Angiosarcoma of the breast, the unknown—a review of the current literature
Introduction
Angiosarcoma is one of the rarest cancers and comprises 2% of all sarcomas (1). Angiosarcomas are classified into cutaneous, visceral, and soft tissue subtypes. The word “angiosarcoma” envelopes two greek words meaning vessel (angios) and flesh (sarcoma), describing a sarcoma subtype originating from vessels and spreading to other organs and whose cells express properties similar to endothelial cells in breast, liver, heart, spleen, soft tissues and bone (2-4). Histologically, angiosarcoma spans from well-differentiated tumor to high-grade spindle cell malignancy. However, there is a specific morphologic subtype of angiosarcoma, in which the malignant endothelial cells have a predominantly epithelioid appearance defined as epithelioid angiosarcoma (5). Epithelioid angiosarcoma often presents with early nodal and solid organ metastasis, especially to the lungs, bone, soft tissue, and skin. Compared to classic mesenchymal-cell derived angiosarcoma (with endothelial differentiation), epithelioid angiosarcomas follow either or both (vascular and lymphatic) endothelial cell lines, so are more likely to spread to lymph nodes.
Management of angiosarcoma consist of surgery, seldom followed by chemotherapy. Whether radiotherapy might be delivered remains an issue as the most of angiosarcomas are caused by previous radiation treatments. The prognosis is often poor (6).
Breast angiosarcoma
Breast is one of the organs most commonly affected by angiosarcoma (7). Breast angiosarcoma, was first described by Schmidt in 1887 (8). It is classified into primary, arising de novo, or secondary, developing as consequence of chronic lymphoedema or breast irradiation after breast conserving surgery (9,10). Primary and secondary angiosarcomas are two clinical distinct entities. Primary breast angiosarcomas represent less than 0.04% of total breast malignancies as their incidence is about 0.0005% (11,12). Wang et al. showed a collection of more than 5,000 cases of breast tumours from 1997 to 2007, including only 11 cases of breast angiosarcomas, among which only one was a primary breast angiosarcoma (13).
Primary angiosarcomas of the breast generally develop during the third and the fourth decades of life (median age 35), although cases in postmenopausal age have been reported (13,14). The lesions of primary angiosarcoma usually arise in the parenchyma of non-irradiated breast. Patient with primary angiosarcoma usually presents a rapidly growing painless and palpable mass (≥4 cm), rarely associated with purple blue skin discolouration. They are typical of young age and are often misdiagnosed by mammography because of dense breast parenchyma (14). Different studies on ultrasound imaging showed that angiosarcomas are mainly characterised by both hyperechogenicity and mixed hyper- and hypoechogenicity while others showed the failing of correct angiosarcoma diagnosis by common ultrasounds in relatively high percentage of cases (12). Good results in early diagnosis may be obtained by breast magnetic resonance imaging (MRI) as shows higher accuracy in diagnosis if compared to mammography (15). Fine needle biopsy may give false negative results in a quite high percentage of cases (7,16), so core biopsy should be recommended. Although the aetiology of the primary angiosarcoma of the breast remains unknown, the possible risk factors include trauma, radiation and lymphedema, but there is no definitive data to support this claim (17). The prognosis in primary breast angiosarcoma is poor with a high risk of recurrence and metastasis. In literature there are conflicting opinions regarding the correlation between tumour size and prognosis. Local recurrence is mainly associated with high grade and positive margins. Most of the authors found no correlation between the size of the primary tumour and the risk of death presenting a survival between 25 and 48 months (17,18).
The best treatment of primary breast angiosarcoma is surgery. Simple mastectomy is recommended although breast conserving surgery can be optioned in selected cases. Axillary clearance is not necessary in all the patients because tumour do not usually follow a lymphatic way of dissemination. Only bulky masses invading the axilla necessitate an axillary node dissection (6,19). Adjuvant chemotherapy and/or radiotherapy seem to improve survival (19).
In contrast to primary breast angiosarcoma that generally affects young women, secondary breast angiosarcoma arises in older women (from 46 to 87 years), with a median of 70 years (11). Secondary angiosarcoma arises in the dermal and subcutaneous layers of the skin of radiated fields and may not necessarily involve the parenchyma. It is frequently associated with purple blue skin discolouration and occurs 7–10 years after radiation therapy (20). Secondary breast angiosarcomas can be distinguished in lymphedema-associated cutaneous angiosarcoma, also known as Stewart-Treves syndrome or post-mastectomy angiosarcoma, and post-irradiation angiosarcoma (21).
In 1948 Stewart and Treves firstly described a case of lymphedema-associated angiosarcoma (21), lately called lymphangiosarcoma. Shon et al. described five cases of angiosarcoma arising in morbidly obese patients suffering from massive localized lymphedema, whom clinical and pathological features were the same of the other lymphedema-associated cutaneous angiosarcomas (22). Lymphangiosarcoma also known as Stewart-Treves syndrome has a longer latency period, with an average of about 10 years (23,24). It develops in long-standing chronic lymphedema mainly after radical mastectomy, but also after breast conserving surgery and axillary dissection (22). Generally, the interval between radiation and the diagnosis of angiosarcoma ranges from 3 to 12 years with a median of 7 years after radiotherapy (25,26). The prognosis is poor with a median overall survival (OS) of 37 months. Tumor resectability plays a central role in terms of survival. Overall survival of patients with irresectable localised disease is significantly shorter than those with resectable disease (median OS 18 vs. 37 months, P<0.001, log-rank test) (25) and is mainly associated with tumour size. Rates of local and distant recurrence are high. The multivariate model by Sher et al. has been shown patients who received prior radiotherapy had 2.71 times the risk of death and 1.56 times the risk of disease recurrence compared with patients who had not received prior radiotherapy, although this effect did not attain statistical significance (27).
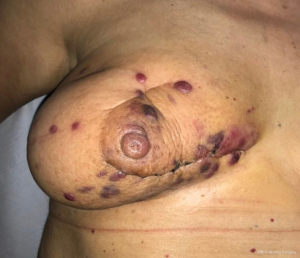
Clinically secondary angiosarcoma, especially radiation-induced angiosarcoma, affects the dermis of the breast and only occasionally develops within the breast parenchyma. This is in contrast with primary angiosarcomas, which arise within the breast parenchyma and only after involve the skin. Secondary angiosarcoma often appears as a rash, ecchymosis, or skin thickening nearby the previous cancer or surgical site. Progressive swelling is common, and in some cases, bluish nodules are visible (Figure 1). The diagnosis may be difficult because of easy misunderstanding with either radiodermatitis or several cutaneous diseases (28,29). Mammography is nonspecific whilst ultrasound scan may not be useful in distinguishing causes of skin changing. MRI has shown curves similar to primary angiosarcoma, but mostly the diagnosis is clinical, whereas all the imaging is negative. The liver and the lungi s the most common site of distant metastases reported by literature (30,31).
Immunohistochemical procedures in diagnosing angiosarcoma
Differential diagnosis of angiosarcoma is often difficult. CD31, CD34, factor VIII-related antigen, Fli-1 and ERG positivity are typical markers of angiosarcomas due to their vascular origin, so they are routinely used by pathologists to establish a diagnosis (32,33). Positivity for CD31 and simultaneous negativity for CD34 are helpful in distinguishing epithelioid variants of angiosarcoma from the most common carcinomas) (32-35). Yet, several ongoing studies are focused on finding more specific molecular markers and genetic alterations.
Molecular pathways involved in angiosarcoma development
BRCA1 and BRCA2
BReast CAncer 1 and 2 (BRCA1 and BRCA2) expression and function are some of the most studied genes because of their involvement in breast cancer onset. BRCA1 and BRCA2 are frequently mutated in breast, ovarian and prostate cancer and the related proteins show an important role in cellular homeostasis (36-39). Point mutations in BRCA2 are supposed to be causes of some secondary breast angiosarcomas (40). The loss of function of BRCA mutated prevents to exert protection against radiation-induced DNA damage hence it is reasonable supposing a role in developing this kind of tumour (37). In 2002 de Bree et al. corroborated with their work the hypothesis that genetic predisposition has a pivotal role in the development of angiosarcoma after breast conserving therapy (41).
More recently, West et al. presented a case report in which a BRCA2 carrier (8540delC) developed a chest wall angiosarcoma after mastectomy (40). In 2013, Kadouri et al. reported the genetic evaluation of three cases of secondary breast angiosarcoma, two BRCA1 and a BRCA2 carrier (185delAG and 6174delT respectively) and one non-carrier (42). They estimated approximately two-fold increased risk of angiosarcoma in BRCA1/2 carriers, but since angiosarcoma is a rare event should not be considered in the decision regarding irradiation treatment in this population. All these data should instead help in monitoring the possible development of angiosarcoma in patients BRCA carriers.
P53 and MDM2
p53 loss of function, mouse double minute 2 (MDM2) and vascular endothelial growing factor (VEGF) overexpression are alterations of molecular pathways which have been found involved in angiosarcoma origin (37,43-46). Studies on p53 expression in angiosarcomas show that often it is possible finding mutations and down-regulation. It has been shown that transgenic p53−/− mice have a high incidence of angiosarcoma (47,48).
In 2006, Domfeh et al. found high percentage (83%) of loss of heterozygosis (49) in locus 17p13 (corresponding to p53) analysing some cases of angiosarcoma. Other papers describe p53 mutations frequently found in angiosarcomas, so it could be conceivable the hypothesis that p53 loss of function exerts an actual role in angiosarcoma development (50-52). To strengthen the hypothesis of inactivation of p53 in angiosarcoma development, successive investigations were performed to evaluate the role exerted by MDM2, a nuclear phosphoprotein that binds and represses p53 transcriptional activity (46).
Zietz et al. in 1998 described a clear functional impairment of the p53/MDM-2 pathway in about 70% of angiosarcomas cases (46). The observed deregulation of p53/MDM-2 expression affects also differentiation and phenotype of endothelial cells up-regulating VEGF expression (50). So, it may be inferred that p53 loss of function should be an important step in angiosarcoma development, since LOH, mutations or overexpression of MDM2 impair the correct cellular homeostasis control performed by p53.
Restoration of wild-type p53 expression was proposed to be an effective therapeutic strategy. Li et al., in 2014, analysed the effects of restoring p53 activity in MDM2-overexpressing angiosarcomas using animal models (53). Data indicate that p53 restoration is able to suppress the growth of MDM2-overexpressing angiosarcomas resulting in tumour stasis and regression in some cases (53). Hence, in the clinical practice, patients with ascertain p53 mutation should be encouraged to annual MRI screening for breast cancer and risk-reducing mastectomy should be discussed (54). If diagnosed with breast cancer patients with p53 mutation should not be treated with mastectomy with or without breast reconstruction. Breast conserving surgery should be avoided considering the risk of radiation-induced angiosarcoma (54).
MYC and FLT4
MYC is a multifunctional, nuclear phosphoprotein that plays a role in cell cycle progression, apoptosis and cellular transformation, as well as stimulates angiogenesis and promotes metastasis (55). It was one of the first protooncogenes to be described and is deregulated in most tumour types (56,57). Its deregulation is generally due not to mutation but rather to gene amplification, translocation, altered ploidy and increased transcription owing to deregulated upstream pathways (57). In a study conducted by Manner et al. in 2010, 22 cases of angiosarcomas, both primary and secondary, were analysed by array comparative genomic hybridization (57). Data showed amplifications on chromosome 8q24.21 and the candidate gene in this chromosomal region was MYC.
Fluorescence in situ hybridization confirmed this assumption. MYC high-level gene amplifications were observed in all secondary angiosarcoma cases but not in primary ones, suggesting that, despite their identical morphology, secondary angiosarcomas are genetically different from primary ones (57). Almost identical results were yield by Mentzel et al. (58). The presence of MYC amplification was seen only in post-irradiation angiosarcoma and not in atypical vascular lesions (AVLs) after radiotherapy. Manner et al. also found co-amplification of chromosome 5q35 (57). Guo et al. in 2011 hypothesized that Fms-related tyrosine kinase (FLT4), encoding a tyrosine kinase receptor for vascular endothelial growth factors involved in lymphangiogenesis, may be a potential candidate for this gene amplification (59). High-level gene amplification pattern was detected in 25% radiation-induced angiosarcoma and in one post-lymphedema angiosarcoma analysed and always co-amplified with MYC.
This result suggests that FLT4 over-expression may represent a second step to progression of secondary angiosarcomas. All these findings suggest that MYC can be considered a hallmark of secondary angiosarcoma and may have implications both for the diagnosis and treatment of these tumours.
PIK3CA/AKT/mTOR
PIK3CA/AKT/mTOR pathway is either directly or indirectly involved in breast angiosarcoma onset. mTOR has been found to play a major role in cancer progression by acting as a master switch for cellular catabolism and anabolism. mTOR enhances cancer cell growth and proliferation and induce cell cycle progression. Thus, mTOR may represent a possible target for angiosarcoma therapy (60). In 2015, Wada et al. investigated the sensitivity of inhibitors for the PI3K/AKT/mTOR pathway in two cutaneous angiosarcoma cell lines (61). Data showed that both PI3K inhibitor and mTOR inhibitor inhibited the growth of both cell lines in a dose-dependent manner.
Treatment of angiosarcoma
Angiosarcoma is a rare pathology and well trialed therapy has been consolidated yet. Breast angiosarcoma is treated by surgical resection. It is generally preferred to perform a total mastectomy sparing axillary surgery (6,19,23,27), unless axillary lymph nodes are enlarged and palpable (62). It has been found that lymph node metastases may occur among high-grade sarcomas in about 3–25% of cases and subtypes keener on regional metastatic diffusion are epithelioid sarcomas (63-66). Sometimes, after surgical resection adjuvant chemotherapy is given, although the rarity of the disease does not allow scheduling chemotherapy according to either biological factors or gene profile as we do with epithelial breast carcinomas (67). Lahat and colleagues show favourable outcomes amongst patients suffering from angiosarcoma with isolated lymphatic spread treated with taxol-based chemotherapeutic regimens (68). More recently, Mocerino et al. report a case of radiation-induced angiosarcoma of the breast in a 77-year-old woman treated with surgery, chemotherapy, radiotherapy and electrochemotherapy, which induced significant objective responses prolonging survival whilst improving patient quality of life (69). Consistent with these studies we have some unpublished experience in treating recurrent breast angiosarcoma with bleomicin-based electrochemotherapy with encouraging results within our institution.
There is little agreement on the choice of chemotherapeutic agents for angiosarcoma after resection. The most common chemotherapeutic drugs are adriamycin, ifosfamide, cyclophosphamide, vincristine, and paclitaxel, typically administered weekly whereas kinase inhibitors are not conventional drugs in angiosarcoma therapy. Three phase II trials investigating weekly paclitaxel, sorafenib and imatinib, demonstrated the efficacy of this treatment for such rare disease (70). Tailored therapy might be the future overcoming resistance to the principal drugs that are used to treat certain malignancies.
Although the pathogenic pathways underlying angiosarcoma are not fully understood, several groups have become interested in exploring the potential of antiangiogenic molecules in the treatment of angiosarcomas. Namely, bevacizumab, a vascular endothelial growing factor (VEGF) monoclonal antibody, which blocks VEGF activities dose-dependently, seems to be an effective and well-tolerated treatment for metastatic or locally advanced angiosarcoma and epithelioid subtype alone or in combination with radio and/or chemotherapy (71). By contrast, the study by Ray-Coquard et al. contradicts the previous preclinical and clinical evidence regarding the use of antiangiogenic therapy in angiosarcoma, although sample size of this study was noteworthy small (50 patients) (72). Ray-Coquard et al. showed evaluated the addition of bevacizumab to weekly paclitaxel was studied in a randomised phase II study (72). The response rate was lower with combination therapy (29% vs. 46%), but the progression free survival rate was identical. Furthermore, the addition of bevacizumab to paclitaxel was associated with higher rates of serious toxicity (44% vs. 22%), and more patients receiving combination treatment required dose reductions (56% vs. 35%). The addition of bevacizumab to weekly paclitaxel is therefore not recommended.
Thus, large resection, when reliable, represents the best choice for localized angiosarcomas. Although clear tumor-free margins are rarely achieved, surgery followed by adjuvant radiotherapy is often mandatory (73,74). There are no convincing data supporting the administration of adjuvant chemotherapy after definitive surgery and radiotherapy. For metastatic or locally advanced disease, doxorubicin-based chemotherapy remains the first-line standard treatment of metastatic or unresectable angiosarcoma showing a progression-free survival of 3.7–5.4 months (75), although weekly paclitaxel has been shown to be well tolerated and active in this setting in some experiences (76).
Conclusions
Angiosarcoma of the breast is a rare and aggressive tumour. It is characterized by rapid growth and high metastatic hematogenous potential. Aetiology and molecular pathways are only partially known and still under investigation. Most of the altered pathways involved in angiosarcoma onset are similar to those involved in other tumours, but effective drugs are few if compared with other neoplasms. In addition to this, the rarity of breast angiosarcoma contributes to a great difficulty in establishing a well trialled therapy. Although some authors report quite good results with different chemotherapy regime, angiosarcoma is preferentially treated with surgery, despite complete surgical resection is often impossible. Electrochemotherapy has been shown to represent a new option, especially in the recurrent setting, but more solid data are warranted.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the focused issue “Rare Tumors of the Breast”. This article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.38). The focused issue “Rare Tumors of the Breast” was commissioned by the editorial office without any funding or sponsorship. EE served as the unpaid Guest Editor for the focused issue and serves as the unpaid editorial board member of Translational Cancer Research from May 2018 to Apr 2020. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mark RJ, Poen JC, Tran LM, et al. Angiosarcoma. A report of 67 patients and a review of the literature. Cancer 1996;77:2400-6. [Crossref] [PubMed]
- Moore MP, Kinne DW. Breast Sarcoma. Surg Clin North Am 1996;76:383-92. [Crossref] [PubMed]
- Simpson L, Kumar SK, Okuno SH, et al. Malignant primary cardiac tumors: review of a single institution experience. Cancer 2008;112:2440-6. [Crossref] [PubMed]
- Choi JJ, Murphey MD. Angiomatous skeletal lesions. Semin Musculoskelet Radiol 2000;4:103-12. [Crossref] [PubMed]
- CDM. F. Diagnostic Histopathology of Tumors. Elsevier Limited 2007;1:66-7.
- Toesca A, Spitaleri G, De Pas T, et al. Sarcoma of the breast: outcome and reconstructive options. Clin Breast Cancer 2012;12:438-44. [Crossref] [PubMed]
- Chen KT, Kirkegaard DD, Bocian JJ. Angiosarcoma of the breast. Cancer 1980;46:368-71. [Crossref] [PubMed]
- Schmidt. Ueber das Angiosarkom der Mamma. Arch Klin Chir 1887;421-7.
- Brenn T, Fletcher CD. Postradiation vascular proliferations: an increasing problem. Histopathology 2006;48:106-14. [Crossref] [PubMed]
- Glazebrook KN, Magut MJ, Reynolds C. Angiosarcoma of the breast. AJR Am J Roentgenol 2008;190:533-8. [Crossref] [PubMed]
- Strobbe LJ, Peterse HL, van Tinteren H, et al. Angiosarcoma of the breast after conservation therapy for invasive cancer, the incidence and outcome. An unforseen sequela. Breast Cancer Res Treat 1998;47:101-9. [Crossref] [PubMed]
- Liberman L, Dershaw DD, Kaufman RJ, et al. Angiosarcoma of the breast. Radiology 1992;183:649-54. [Crossref] [PubMed]
- Wang XY, Jakowski J, Tawfik OW, et al. Angiosarcoma of the breast: a clinicopathologic analysis of cases from the last 10 years. Ann Diagn Pathol 2009;13:147-50. [Crossref] [PubMed]
- Rohan VS, Hanji AM, Patel JJ, et al. Primary angiosarcoma of the breast in a postmenopausal patient. J Cancer Res Ther 2010;6:120-2. [Crossref] [PubMed]
- Yang WT, Hennessy BT, Dryden MJ, et al. Mammary angiosarcomas: imaging findings in 24 patients. Radiology 2007;242:725-34. [Crossref] [PubMed]
- Mantilla JG, Koenigsberg T, Reig B, et al. Core Biopsy of Vascular Neoplasms of the Breast: Pathologic Features, Imaging, and Clinical Findings. Am J Surg Pathol 2016;40:1424-34. [Crossref] [PubMed]
- Rosen PP, Kimmel M, Ernsberger D. Mammary angiosarcoma. The prognostic significance of tumor differentiation. Cancer 1988;62:2145-51. [Crossref] [PubMed]
- Bousquet G, Confavreux C, Magne N, et al. Outcome and prognostic factors in breast sarcoma: a multicenter study from the rare cancer network. Radiother Oncol 2007;85:355-61. [Crossref] [PubMed]
- Bordoni D, Bolletta E, Falco G, et al. Primary angiosarcoma of the breast. Int J Surg Case Rep 2016;20S:12-5. [Crossref] [PubMed]
- Kelly NP, Siziopikou K. Pathologic quiz case: a 68-year-old woman with bluish discoloration of the skin of the breast. Arch Pathol Lab Med 2002;126:989-90. [PubMed]
- Stewart FW, Treves N. Lymphangiosarcoma in postmastectomy lymphedema; a report of six cases in elephantiasis chirurgica. Cancer 1948;1:64-81. [Crossref] [PubMed]
- Shon W, Ida CM, Boland-Froemming JM, et al. Cutaneous angiosarcoma arising in massive localized lymphedema of the morbidly obese: a report of five cases and review of the literature. J Cutan Pathol 2011;38:560-4. [Crossref] [PubMed]
- Hodgson NC, Bowen-Wells C, Moffat F, et al. Angiosarcomas of the breast: a review of 70 cases. Am J Clin Oncol 2007;30:570-3. [Crossref] [PubMed]
- Vorburger SA, Xing Y, Hunt KK, et al. Angiosarcoma of the breast. Cancer 2005;104:2682-8. [Crossref] [PubMed]
- Cohen-Hallaleh RB, Smith HG, Smith RC, et al. Radiation induced angiosarcoma of the breast: outcomes from a retrospective case series. Clin Sarcoma Res 2017;7:15. [Crossref] [PubMed]
- Monroe AT, Feigenberg SJ, Mendenhall NP. Angiosarcoma after breast-conserving therapy. Cancer 2003;97:1832-40. [Crossref] [PubMed]
- Sher T, Hennessy BT, Valero V, et al. Primary angiosarcomas of the breast. Cancer 2007;110:173-8. [Crossref] [PubMed]
- Requena L, Santonja C, Stutz N, et al. Pseudolymphomatous cutaneous angiosarcoma: a rare variant of cutaneous angiosarcoma readily mistaken for cutaneous lymphoma. Am J Dermatopathol 2007;29:342-50. [Crossref] [PubMed]
- Muzumder S, Das P, Kumar M, et al. Primary epithelioid angiosarcoma of the breast masquerading as carcinoma. Curr Oncol 2010;17:64-9. [Crossref] [PubMed]
- Kunkiel M, Maczkiewicz M, Jagiello-Gruszfeld A, et al. Primary angiosarcoma of the breast-series of 11 consecutive cases-a single-centre experience. Curr Oncol 2018;25:e50-e3. [Crossref] [PubMed]
- Arora TK, Terracina KP, Soong J, et al. Primary and secondary angiosarcoma of the breast. Gland Surg 2014;3:28-34. [PubMed]
- Miettinen M, Wang Z, Sarlomo-Rikala M, et al. ERG expression in epithelioid sarcoma: a diagnostic pitfall. Am J Surg Pathol 2013;37:1580-5. [Crossref] [PubMed]
- Miettinen M, Lindenmayer AE, Chaubal A. Endothelial cell markers CD31, CD34, and BNH9 antibody to H- and Y-antigens--evaluation of their specificity and sensitivity in the diagnosis of vascular tumors and comparison with von Willebrand factor. Mod Pathol 1994;7:82-90. [PubMed]
- Folpe AL, Chand EM, Goldblum JR, et al. Expression of Fli-1, a nuclear transcription factor, distinguishes vascular neoplasms from potential mimics. Am J Surg Pathol 2001;25:1061-6. [Crossref] [PubMed]
- De Young BR, Frierson HF Jr, Ly MN, et al. CD31 immunoreactivity in carcinomas and mesotheliomas. Am J Clin Pathol 1998;110:374-7. [Crossref] [PubMed]
- Milne RL, Osorio A, Ramon y Cajal T, et al. Parity and the risk of breast and ovarian cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat 2010;119:221-32. [Crossref] [PubMed]
- Easton DF, Bishop DT, Ford D, et al. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1993;52:678-701. [PubMed]
- Lancaster JM, Wooster R, Mangion J, et al. BRCA2 mutations in primary breast and ovarian cancers. Nat Genet 1996;13:238-40. [Crossref] [PubMed]
- Wooster R, Weber BL. Breast and ovarian cancer. N Engl J Med 2003;348:2339-47. [Crossref] [PubMed]
- West JG, Weitzel JN, Tao ML, et al. BRCA mutations and the risk of angiosarcoma after breast cancer treatment. Clin Breast Cancer 2008;8:533-7. [Crossref] [PubMed]
- de Bree E, van Coevorden F, Peterse JL, et al. Bilateral angiosarcoma of the breast after conservative treatment of bilateral invasive carcinoma: genetic predisposition? Eur J Surg Oncol 2002;28:392-5. [Crossref] [PubMed]
- Kadouri L, Sagi M, Goldberg Y, et al. Genetic predisposition to radiation induced sarcoma: possible role for BRCA and p53 mutations. Breast Cancer Res Treat 2013;140:207-11. [Crossref] [PubMed]
- Hirata A, Tsukamoto T, Yamamoto M, et al. Organ-specific susceptibility of p53 knockout mice to N-bis(2-hydroxypropyl)nitrosamine carcinogenesis. Cancer Lett 2006;238:271-83. [Crossref] [PubMed]
- Damo LA, Snyder PW, Franklin DS. Tumorigenesis in p27/p53- and p18/p53-double null mice: functional collaboration between the pRb and p53 pathways. Mol Carcinog 2005;42:109-20. [Crossref] [PubMed]
- Weihrauch M, Markwarth A, Lehnert G, et al. Abnormalities of the ARF-p53 pathway in primary angiosarcomas of the liver. Hum Pathol 2002;33:884-92. [Crossref] [PubMed]
- Zietz C, Rossle M, Haas C, et al. MDM-2 oncoprotein overexpression, p53 gene mutation, and VEGF up-regulation in angiosarcomas. Am J Pathol 1998;153:1425-33. [Crossref] [PubMed]
- Donehower LA, Harvey M, Slagle BL, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 1992;356:215-21. [Crossref] [PubMed]
- Chen CM, Chang JL, Behringer RR. Tumor formation in p53 mutant ovaries transplanted into wild-type female hosts. Oncogene 2004;23:7722-5. [Crossref] [PubMed]
- Domfeh AB, Fichera M, Hunt JL. Allelic loss of 3 different tumor suppressor gene loci in benign and malignant endothelial tumors of the head and neck. Arch Pathol Lab Med 2006;130:1184-7. [PubMed]
- Naka N, Tomita Y, Nakanishi H, et al. Mutations of p53 tumor-suppressor gene in angiosarcoma. Int J Cancer 1997;71:952-5. [Crossref] [PubMed]
- Zu Y, Perle MA, Yan Z, et al. Chromosomal abnormalities and p53 gene mutation in a cardiac angiosarcoma. Appl Immunohistochem Mol Morphol 2001;9:24-8. [Crossref] [PubMed]
- Momand J, Zambetti GP, Olson DC, et al. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 1992;69:1237-45. [Crossref] [PubMed]
- Li Q, Zhang Y, El-Naggar AK, et al. Therapeutic efficacy of p53 restoration in Mdm2-overexpressing tumors. Mol Cancer Res 2014;12:901-11. [Crossref] [PubMed]
- NCCN Flash Update: NCCN Guidelines® for Pancreatic Adenocarcinoma & Genetic/Familial High-Risk Assessment: Breast and Ovarian. 2019.
- Brooks TA, Hurley LH. The role of supercoiling in transcriptional control of MYC and its importance in molecular therapeutics. Nat Rev Cancer 2009;9:849-61. [Crossref] [PubMed]
- Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer 2008;8:976-90. [Crossref] [PubMed]
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol 2010;176:34-9. [Crossref] [PubMed]
- Mentzel T, Schildhaus HU, Palmedo G, et al. Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological, immunohistochemical and molecular analysis of 66 cases. Mod Pathol 2012;25:75-85. [Crossref] [PubMed]
- Guo T, Zhang L, Chang NE, et al. Consistent MYC and FLT4 gene amplification in radiation-induced angiosarcoma but not in other radiation-associated atypical vascular lesions. Genes Chromosomes Cancer 2011;50:25-33. [Crossref] [PubMed]
- Lahat G, Dhuka AR, Hallevi H, et al. Angiosarcoma: clinical and molecular insights. Ann Surg 2010;251:1098-106. [Crossref] [PubMed]
- Wada M, Horinaka M, Yasuda S, et al. PDK1 is a potential therapeutic target against angiosarcoma cells. J Dermatol Sci 2015;78:44-50. [Crossref] [PubMed]
- Losanoff JE, Jaber S, Esuba M, et al. Primary angiosarcoma of the breast: do enlarged axillary nodes matter? Breast J 2006;12:371-4. [Crossref] [PubMed]
- Skinner KA, Eilber FR. Soft tissue sarcoma nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am 1996;5:121-7. [Crossref] [PubMed]
- Potter DA, Glenn J, Kinsella T, et al. Patterns of recurrence in patients with high-grade soft-tissue sarcomas. J Clin Oncol 1985;3:353-66. [Crossref] [PubMed]
- Vezeridis MP, Moore R, Karakousis CP. Metastatic patterns in soft-tissue sarcomas. Arch Surg 1983;118:915-8. [Crossref] [PubMed]
- Naka N, Ohsawa M, Tomita Y, et al. Prognostic factors in angiosarcoma: a multivariate analysis of 55 cases. J Surg Oncol 1996;61:170-6. [Crossref] [PubMed]
- Kiluk JV, Yeh KA. Primary angiosarcoma of the breast. Breast J 2005;11:517-8. [Crossref] [PubMed]
- Lahat G, Dhuka AR, Lahat S, et al. Outcome of locally recurrent and metastatic angiosarcoma. Ann Surg Oncol 2009;16:2502-9. [Crossref] [PubMed]
- Mocerino C, Iannaci G, Sapere P, et al. Multidisciplinary approach to breast angiosarcoma in an elderly patient: Repeated local relapses and significant objective responses. Int J Immunopathol Pharmacol 2016;29:537-42. [Crossref] [PubMed]
- Penel N, Marreaud S, Robin YM, et al. Angiosarcoma: state of the art and perspectives. Crit Rev Oncol Hematol 2011;80:257-63. [Crossref] [PubMed]
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol 2013;24:257-63. [Crossref] [PubMed]
- Ray-Coquard IL, Domont J, Tresch-Bruneel E, et al. Paclitaxel Given Once Per Week With or Without Bevacizumab in Patients With Advanced Angiosarcoma: A Randomized Phase II Trial. J Clin Oncol 2015;33:2797-802. [Crossref] [PubMed]
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol 2010;11:983-91. [Crossref] [PubMed]
- Young RJ, Woll PJ. Anti-angiogenic therapies for the treatment of angiosarcoma: a clinical update. Memo 2017;10:190-3. [Crossref] [PubMed]
- Fury MG, Antonescu CR, Van Zee KJ, et al. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J 2005;11:241-7. [Crossref] [PubMed]
- Apice G, Pizzolorusso A, Di Maio M, et al. Confirmed Activity and Tolerability of Weekly Paclitaxel in the Treatment of Advanced Angiosarcoma. Sarcoma 2016;2016:6862090. [Crossref] [PubMed]