (OS) was defined as the time from the DEB-TACE operation until the date of death from any causes. The median follow-up duration was 171 days (range, 38–404 days), and the last follow-up date was December 28th, 2016.
Safety assessment
Liver function indexes including ALB, TP, TBIL, TBA, ALT, AST, and ALP were recorded before, 1-week post and 1–3 months post-DEB-TACE treatment to evaluate the influence of DEB-TACE on liver function. Adverse events (AEs) were recorded during DEB-TACE operation and 1 month after DEB-TACE operation.
Statistics
Statistical analyses were performed using SPSS 22.0 (IBM, USA) and Microsoft Office 2010 software (Microsoft, USA). Data were shown as mean ± standard deviation, median (25th–75th) or count (%). The Chi-Square test determined comparison between the two groups, and the McNemar test performed the comparison of liver function indexes between each visit. Kaplan-Meier (K-M) curves and log-rank test were performed to compare OS in the different groups. Factors affecting ORR achievement were determined by univariate logistic regression analysis, while all factors with a P value less than 0.1 were further detected by multivariate logistic regression analysis. Univariate Cox analysis determined factors affecting OS. A P value <0.05 was considered significant.
Results
Baseline characteristics
As listed in Table 1, the mean age of those 55 patients with secondary liver cancer undergoing DEB-TACE treatment was 64.33±10.96 years. Female and male patient sample sizes were 25 and 30 respectively.
Table 1
| Parameters | Patients (n=55) |
|---|---|
| Age (years) | 64.33±10.96 |
| Gender (female/male) | 25/30 |
| Tumor distribution, n (%) | |
| Multifocal | 39 (70.9) |
| Unifocal | 16 (29.1) |
| Tumor location, n (%) | |
| Left liver | 6 (10.9) |
| Right liver | 20 (36.4) |
| Bilobar | 29 (52.7) |
| Largest nodule size (cm) | 5.0 (2.6–6.7) |
| Portal vein invasion, n (%) | 6 (10.9) |
| Hepatic vein invasion, n (%) | 6 (10.9) |
| ECOG performance status, n (%) | |
| 0 | 12 (21.8) |
| 1 | 29 (52.7) |
| 2 | 11 (20.0) |
| 3 | 3 (5.5) |
| Primary cancer, n (%) | |
| Intestinal cancer | 34 (61.8) |
| Gastric cancer | 5 (9.1) |
| Pancreatic cancer | 7 (12.7) |
| Others (lung cancer, breast cancer and so on), n (%) | 9 (16.4) |
| Cycles of DEB-TACE treatment, n (%) | |
| 1 cycle | 48 (87.3) |
| 2 or more cycles | 7 (12.7) |
| Blood routine | |
| WBC (×109 cell/L) | 5.6 (4.3–7.6) |
| RBC (×1012 cell/L) | 3.9 (3.6–4.3) |
| ANC% | 62.7 (53.3–70.6) |
| Hb (g/L) | 116 (100–128) |
| PLT (×109 cell/L) | 159 (120.5–226.5) |
| Liver function | |
| ALB (g/L) | 38.9 (36.7–42.7) |
| TP (g/L) | 68.9 (64.3–73.6) |
| TBIL (μmol/L) | 11.8 (8.0–15.9) |
| TBA (I/L) | 6.0 (3.8–10.6) |
| ALT (U/L) | 18.0 (11.5–28.0) |
| AST (U/L) | 27.0 (21.0–37.5) |
| ALP (U/L) | 112 (83–155) |
| Kidney function | |
| BCr (μmol/L) | 60 (49.4–73.1) |
| BUN (mmol/L) | 4.5 (3.6–5.4) |
| Tumor markers | |
| AFP (μg/L) | 2.6 (2.2–3.9) |
| CEA (μg/L) | 12.1 (2.9–89.7) |
| CA199 (kU/L) | 20.5 (7.3–77.8) |
| Previous treatments, n (%) | |
| cTACE | 11 (20.0) |
| Surgery | 32 (58.2) |
| Systematic chemotherapy | 33 (60.0) |
| Radiofrequency ablation | 12 (21.8) |
| Targeted therapy | 4 (7.3) |
| Chemoembolization reagents (62 DEB-TACE records), n (%) | |
| Anthracyclines | 10 (16.1) |
| Irinotecan | 52 (83.9) |
| Combination of ordinary embolization agent, n (%) | 8 (14.5) |
Data was presented as mean ± standard deviation, median (25th–75th) or count (%). DEB-TACE, drug-eluting bead transarterial chemoembolization; ECOG, Eastern Cooperative Oncology Group; WBC, white blood cell; RBC, red blood cell; ANC, absolute neutrophil count; Hb, hemoglobin; PLT, platelet; ALB, albumin; TP, total protein; TBIL, total bilirubin; TBA, total bile acid; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; BCr, blood creatinine; BUN, blood urea nitrogen; AFP, alpha fetoprotein; CEA, carcino-embryonic antigen; CA199, carbohydrate antigen199; cTACE, conventional transarterial chemo-embolization.
There were 39 (70.9%) patients with multifocal tumor distribution and 16 (29.1%) patients with unifocal tumor distribution. The percentage of patients with portal vein invasion and patients with hepatic vein invasion was the same at 10.9% (N=6). As for primary cancer, the numbers of patients with intestinal cancer, gastric cancer, pancreatic cancer, and other cancers were 34 (61.8%), 5 (9.1%), 7 (12.7%), and 9 (16.4%) respectively. Forty-eight (87.3%) patients achieved 1 cycle of DEB-TACE treatment, while 7 (12.7%) patients achieved 2 or more cycles. The median levels of ALB, TP, TBIL, TBA, ALT, AST, and ALP were 38.9 (interquartile range, 36.7–42.7) g/L, 68.9 (interquartile range, 64.3–73.6) g/L, 11.8 (interquartile range, 8.0–15.9) µmol/L, 6.0 (interquartile range, 3.8–10.6) I/L, 18.0 (interquartile range, 11.5–28.0) U/L, 27.0 (interquartile range, 21.0–37.5) U/L, and 112 (interquartile range, 83–155) U/L, respectively. As for tumor markers, the median levels of AFP, CEA, and CA199 were 2.6 (interquartile range, 2.2–3.9) µg/L, 12.1 (interquartile range, 2.9–89.7) µg/L, and 20.5 (interquartile range, 7.3–77.8) kU/L. Moreover, 11 (20.0%), 32 (58.2%), 33 (60.0%), 12 (21.8%), and 4 (7.3%) patients were previously treated with cTACE, surgery, systematic chemotherapy, radiofrequency ablation, and targeted therapy respectively. There were 62 DEB-TACE records, including 10 (16.1%) records used with anthracyclines and 52 (83.9%) records used with Irinotecan. Other baseline characteristics are shown in Table 1.
Treatment response of DEB-TACE treatment
After DEB-TACE operation, the ORR of these 55 patients was 67.2%, containing 12.7% of patients who achieved CR and 54.5% of patients who achieved PR. The SD rate was 23.6%, and the PD rate was 9.1% (Figure 1A). Among the patients who reached PR, the percentages of patients with necrosis rate of ≥80%, 50% to 80% and <50% were 23.3%, 70.0% and 6.7% respectively (Figure 1B).
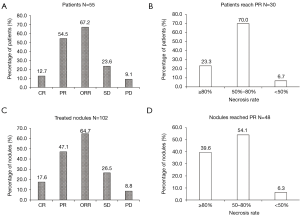
As for treated nodules (N=102), the ORR was 64.7%, which consisted of CR (17.6%) and PR (47.1%), and 26.5% patients achieved SD and 8.8% patients achieved PD (Figure 1C). For those nodules reaching PR (N=48), 39.6% of nodules had a necrosis rate ≥80%, 54.1% of nodules had a necrosis rate of 50% to 80%, while 6.3% of nodules had a necrosis rate <50% (Figure 1D).
OS of DEB-TACE treatment in secondary liver cancer patients
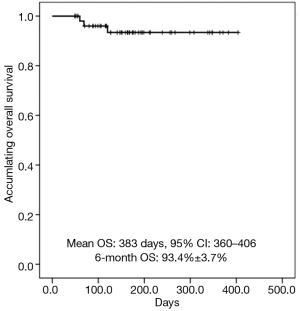
K-M curves showed that mean OS of secondary liver cancer patients treated with DEB-TACE was 383 d (95% CI: 360–406), and the percentage of 6-month OS was 93.4%±3.7% (Figure 2).
Comparison of ORR between/among subgroups
Chi-Square test was used to compare ORR in subgroups divided by demographic and clinical characteristics. Previous cTACE treatment was correlated with worse ORR (P=0.028), while no difference of ORR in subgroups divided by other demographic and clinical characteristics was found (Table 2).
Table 2
| Parameters | n | Not ORR | ORR | P value |
|---|---|---|---|---|
| Age, n (%) | 0.196 | |||
| ≥60 years | 37 | 10 (27.0) | 27 (73.0) | |
| <60 years | 18 | 8 (44.4) | 10 (55.6) | |
| Gender, n (%) | 0.495 | |||
| Male | 30 | 11 (36.7) | 19 (63.3) | |
| Female | 25 | 7 (28.0) | 18 (72.0) | |
| Tumor distribution, n (%) | 0.157 | |||
| Multifocal | 39 | 15 (38.5) | 24 (61.5) | |
| Unifocal | 16 | 3 (18.8) | 13 (81.3) | |
| Tumor location, n (%) | 0.569 | |||
| Left liver | 6 | 1 (16.7) | 5 (83.3) | |
| Right liver | 20 | 6 (30.0) | 14 (70.0) | |
| Bilobar | 29 | 11 (37.9) | 18 (62.1) | |
| Largest nodule size, n (%) | 0.631 | |||
| ≥5 cm | 28 | 10 (35.7) | 18 (64.3) | |
| <5 cm | 27 | 8 (29.6) | 19 (70.4) | |
| Portal vein invasion, n (%) | 1.000 | |||
| Yes | 6 | 2 (33.3) | 4 (66.7) | |
| No | 49 | 16 (32.7) | 33 (67.3) | |
| Hepatic vein invasion, n (%) | 0.381 | |||
| Yes | 6 | 3 (50.0) | 3 (50.0) | |
| No | 49 | 15 (30.6) | 34 (69.4) | |
| ECOG performance status | 0.505 | |||
| 0 | 12 | 4 (33.3) | 8 (66.7) | |
| 1 | 29 | 8 (27.6) | 21 (72.4) | |
| 2 | 11 | 4 (36.4) | 7 (63.6) | |
| 3 | 3 | 2 (66.7) | 1 (33.3) | |
| Primary cancer, n (%) | 0.321 | |||
| Intestinal cancer | 34 | 10 (29.4) | 24 (70.6) | |
| Gastric cancer | 5 | 3 (60.0) | 2 (40.0) | |
| Pancreatic cancer | 7 | 1 (14.3) | 6 (85.7) | |
| Others | 9 | 4 (44.4) | 5 (55.6) | |
| Cycles of DEB-TACE treatment, n (%) | 0.671 | |||
| 1 cycle | 48 | 15 (31.3) | 33 (68.8) | |
| 2 or more cycles | 7 | 3 (42.9) | 4 (57.1) | |
| Previous cTACE treatment, n (%) | 0.028 | |||
| Yes | 11 | 7 (63.6) | 4 (36.4) | |
| No | 44 | 11 (25.0) | 33 (75.0) | |
| Previous surgery, n (%) | 0.561 | |||
| Yes | 32 | 9 (28.1) | 23 (71.9) | |
| No | 23 | 9 (39.1) | 14 (60.9) | |
| Previous systematic chemotherapy, n (%) | 0.481 | |||
| Yes | 33 | 12 (36.4) | 21 (63.6) | |
| No | 22 | 6 (27.3) | 16 (72.7) | |
| Previous radiofrequency ablation, n (%) | 1.000 | |||
| Yes | 12 | 4 (33.3) | 8 (66.7) | |
| No | 43 | 14 (32.6) | 29 (67.4) | |
| Previous targeted therapy, n (%) | 0.291 | |||
| Yes | 4 | 0 (0.0) | 4 (100) | |
| No | 51 | 18 (35.3) | 33 (64.7) | |
| Combination of ordinary embolization agent, n (%) | 1.000 | |||
| Yes | 8 | 2 (25.0) | 6 (75.0) | |
| No | 47 | 16 (34.0) | 31 (66.0) |
Data was presented as count (%). Comparison between two groups was determined by Chi-square test. P<0.05 was considered significant. ORR, objective response rate; ECOG, Eastern Cooperative Oncology Group; DEB-TACE, drug-eluting bead transarterial chemoembolization; cTACE, conventional transarterial chemo-embolization.
As to subgroups divided by biochemical indexes, abnormal AST (P=0.080), ALP (P=0.073), CEA (P=0.076), and CA199 (P=0.059) were correlated with numerically worse ORR but without statistical significance (Table 3). No difference of ORR between/among subgroups divided by other biochemical indexes was observed (Table 3).
Table 3
| Parameters | n | Not ORR | ORR | P value |
|---|---|---|---|---|
| Blood routine | ||||
| WBC | 0.244 | |||
| Abnormal | 9 | 1 (11.1) | 8 (88.9) | |
| Normal | 46 | 17 (37.0) | 29 (63.0) | |
| RBC | 0.495 | |||
| Abnormal | 30 | 11 (36.7) | 19 (63.3) | |
| Normal | 25 | 7 (28.0) | 18 (72.0) | |
| ANC | 0.331 | |||
| Abnormal | 17 | 4 (23.5) | 13 (76.5) | |
| Normal | 38 | 14 (36.8) | 24 (63.2) | |
| Hb | 0.933 | |||
| Abnormal | 31 | 10 (32.3) | 21 (67.7) | |
| Normal | 24 | 8 (33.3) | 16 (66.7) | |
| PLT | 0.701 | |||
| Abnormal | 14 | 4 (28.6) | 10 (71.4) | |
| Normal | 41 | 14 (34.1) | 27 (65.9) | |
| Liver function | ||||
| ALB | 0.925 | |||
| Abnormal | 27 | 9 (33.3) | 18 (66.7) | |
| Normal | 28 | 9 (32.1) | 19 (67.9) | |
| TP | 0.434 | |||
| Abnormal | 16 | 4 (25.0) | 12 (75.0) | |
| Normal | 39 | 14 (35.9) | 25 (64.1) | |
| TBIL | 0.200 | |||
| Abnormal | 7 | 4 (57.1) | 3 (42.9) | |
| Normal | 48 | 14 (29.2) | 34 (70.8) | |
| TBA | 1.000 | |||
| Abnormal | 6 | 2 (33.3) | 4 (66.7) | |
| Normal | 41 | 12 (29.3) | 29 (70.7) | |
| ALT | 1.000 | |||
| Abnormal | 11 | 4 (36.4) | 7 (63.6) | |
| Normal | 44 | 14 (31.8) | 30 (68.2) | |
| AST | 0.080 | |||
| Abnormal | 16 | 8 (50.0) | 8 (50.0) | |
| Normal | 39 | 10 (25.6) | 29 (74.4) | |
| ALP | 0.073 | |||
| Abnormal | 20 | 10 (50.0) | 10 (50.0) | |
| Normal | 34 | 8 (23.5) | 26 (76.5) | |
| Kidney function | ||||
| BCr | 1.000 | |||
| Abnormal | 6 | 2 (33.3) | 4 (66.7) | |
| Normal | 49 | 16 (32.7) | 33 (67.3) | |
| BUN | 1.000 | |||
| Abnormal | 8 | 3 (37.5) | 5 (62.5) | |
| Normal | 47 | 15 (31.9) | 32 (68.1) | |
| Tumor markers | ||||
| AFP | 1.000 | |||
| Abnormal | 6 | 2 (33.3) | 4 (66.7) | |
| Normal | 40 | 11 (27.5) | 29 (72.5) | |
| CEA | 0.076 | |||
| Abnormal | 33 | 13 (39.4) | 20 (60.6) | |
| Normal | 19 | 3 (15.8) | 16 (84.2) | |
| CA199 | 0.059 | |||
| Abnormal | 20 | 9 (45.0) | 11 (55.0) | |
| Normal | 30 | 6 (20.0) | 24 (80.0) |
Data was presented as count (%). Comparison between two groups was determined by Chi-Square test. P<0.05 was considered significant. ORR, objective response rate; WBC, white blood cell; RBC, red blood cell; ANC, absolute neutrophil count; Hb, hemoglobin; PLT, platelet; ALB, albumin; TP, total protein; TBIL, total bilirubin; TBA, total bile acid; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; BCr, blood creatinine; BUN, blood urea nitrogen; AFP, alpha fetoprotein; CEA, carcino-embryonic antigen; CA199, carbohydrate antigen199.
Factors affecting ORR achievement by logistic regression model analysis
Univariate logistic regression was used to analyze the factors affecting ORR achievement, which indicated that previous cTACE treatment (P=0.021) was negatively correlated with ORR achievement (Table 4). Multivariate logistic regression was further performed to analyze all factors with a P value no more than 0.1. It revealed that previous cTACE treatment (P=0.057) and CEA (P=0.096) seemed to be independent factors for ORR achievement in secondary liver cancer patients but without statistical significance.
Table 4
| Parameters | Univariate logistic regression | Multivariate logistic regression | |||||||
|---|---|---|---|---|---|---|---|---|---|
| P value | OR | 95% CI | P value | OR | 95% CI | ||||
| Lower | Higher | Lower | Higher | ||||||
| Age ≥60 years | 0.201 | 2.160 | 0.664 | 7.025 | – | – | – | – | |
| Male | 0.496 | 0.672 | 0.214 | 2.113 | – | – | – | – | |
| Multifocal disease | 0.166 | 0.369 | 0.090 | 1.515 | – | – | – | – | |
| Tumor location-left liver | 0.390 | 2.656 | 0.287 | 24.608 | – | – | – | – | |
| Tumor location-right liver | 0.745 | 1.217 | 0.373 | 3.978 | – | – | – | – | |
| Tumor location-Bilobar | 0.387 | 0.603 | 0.192 | 1.897 | – | – | – | – | |
| Largest nodule size ≥5 cm | 0.631 | 0.758 | 0.244 | 2.349 | – | – | – | – | |
| Portal vein invasion | 0.973 | 0.970 | 0.160 | 5.862 | – | – | – | – | |
| Hepatic vein invasion | 0.349 | 0.441 | 0.080 | 2.443 | – | – | – | – | |
| Higher ECOG performance status | 0.395 | 0.735 | 0.361 | 1.495 | – | – | – | – | |
| Primary intestinal cancer | 0.506 | 1.477 | 0.468 | 4.659 | – | – | – | – | |
| Primary gastric cancer | 0.194 | 0.286 | 0.043 | 1.889 | – | – | – | – | |
| Primary pancreatic cancer | 0.288 | 3.290 | 0.365 | 29.638 | – | – | – | – | |
| 2 or more cycles of DEB-TACE treatment | 0.544 | 0.606 | 0.120 | 3.052 | – | – | – | – | |
| Previous cTACE treatment | 0.021 | 0.190 | 0.047 | 0.776 | 0.057 | 0.153 | 0.022 | 1.056 | |
| Previous Surgery | 0.393 | 1.643 | 0.526 | 5.127 | – | – | – | – | |
| Previous systematic chemotherapy | 0.483 | 0.656 | 0.202 | 2.128 | – | – | – | – | |
| Previous radiofrequency ablation | 0.960 | 0.966 | 0.248 | 3.759 | – | – | – | – | |
| Previous targeted therapy | – | – | – | – | – | – | – | – | |
| Combination of ordinary embolization agent | 0.616 | 1.548 | 0.280 | 8.563 | – | – | – | – | |
| WBC abnormal | 0.161 | 4.690 | 0.539 | 40.801 | – | – | – | – | |
| RBC abnormal | 0.496 | 0.672 | 0.214 | 2.113 | – | – | – | – | |
| ANC abnormal | 0.335 | 1.896 | 0.517 | 6.957 | – | – | – | – | |
| Hb abnormal | 0.933 | 1.050 | 0.338 | 3.265 | – | – | – | – | |
| PLT abnormal | 0.702 | 1.296 | 0.344 | 4.887 | – | – | – | – | |
| ALB abnormal | 0.925 | 0.947 | 0.307 | 2.923 | – | – | – | – | |
| TP abnormal | 0.437 | 1.680 | 0.455 | 6.208 | – | – | – | – | |
| TBIL abnormal | 0.155 | 0.309 | 0.061 | 1.562 | – | – | – | – | |
| TBA abnormal | 0.839 | 0.828 | 0.133 | 5.138 | – | – | – | – | |
| ALT abnormal | 0.774 | 0.817 | 0.205 | 3.255 | – | – | – | – | |
| AST abnormal | 0.086 | 0.345 | 0.102 | 1.163 | 0.309 | 0.404 | 0.071 | 2.316 | |
| ALP abnormal | 0.051 | 0.308 | 0.094 | 1.003 | 0.567 | 0.643 | 0.142 | 2.913 | |
| BCr abnormal | 0.973 | 0.970 | 0.160 | 5.862 | – | – | – | – | |
| BUN abnormal | 0.756 | 0.781 | 0.165 | 3.707 | – | – | – | – | |
| AFP abnormal | 0.768 | 0.759 | 0.121 | 4.747 | – | – | – | – | |
| CEA abnormal | 0.086 | 0.288 | 0.070 | 1.190 | 0.096 | 0.176 | 0.023 | 1.358 | |
| CA199 abnormal | 0.064 | 0.306 | 0.087 | 1.072 | 0.119 | 0.301 | 0.067 | 1.362 | |
Data was presented as P value, odds ratio (OR) and 95% confidence interval (CI). Factors affecting objective response rate (ORR) achievement were determined by univariate logistic regression analysis, while all factors with P value no more than 0.1 were further detected by multivariate logistic regression analysis. P value <0.05 was considered significant. ECOG, Eastern Cooperative Oncology Group; DEB-TACE, drug-eluting bead transarterial chemoembolization; cTACE, conventional transarterial chemo-embolization; WBC, white blood cell; RBC, red blood cell; ANC, absolute neutrophil count; Hb, hemoglobin; PLT, platelet; ALB, albumin; TP, total protein; TBIL, total bilirubin; TBA, total bile acid; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; BCr, blood creatinine; BUN, blood urea nitrogen; AFP, alpha fetoprotein; CEA, carcino-embryonic antigen; CA199, carbohydrate antigen199.
Comparison of OS between/among subgroups
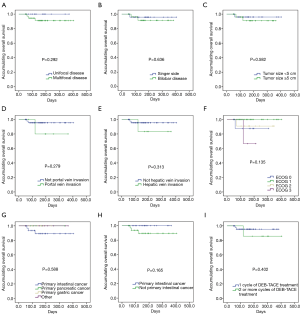
No difference in OS was found between or among the subgroups divided by clinicopathological characteristics, including unifocal disease and multifocal disease (P=0.292); single-side and bilobar disease (P=0.636); tumor size <5 cm and tumor size ≥5 cm (P=0.582); no portal vein invasion and portal vein invasion (P=0.279); no hepatic vein invasion and hepatic vein invasion (P=0.313); ECOG 0 to 3 performance status (P=0.135); primary intestinal cancer, primary pancreatic cancer, primary gastric cancer and other disease (P=0.588); primary intestinal cancer and no primary intestinal cancer (P=0.165); and 1 cycle of DEB-TACE treatment and 2 or more cycles of DEB-TACE treatment (P=0.402) subgroups (Figure 3).
Factors affecting OS
Factors affecting OS were determined by the univariate Cox proportional hazards regression analysis, which suggested that no factor (all P>0.05) could predict OS in patients with secondary liver cancer post-DEB-TACE. Owing to no factor with P<0.1 in univariate Cox proportional hazards regression analysis, multivariate Cox proportional hazards regression analysis was not performed (Table 5).
Table 5
| Parameters | Univariate Cox’s regression | |||
|---|---|---|---|---|
| P value | HR | 95% CI | ||
| Lower | Higher | |||
| Age ≥60 years | 0.912 | 1.146 | 0.103 | 12.691 |
| Male | 0.651 | 1.740 | 0.158 | 19.213 |
| Multifocal disease | 0.526 | 33.047 | 0.001 | 1,642,247 |
| Tumor location-left liver | 0.673 | 0.040 | 0.000 | 120,151 |
| Tumor location-right liver | 0.970 | 0.954 | 0.086 | 10.538 |
| Tumor location-Bilobar | 0.641 | 1.771 | 0.161 | 19.538 |
| Largest nodule size ≥5 cm | 0.589 | 1.938 | 0.176 | 21.375 |
| Portal vein invasion | 0.309 | 3.475 | 0.315 | 38.362 |
| Hepatic vein invasion | 0.341 | 3.225 | 0.290 | 35.813 |
| Higher ECOG performance status | 0.318 | 1.968 | 0.522 | 7.426 |
| Primary intestinal cancer | 0.437 | 45.056 | 0.003 | 671,987 |
| Primary gastric cancer | 0.656 | 0.040 | 0.000 | 60,048 |
| Primary pancreatic cancer | 0.717 | 0.043 | 0.000 | 1,056,651 |
| 2 or more cycles of DEB-TACE treatment | 0.420 | 2.691 | 0.242 | 29.901 |
| Previous cTACE treatment | 0.671 | 1.682 | 0.152 | 18.561 |
| Previous Surgery | 0.414 | 0.366 | 0.033 | 4.074 |
| Previous systematic chemotherapy | 0.749 | 1.481 | 0.133 | 16.480 |
| Previous radiofrequency ablation | 0.558 | 0.033 | 0.000 | 2,992 |
| Previous targeted therapy | 0.738 | 0.044 | 0.000 | 3,864,701 |
| Combination of ordinary embolization agent | 0.500 | 2.291 | 0.206 | 25.467 |
| WBC abnormal | 0.405 | 2.777 | 0.251 | 30.705 |
| RBC abnormal | 0.620 | 1.836 | 0.166 | 20.255 |
| ANC abnormal | 0.349 | 236.360 | 0.003 | 2,174,933 |
| Hb abnormal | 0.651 | 1.740 | 0.158 | 19.213 |
| PLT abnormal | 0.558 | 0.033 | 0.000 | 2,992 |
| ALB abnormal | 0.355 | 84.331 | 0.007 | 1,012,513 |
| TP abnormal | 0.408 | 605.75 | 0.000 | 2,372,162 |
| TBIL abnormal | 0.673 | 0.040 | 0.000 | 120,151 |
| TBA abnormal | 0.700 | 0.042 | 0.000 | 417,666 |
| ALT abnormal | 0.540 | 2.119 | 0.192 | 23.381 |
| AST abnormal | 0.487 | 0.027 | 0.000 | 719.253 |
| ALP abnormal | 0.305 | 3.511 | 0.318 | 38.785 |
| BCr abnormal | 0.673 | 0.040 | 0.000 | 120,151 |
| BUN abnormal | 0.630 | 0.038 | 0.000 | 23,118 |
| AFP abnormal | 0.662 | 0.040 | 0.000 | 74,272 |
| CEA abnormal | 0.846 | 1.269 | 0.115 | 14.014 |
| CA199 abnormal | 0.854 | 0.799 | 0.072 | 8.817 |
Data was presented as P value, HR (hazards ratio) and 95% CI (confidence interval). P Value <0.05 was considered significant. ECOG, Eastern Cooperative Oncology Group; DEB-TACE, drug-eluting bead transarterial chemoembolization; cTACE, conventional transarterial chemo-embolization; WBC, white blood cell; RBC, red blood cell; ANC, absolute neutrophil count; Hb, hemoglobin; PLT, platelet; ALB, albumin; TP, total protein; TBIL, total bilirubin; TBA, total bile acid; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; BCr, blood creatinine; BUN, blood urea nitrogen; AFP, alpha fetoprotein; CEA, carcino-embryonic antigen; CA199, carbohydrate antigen199.
Liver function before and after DEB-TACE treatment
Compared to baseline, the percentages of abnormal TP (P=0.031), TBIL (P=0.022), ALT (P=0.002), and AST (P=0.035) at 1 week post-DEB-TACE were increased, while these four indexes returned to the percentage at baseline (all P>0.05) 1–3 months post-DEB-TACE (Table 6). However, abnormal ALP (P=0.006) at 1 week post-DEB-TACE was similar to baseline, while it was increased at 1–3 months post-DEB-TACE compared to baseline levels. The percentages of ALB and TBA in 1 week and 1–3 months were similar to those of baseline (all P>0.05) in secondary liver cancer patients.
Table 6
| Variables | Baseline | 1 week post DEB-TACE | 1–3 months post DEB-TACE | P value* | P value# |
|---|---|---|---|---|---|
| ALB abnormal, n/N (%) | 31/62 (50.0) | 31/51 (60.8) | 30/57 (52.6) | 0.227 | 0.754 |
| TP abnormal, n/N (%) | 20/62 (32.3) | 27/51 (52.9) | 17/57 (29.8) | 0.031 | 0.774 |
| TBIL abnormal, n/N (%) | 7/62 (11.3) | 15/51 (29.4) | 11/57 (19.3) | 0.022 | 0.424 |
| TBA abnormal, n/N (%) | 7/53 (13.2) | 9/42 (21.4) | 13/48 (27.1) | 0.774 | 0.180 |
| ALT abnormal, n/N (%) | 13/62 (21.0) | 26/51 (51.0) | 21/57 (36.8) | 0.002 | 0.093 |
| AST abnormal, n/N (%) | 17/62 (27.4) | 23/49 (46.9) | 26/56 (46.4) | 0.035 | 0.052 |
| ALP abnormal, n/N (%) | 25/61 (41.0) | 24/48 (50.0) | 33/56 (58.9) | 0.219 | 0.006 |
Data was presented as count. Comparison among groups was determined by McNemar test. P<0.05 was considered significant. Analysis was based on 62 DEB-TACE records. *, P value of liver function related biochemical indexes of patients from baseline to 1 week post treatment. #, P value of liver function related biochemical indexes of patients from baseline to 1–3 months post treatment. DEB-TACE, drug-eluting bead transarterial chemoembolization; ALB, albumin; TP, total protein; TBIL, total bilirubin; TBA, total bile acid; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase.
Safety profiles of DEB-TACE treatment
As for the safety profiles, 41 (66.1%), 28 (45.2%), 17 (27.4%), 8 (12.9%), and 6 (9.7%) cases occurred with pain, vomiting, fever, nausea and other AEs during DEB-TACE respectively, while 26 (41.9%), 9 (14.5%), 8 (12.9%), 4 (6.5%), 1 (1.6%), and 2 (3.2%) cases occurred with pain, fever, vomiting, nausea, bone marrow toxicity and other AEs after 1-month DEB-TACE operaEtion respectively (Table 7).
Table 7
| Parameters | N (%) |
|---|---|
| During DEB-TACE operation | |
| Pain | 41 (66.1) |
| Vomiting | 28 (45.2) |
| Fever | 17 (27.4) |
| Nausea | 8 (12.9) |
| Others | 6 (9.7) |
| 1 month after DEB-TACE operation | |
| Pain | 26 (41.9) |
| Fever | 9 (14.5) |
| Vomiting | 8 (12.9) |
| Nausea | 4 (6.5) |
| bone marrow toxicity | 1 (1.6) |
| Others | 2 (3.2) |
Description was based on 62 DEB-TACE records. DEB-TACE, drug-eluting bead transarterial chemoembolization.
Discussion
In the present study, we observed that in patients with secondary liver cancer after DEB-TACE treatment, (I) the CR and ORR were 12.7% and 67.3% respectively, and the mean OS was 383 d (95% CI: 360–406). (II) Also, previous cTACE treatment was associated with a worse ORR, and univariate and multivariate logistic analyses revealed that previous cTACE treatment and CEA were likely to be independent risk factors for ORR but without statistical significance, while Cox analysis indicated that no predictive factor of OS in patients with secondary liver cancer was found. (III) Additionally, liver function indexes were deteriorative 1 week post-DEB-TACE, while at 1–3 months post-DEB-TACE, most of liver function indexes returned to baseline levels.
Liver metastases frequently occured in a large number of patients with primary cancers; these patients’ metastatic process typically occurs with invasive tumor cells being separated from the primary site into circulation, with these circulating cancer cells subsequently adhering to sinusoidal endothelial cells by specific sets of adhesion molecules, thereby arresting at the site of the liver and finally colonizing in the liver (19). TACE with drug-eluting microspheres, serving as a new therapeutic approach, is characterized by delivering the chemotherapeutics through microspheres to achieve ischemic tumor effect, and a more controlled release of chemotherapeutic and sustained drug concentration (16,17). However, few studies have explored the role of DEB-TACE in patients with secondary liver cancer; thus, our study enrolled 55 patients with secondary liver cancer and evaluated the efficacy of DEB-TACE in treatment response and OS.
Prior studies have found a CR and ORR of 28.6% and 71.4% respectively, in HCC patients 1–3 months after DEB-TACE (20). Also, DEB-TACE achieved a CR of 40% and ORR of 73.3% in HCC patient at 1–3 months (21). Another interesting study reported that at 1-month follow up, CR and ORR were 48% and 64% respectively in HCC patients receiving DEB-TACE (22). However, knowledge about the treatment response of DEB-TACE in patients with secondary liver cancer is lacking. In our study, the CR was 12.7% and the ORR was 67.3% in patients with secondary liver cancer at 1 to 3 months post-DEB-TACE, which were numerically lower compared to HCC patients treated with DEB-TACE in previous studies. This might result from the fact that patients in our study were all secondary liver cancer patients, while all patients enrolled in those previous studies were primary liver cancers, which would lead to the difference in treatment response of DEB-TACE.
Regarding OS, a previous short-term study with a 12-month follow-up duration revealed that DEB-TACE (loaded with irinotecan) led to a mean OS of 11.7 months, which is better than a mean OS of 5.7 months in HCC patients treated with cTACE (23). In another study, survival rates of HCC patients receiving DEB-TACE at 1 and 2 years were 65% and 55% respectively (24). As for patients with colorectal liver metastasis, the percentage of 1-year OS occurring post-DEB-TACE was found to be 75% (25). However, for patients with secondary liver cancer treated with DEB-TACE, we observed a mean OS of 383 days (95% CI: 360–406), and a rate of 6-month OS of 93.4%±3.7%. Therefore, OS observed in this study was numerically similar to that in HCC patients after DEB-TACE, while it was numerically better compared to patients with liver metastasis in previous studies. However, the follow-up duration was short in the present study. Thus, a longer follow-up duration is much needed in further study.
In the present study, we also observed that previous cTACE treatment was a risk factor for ORR achievement. The possible reasons were as follows: (I) retreatment with TACE might accumulate liver deterioration, leading to worse treatment response; (II) previous cTACE treatment might increase drug resistance, thereby resulting in worse treatment response. However, no other predictive factor for ORR achievement was identified in this study apart from previous cTACE treatment in secondary liver cancer patients post-DEB-TACE. Recent data illustrates that tumor size and tumor location serve as independent predictive factors for treatment response to DEB-TACE in HCC patients (26). The possible reasons are that the sample size in our study was relatively small, leading to lower statistical efficiency compared to more extensive sample size studies. Thus, the predictive effects of these baseline factors were not apparent. However, we did not find any potential factor predicting OS in patients with secondary liver cancer post-DEB-TACE. These might partially result from the relatively small sample size and short follow-up duration, leading to a lack of death events.
The effect of DEB-TACE on liver function was also evaluated, which suggested abnormal TP, TBIL, ALT, and AST were increased 1 week post-DEB-TACE compared to baseline, while, at 1–3 months post-DEB-TACE, these four indexes returned to baseline. This rapid worsening of liver function in patients might result from the fact that liver function can be impaired by invasion, and healthy liver tissue can be damaged by vascular occlusion during a DEB-TACE procedure. On the other hand, the liver has the self-recovery capability and regeneration capacity that contributes to the recovery of liver impairment caused during DEB-TACE procedure; thus, liver function indexes would return to baseline after the short-term deterioration. Moreover, we also observed that the percentage of abnormal ALP was similar to baseline, while it notably increased at 1–3 months post-DEB-TACE. ALP is an important enzyme known to catalyze the hydrolytic removal of phosphate from a variety of molecules, and its concentration may be affected by some drugs, such as anthracyclines and irinotecan which were loaded into the DEBs used in this study; these might partially explain the increase of ALP post procedures. However, according to a previous meta-analysis, the results of liver function indexes, including AST, TBIL, ALB, and prothrombin (PT), increased compared to baseline in HCC patients after the procedure of both DEB-TACE and cTACE (27).
It has been demonstrated that, in safety profiles for post-DEB-TACE, the most common AEs were abdominal pain, transient fever, fatigue, and nausea (16,17). In line with these studies, we also observed that during DEB-TACE, pain, vomiting, and fever were the most frequent AEs, while at 1-month DEB-TACE operation pain was the most common AE and only 1 case of bone marrow toxicity occurred. There were no severe AEs during and 1 month after DEB-TACE operation. Therefore, these results suggest that DEB-TACE was well-tolerated in patients with secondary liver cancer.
Despite these new findings, some limitations still exist. First, the sample size in this study was relatively small, and so further study should recruit more patients with secondary liver cancer. Second, this was a cohort study with a single arm; an RCT study enrolling more than 1,000 patients that explores the comparison of effects in treatment responses and OS between DEB-TACE and cTACE is much needed. Third, the follow-up duration was relatively short; analysis of the long-term efficacy of DEB-TACE in patients with secondary liver cancer is necessary.
In conclusion, DEB-TACE was efficient and well tolerated in treating patients with secondary liver cancer.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.06.44). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics committee of the Zhejiang Cancer Hospital. All the patients or their legal guardian provided the written informed consent. This study was conducted according to the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferreira A, Pereira P, Rolanda C. Diffuse hepatic metastasis--or not? Gastroenterology 2012;142:1070, 257.
- Reddy SK, Clary BM. Neuroendocrine liver metastases. Surg Clin North Am 2010;90:853-61. [Crossref] [PubMed]
- De Greef K, Rolfo C, Russo A, et al. Multisciplinary management of patients with liver metastasis from colorectal cancer. World J Gastroenterol 2016;22:7215-25. [Crossref] [PubMed]
- Negoi I, Runcanu A, Paun S, et al. Resection of Large Metachronous Liver Metastasis with Gastric Origin: Case Report and Review of the Literature. Cureus 2016;8:e814. [PubMed]
- Fukumitsu N, Okumura T, Numajiri H, et al. Follow-up study of liver metastasis from breast cancer treated by proton beam therapy. Mol Clin Oncol 2017;7:56-60. [Crossref] [PubMed]
- Sieghart W, Hucke F, Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol 2015;62:1187-95. [Crossref] [PubMed]
- Imai N, Ishigami M, Ishizu Y, et al. Transarterial chemoembolization for hepatocellular carcinoma: A review of techniques. World J Hepatol 2014;6:844-50. [Crossref] [PubMed]
- Korean Liver Cancer Study G. National Cancer Center K. 2014 KLCSG-NCC Korea Practice Guideline for the Management of Hepatocellular Carcinoma. Gut Liver 2015;9:267-317. [PubMed]
- Xie ZB, Wang XB, Peng YC, et al. Systematic review comparing the safety and efficacy of conventional and drug-eluting bead transarterial chemoembolization for inoperable hepatocellular carcinoma. Hepatol Res 2015;45:190-200. [Crossref] [PubMed]
- Kim JH, Sinn DH, Shin SW, et al. The role of scheduled second TACE in early-stage hepatocellular carcinoma with complete response to initial TACE. Clin Mol Hepatol 2017;23:42-50. [Crossref] [PubMed]
- Zhou X, Tang Z, Wang J, et al. Doxorubicin-eluting beads versus conventional transarterialchemoembolization for the treatment of hepatocellular carcinoma: a meta-analysis. Int J Clin Exp Med 2014;7:3892-903. [PubMed]
- Kettenbach J, Stadler A, Katzler IV, et al. Drug-loaded microspheres for the treatment of liver cancer: review of current results. Cardiovasc Intervent Radiol 2008;31:468-76. [Crossref] [PubMed]
- Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010;33:41-52. [Crossref] [PubMed]
- Cucchetti A, Trevisani F, Cappelli A, et al. Cost-effectiveness of doxorubicin-eluting beads versus conventional trans-arterial chemo-embolization for hepatocellular carcinoma. Dig Liver Dis 2016;48:798-805. [Crossref] [PubMed]
- Suk Oh J, Jong Chun H, Gil Choi B, et al. Transarterial chemoembolization with drug-eluting beads in hepatocellular carcinoma: usefulness of contrast saturation features on cone-beam computed tomography imaging for predicting short-term tumor response. J Vasc Interv Radiol 2013;24:483-9. [Crossref] [PubMed]
- Facciorusso A, Mariani L, Sposito C, et al. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Gastroenterol Hepatol 2016;31:645-53. [Crossref] [PubMed]
- Song MJ, Chun HJ, Song DS, et al. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol 2012;57:1244-50. [Crossref] [PubMed]
- Zhang X, Zhou J, Zhu DD, et al. CalliSpheres(R) drug-eluting beads (DEB) transarterial chemoembolization (TACE) is equally efficient and safe in liver cancer patients with different times of previous conventional TACE treatments: a result from CTILC study. Clin Transl Oncol 2019;21:167-77. [Crossref] [PubMed]
- Ma R, Feng Y, Lin S, et al. Mechanisms involved in breast cancer liver metastasis. J Transl Med 2015;13:64. [Crossref] [PubMed]
- Kloth C, Thaiss WM, Kargel R, et al. Evaluation of Texture Analysis Parameter for Response Prediction in Patients with Hepatocellular Carcinoma Undergoing Drug-eluting Bead Transarterial Chemoembolization (DEB-TACE) Using Biphasic Contrast-enhanced CT Image Data: Correlation with Liver Perfusion CT. Acad Radiol 2017;24:1352-63. [Crossref] [PubMed]
- Yu CY, Ou HY, Weng CC, et al. Drug-Eluting Bead Transarterial Chemoembolization as Bridge Therapy for Hepatocellular Carcinoma Before Living-Donor Liver Transplantation. Transplant Proc 2016;48:1045-8. [Crossref] [PubMed]
- Grosso M, Vignali C, Quaretti P, et al. Transarterial chemoembolization for hepatocellular carcinoma with drug-eluting microspheres: preliminary results from an Italian multicentre study. Cardiovasc Intervent Radiol 2008;31:1141-9. [Crossref] [PubMed]
- Kuhlmann JB, Euringer W, Spangenberg HC, et al. Treatment of unresectable cholangiocarcinoma: conventional transarterial chemoembolization compared with drug eluting bead-transarterial chemoembolization and systemic chemotherapy. Eur J Gastroenterol Hepatol 2012;24:437-43. [PubMed]
- Reyes DK, Vossen JA, Kamel IR, et al. Single-center phase II trial of transarterial chemoembolization with drug-eluting beads for patients with unresectable hepatocellular carcinoma: initial experience in the United States. Cancer J 2009;15:526-32. [Crossref] [PubMed]
- Martin RC, Joshi J, Robbins K, et al. Hepatic intra-arterial injection of drug-eluting bead, irinotecan (DEBIRI) in unresectable colorectal liver metastases refractory to systemic chemotherapy: results of multi-institutional study. Ann Surg Oncol 2011;18:192-8. [Crossref] [PubMed]
- Cavalcante RN, Nasser F, Motta-Leal-Filho JM, et al. Occurrence of Vascular Lake Phenomenon as a Predictor of Improved Tumor Response in HCC Patients That Underwent DEB-TACE. Cardiovasc Intervent Radiol 2017;40:1044-51. [Crossref] [PubMed]
- Huang K, Zhou Q, Wang R, et al. Doxorubicin-eluting beads versus conventional transarterial chemoembolization for the treatment of hepatocellular carcinoma. J Gastroenterol Hepatol 2014;29:920-5. [Crossref] [PubMed]


