
Role of PI3K/Akt pathway in Benzidine-induced proliferation in SV-40 immortalized human uroepithelial cell
Introduction
Bladder cancer has been recognized as the frequently-seen tumor in urinary track worldwide. Statistic showed that there were 72,570 new cases of bladder cancer in 2013, accounts for 51.68% of the urinary system, increased to 74,000 cases at the ratio of 53.35%, and resulted in 16,000 death in 2015 in the Unite State alone (1,2). Epidemiology of bladder cancer indicated that occupational exposures, cigarette smoking, arsenic, some medications, and genetic variation are the major risk factors associated with the disease, and aromatic amine is the notable risk factor of bladder cancer development. Benzidine (BZ) is a kind of aromatic amine, which is divided as definite human carcinogen according to the International Agency for Research on Cancer (IARC), among them, urinary bladder cancer has been the frequently-occurring cancer type caused by exposure to it (3,4). BZ can be found from rubber industry, chemical dye, fungicides, hair dyes, cigarette smoke, discharge of industrial pollutants, diet and automobile exhaust (5,6). According to prior research, food dyes (including yellow 6, yellow 5, and red 40) have been found to be contaminated with BZ or other carcinogens (7). So BZ continues to be a risk factor of public health.
Previously, the epidemiology of BZ has been extensively investigated, but little research has been conducted to research how BZ leads to bladder tumor. Our previous study has showed that BZ could meditate the epithelial-mesenchymal transition (EMT) among SV-40 human uroepithelial cells (SV-HUC-1) via ERK1/2 signaling (8). However, there were few researches designed to explore the effect and mechanism of BZ-induced cell proliferation. As we all know, cancer should escape the regulation over normal cell cycle for its development; besides, it should obtain the immortalized cell proliferation ability, proliferation capacity without any suitable signals, as well as the ability to neglect the proliferation suppression, while apoptosis induction-related signals. Therefore, we try to characterize the possible carcinogenic mechanism via BZ-induced cell proliferation.
PI3K/Akt pathway is an intracellular signaling pathway, which exerts a vital part in multiple biological processes like invasion, proliferation, and metastasis of cells, as well as progression of the cell cycle (9-11). Being a leading effector in the downstream of G protein-coupled receptors and the receptor tyrosine kinases (RTK), PI3K can transduce signals derived from a variety of cytokines and growth factors as the intracellular messages through producing phospholipids, thereby activating the downstream effectors, such as Akt (12). The activated Akt can result in translation of proteins and progression of cell cycle (13) and could phosphorylate and disable glycogen synthase kinase 3β (GSK3β), which leads to the up-regulation of Myc and cyclin D1 (14). Moreover, activated Akt could inhibit forkhead box O1, thus up-regulating p21 and p27 (15). Overall, the PI3K/Akt pathway mainly functions to promote cell duplication as well as survival, in the meantime of reducing apoptosis and growth suppression (13).
The current research aimed to examine the impact and mechanism by which cell proliferation was meditated by low concentrations of BZ, and the effect of PI3K/Akt on this process. We sought to examine the potential mechanism in cancer genesis caused by BZ.
Methods
Materials
The immortalized SV-HUC-1 was provided by Shanghai Institutes for Biological Sciences, CAS (Shanghai, China). 4, 4ʹ-diaminobiphenyl (BZ, 99.0%, RT), methanol, as well as dimethyl sulfoxide (DMSO), had been provided by Yboiotech (Shanghai, China). In addition, kinase inhibitor (LY294002) was provided by Cell Signaling Technology (Beverly, MA, USA); whereas growth media (namely, the Ham’s F12 medium), fetal bovine serum (FBS), antibiotics, phosphate-buffered saline (PBS), together with trypsin had been provided by HyClone (Logan, UT, USA). Antibodies to total-Akt, phospho-Akt, P21, PCNA and Cyclin D1 had been provided by Cell Signaling Technology. Moreover, the GAPDH antibody had been provided by Biogot Technology (Nanjing, China). In addition, the primers of Akt, Cyclin D1, P21, GAPDH and PCNA had been prepared from Invitrogen (Carlsbad, CA, USA). The sources of other materials were explained within the manuscript accordingly.
Cell culture and treatments
The SV-HUC-1 cell line was grown within the flasks (25 cm2) at 1×105 cells/mL initially, and cultured within the Ham’s F-12 medium supplemented with 100 units/mL penicillin, 10% FBS, as well as 100 units/mL streptomycin in a humid incubator under 37 °C and 5% CO2 conditions for 12 h. Afterwards, cells would be either subjected to BZ exposure at various contents, or LY294002 (10 µmol/L) treatment. The SV-HUC-1 cell line was then subjected to 6 days of stimulation by BZ or inhibitor. Final BZ concentrations would be set at 0, 0.001, 0.005, 0.01, 0.05, as well as 0.1 µmol/L. Each experiment was repeated trice.
MTT assay
The viability of cells was assayed through MTT assay. Briefly, cells would be individually plated into the 96-well plates at 5×104/well during exponential growth phase. After cultured for 12 h, cells would be subjected to culture medium (100 mL) treatment containing 0.1% DMSO as well as different concentrations of BZ (0.0001–200 µmol/L) and got changed every day for 6 days. After 6 days treatment, 10 µL MTT solution (5 mg/mL) would be put into all wells to incubate for another 4 h. Finally, the original medium would be discarded, followed by the addition of 150 mL dimethylsulphoxide into all wells. Afterwards, the absorbance would be measured at 490 nm by the microplate reader.
Analysis of the cell cycle
SV-HUC-1 cell distribution was detected using flow cytometry at various stages during the cell cycle. Generally, cells (2×105) had been inoculated into the 6 cm dishes to culture using the Ham’s F12 medium supplemented with 10% FBS, 100 units/mL streptomycin and 100 units/mL penicillin for 12 h in the humid incubator under 37 °C and 5% CO2 conditions. Then the cells would be treated with BZ at the concentrations of 0.005, 0.01 µM, and equivalent DMSO was used for 6 days of incubation in the control group. Afterwards, cells would be subjected to trypsin digestion, rinsing by cold PBS for twice, and blending with the 70% ethanol overnight under 4 °C; followed by centrifugation and resuspension within PBS (500 µL). Later, 10 µL of 10 mg/mL RNAse was added, and then cells would be allowed for 30 min of standing in dark under 37 °C, followed by 10 µL propidium iodide (1 mg/mL, PI) staining and immediate evaluation through flow cytometry. Cell percentage at every phase in the cell cycle (including G0/G1, G2/M and S) was determined by MULTICYCLE V.3.0, and at least 1× cells were assessed for every sample.
Western blotting
Cells at different BZ contents were homogenized in the lysate buffer (supplemented with 50 mmol/L Tris, 10 µg/mL aprotinin, 5 mmol/L EDTA, 1% NP-40, 1% SDS, 1% Triton-X 100, 10 µg/mL leupeptin, 1 mM PMSF, and 1% sodium deoxycholate, at pH of 7.5), followed by 20 min of centrifugation at 4 °C. Afterwards, protein content would be calculated by the BCA Protein Assay (Pierce, Rockford, IL, USA); 50 mg protein was separated via 7.5–10% SDS-PAGE, followed by transfer onto the PVDF films (Millipore, Billerica, MA, USA). Subsequently, all PVDF films would be mounted by 5% skim milk, incubated using the primary antibody under 4 °C overnight, followed by incubation by the secondary antibody, with GAPDH being utilized to be the loading reference. Afterward, the protein bands would be measured through the Immobilon Western chemiluminescent HRP substrate kit.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total cellular RNA cultured for 6 days at various BZ contents would be separated through RNAiso Plus in accordance with manufacturer protocols (TaKaRa, Japan). Afterwards, the extracted total cellular RNA would be subject to reverse transcription by the Easy RT-PCR kit in accordance with manufacturer instructions. Then, the relative total-Akt, phospho-Akt, Cyclin D1, P21 and PCNA expression was detected through RT-PCR with GAPDH being utilized to be the loading reference. The reaction mixture (20 mL) was comprised of 7.8 mL dH2O, 0.4 mL Rox, 10 mL SYBR premix, as well as 0.4 mL respective forward and reverse primers and 1 mL cDNA sample. The primer sequences of all target genes have been presented in Table 1. Every sample was repeated for trice. Specifically, the RT-PCR conditions were as follows: 15 s at 95 °C during the initial denaturation process; and then 10 s 95 °C, 30 s at 60 °C, and 30 s at 72 °C for 40 cycles during amplification and quantification processes. All primers had been prepared from Invitrogen (Carlsbad, CA, USA). mRNA levels of all genes had been normalized relative to the GAPDH level. The gene expression fold change was computed according to the 2−ΔΔCt method.
Table 1
| Gene name | Primer sequence |
|---|---|
| Akt | Forward: 5'-GTGCTGGAGGACAATGACTACG-3' |
| Reverse: 5'-AGCAGCCCTGAAAGCAAGGA-3' | |
| Cyclin D1 | Forward: 5'-CGTGGCCTCTAAGATGAAGG-3' |
| Reverse: 5'-TGCGGATGATCTGTTTGTTC-3' | |
| P21 | Forward: 5'-GACACCACTGGAGGGTGACT-3' |
| Reverse: 5'-CAGGTCCACATGGTCTTCCT-3' | |
| PCNA | Forward: 5'-CTGAAGCCGAAACCAGCTAGACT-3' |
| Reverse: 5'-TCGTTGATGAGGTCCTTGAGTGC-3' | |
| GAPDH | Forward: 5'-GCTGCCCAACGCACCGAATA-3' |
| Reverse: 5'-GAGTCAACGGATTTGGTCGT-3' |
Statistical analysis
The SPSS 17.0 was adopted for all statistical analyses. Each value was presented in the form of mean ± standard deviation. Statistical differences between various groups would be detected through one-way ANOVA and LSD test in sequence. Differences between any two groups would be detected through unpaired student t-test. The statistical significance level was set at **P<0.01 or *P<0.05.
Results
Selecting the proliferation-promoted BZ to SV-HUC-1
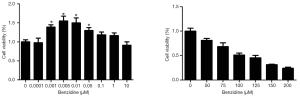
The viability of cells would be detected through MTT assay after cell treatments by different concentrations of BZ. The statistics showed that cell viability was higher at the doses from 0.001 to 0.1 µM compared with the control group and had significant difference, while there was no marked significance between doses above 1 µM and control group (Figure 1). And doses of benzidine higher than 50 µM/L tended to have toxic effect to the cells. Thus, concentrations of BZ for the later experiments were selected at the doses of 0, 0.001, 0.005, 0.01, 0.05, and 0.1 µM.
Cell cycle assessment
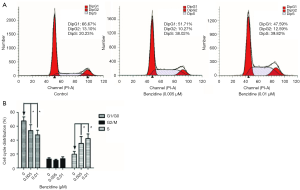
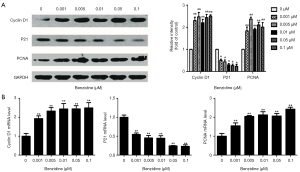
The cell cycle changes between treated groups and the control were evaluated with flow cytometry. The outcome indicated that DipG2 + S increased from 33.33% to 52.41%; DipG1 reduced to 47.59% from 66.67% (Figure 2A,B) and the differences had statistical significance. Expression of specific cell cycle markers had been measured, so as to examine the presence/absence of cell cycle molecular alteration in SV-HUC-1 under BZ treatment. Cells exposed to BZ manifested higher PCNA and Cyclin D1 levels, whereas lower P21 level than those of control (Figure 3A). In addition, the PCNA, P21, and Cyclin D1 mRNA expression was evaluated by RT-PCR, and the results manifested decreasing level of P21 and increasing level of PCNA and Cyclin D1 (Figure 3B).
BZ activated PI3K/Akt signaling pathway
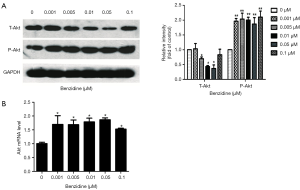
Total-Akt and phospho-Akt were detected to test whether BZ could activate PI3K/Akt signal pathway. Results show that level of phospho-Akt increased, meanwhile total-Akt was reduced (Figure 4A) which indicated that BZ could activate PI3K/Akt pathway. Consistence with the results of Western blots, the mRNA level of Akt was shown in Figure 4B.
PI3K/Akt signal pathway was involved in BZ-meditated cell proliferation
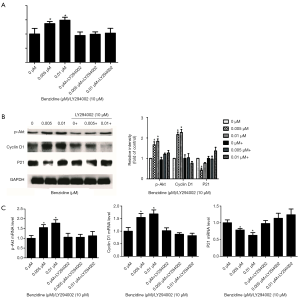
The PI3K/Akt inhibitor (LY294002) was used to culture cells treated with BZ to confirm the relation between PI3K/Akt activation and BZ-triggered cell proliferation. Results revealed that the inhibitor could reverse BZ-meditated cell proliferation as manifested by the results of MTT assay of cell viability (Figure 5A) and Western blot of P21, Cyclin D1 and pAkt (Figure 5B), and RT-PCR for the P21, Akt, and Cyclin D1 mRNA expression (Figure 5C).
Discussion
Although occupational exposure to amylamine like BZ and aminobiphenyl has been manifested as a high risk of bladder cancer, the mechanism of tumor formation to these carcinogens remains to be completely interpreted. According to our findings, low concentrations of BZ could induce cell proliferation in SV-HUC-1 and first demonstrated for the important effect of the PI3K/Akt signal transduction pathway on the BZ-mediated cell proliferation. These findings may provide critical prove for the mechanism by which BZ induced tumor genesis at molecular level, and contribute to mining the possible interventional target for bladder tumor.
More convincing evidences confirmed that the deregulation of normal cell proliferation pathway is central to cancer initiation (16) and escape from regulation of the cell dividing cycle; besides, the uncontrolled proliferation may exert a vital part in initiating tumor genesis (17). Previous study showed that persistent hyperplasia of epithelial cells, together with squamous metaplasia, is recognized to be the preneoplastic lesion in lung cancer genesis (18). Brait et al. also revealed that exposure to tobacco smoke extract induced proliferation in urothelial cell (19).
To elucidate the possible molecular mechanisms of BZ-induced proliferation, the PI3K/Akt pathway regulating the proliferation process has been investigated in our study. In recent years, the association of the PI3K/Akt pathway with tumorigenesis has been a focus of researchers. Abnormal expression of PI3K/Akt pathway proteins and their regulatory molecules could be detected in several of malignant tumors, and overexpression of Akt or inactivation of negative regulation of PTEN is closely related to the formation of malignant tumor (20,21). Xu et al. revealed that the regulation of PI3K/Akt was involved in PIG3-triggered tumorigenesis in papillary thyroid cancer (22). Zhuo et al. found that the activation of this signaling pathway could suppress apoptosis while promoting the development of pediatric osteosarcoma; besides, dual inhibition of PI3K/mTOR could restrain the proliferation and cause cell apoptosis in Burkitt lymphoma cells (23). There was also study revealed that the PI3K/Akt signaling exerted positive effect on cellular motility and, by extension, invasion, and suppressing the PI3K-mediated signal transduction can remarkably affect the proliferation of glioblastoma cells (24). In agreement with previous reports, our study ascertained that the PI3K/Akt was associated with the regulation of cell viability and proliferation in BZ-cultured SV-HUC-1 cells. Therefore, selective inhibitor of PI3K/Akt may be chose to block this signaling pathway to interfere precancerous lesion and target therapy in treatment of bladder cancer.
Our data also showed abnormal expression of cyclin D1, PCNA and P21, whose encode gene were downstream target genes of the PI3K/Akt. The role of this pathway in regulation of encode gene of three periodical proteins mentioned above was confirmed by treating SV-HUC-1 cells with blocker LY294002. Cyclin D1 is a G1 cyclin and its abnormal expression has intimate relationship with the formation and progress as well as the prognosis of tumor. The upregulation of Cyclin D1 could promote cell proliferation, while downregulation of Cyclin D1 inhibits proliferation (25,26). According to former research, PI3K/Akt participates in regulating Cyclin D1 (27). On the other hand, proliferating cell nuclear antigen (PCNA), one of the nuclear nonhistone proteins, is essential to the synthesis of DNA, which can act as an objective indicator of cell proliferative activity (28,29). Former manifested high expression of PCNA is associated with cancer cell proliferation (30). P21, one of the CKIs during the transition from G1 stage to S stage, has been recognized to be the mediator for the downstream p53 (31). Its upregulation can suppress the G1 cyclin-CDK complex kinase activity, thereby avoiding pRB hyperphosphorylation and suppressing the progression of cell cycle and downregulation of it could meditate the promotion of cell proliferation (32). Consistent with previous studies, our experiments found that BZ could activate Akt pathway, promote cell proliferation, and lead to up-regulated cyclin D1 and PCNA, whereas down-regulated P21, as manifested by outcomes of flow cytometry, RT-PCR and Western blotting. In the meantime, we confirmed the role of PI3K/Akt in BZ induced proliferation as showed by the reversed results after the usage of the PI3K/Akt blocker.
Conclusions
We found that low concentrations of BZ promote the proliferation of cells through the PI3K/Akt pathway. Findings in this study indicate that, the PI3K/Akt signal transduction pathway has played a vital part in the BZ-meditated pathologies, which can also shed more light on the underlying mechanisms of BZ- or aromatic amine compound-induced diseases, including bladder diseases and cancer, at molecular level.
Acknowledgments
Funding: This work was financially supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.14). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The current research protocol had been verified by Ethics Committee of Anhui Medical University, Hefei of Anhui Province (Approval No. 20150304) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Narayan VM, Adejoro O, Schwartz I, et al. The Prevalence and Impact of Urinary Marker Testing in Patients with Bladder Cancer. J Urol 2018;199:74-80. [Crossref] [PubMed]
- Choudhary G. Human health perspectives on environmental exposure to hexachlorobutadiene: A review. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 1995;13:179-203. [Crossref]
- Iorio R, Castellucci A, Ventriglia G, et al. Ovarian toxicity: from environmental exposure to chemotherapy. Curr Pharm Des 2014;20:5388-97. [Crossref] [PubMed]
- Tomioka K, Obayashi K, Saeki K, et al. Increased risk of lung cancer associated with occupational exposure to benzidine and/or beta-naphthylamine. Int Arch Occup Environ Health 2015;88:455-65. [Crossref] [PubMed]
- Yu MC, Skipper PL, Tannenbaum SR, Chan KK, Ross RK. Arylamine exposures and bladder cancer risk. Mutat Res 2002;506-507:21-8. [Crossref] [PubMed]
- Kobylewski S, Jacobson MF. Toxicology of food dyes. Int J Occup Environ Health 2012;18:220-46. [Crossref] [PubMed]
- Zhao L, Geng H, Liang ZF, et al. Benzidine induces epithelial-mesenchymal transition in human uroepithelial cells through ERK1/2 pathway. Biochem Biophys Res Commun 2015;459:643-9. [Crossref] [PubMed]
- Rao H, Fuyun WU, Chen D. Effects of Icariin on Signaling Pathways of Phosphatidylinositol 3-kinase (PI3K)/Protein Kinase B (Akt) and E-cadherin in Prostate Cancer Tissues of BALB/c-nu Nude Mice. J Emerg Tradit Chin Med 2018;27:789-92,96.
- Takagi S, Takemoto A, Takami M, et al. Platelets promote osteosarcoma cell growth through activation of the platelet-derived growth factor receptor-Akt signaling axis. Cancer Sci 2014;105:983-8. [Crossref] [PubMed]
- D'Arcy P, Maruwge W, Wolahan B, et al. Oncogenic functions of the cancer-testis antigen SSX on the proliferation, survival, and signaling pathways of cancer cells. PLoS One 2014;9:e95136. [Crossref] [PubMed]
- Chiappini PBO, de Medeiros IUD, Lima LGC, et al. Prognostic implications of phosphatidylinositol 3-kinase/AKT signaling pathway activation in gastric carcinomas. Arch Med Sci 2017;13:1262-8. [Crossref] [PubMed]
- Guo YS, Zhao R, Ma J, et al. βig-h3 promotes human osteosarcoma cells metastasis by interacting with integrin α2β1 and activating PI3K signaling pathway. PLoS One 2014;9:e90220. [Crossref] [PubMed]
- Mabuchi S, Kuroda H, Takahashi R, et al. The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol Oncol 2015;137:173-9. [Crossref] [PubMed]
- Zhang J, Yu XH, Yan YG, Wang C, Wang WJ. PI3K/Akt signaling in osteosarcoma. Clin Chim Acta 2015;444:182-92. [Crossref] [PubMed]
- Zhao L, Zhang T, Geng H, et al. MAPK/AP-1 pathway regulates benzidine-induced cell proliferation through the control of cell cycle in human normal bladder epithelial cells. Oncol Lett 2018;16:4628-34. [PubMed]
- Kiriluk KJ, Prasad SM, Patel AR, Steinberg GD, Smith ND. Bladder cancer risk from occupational and environmental exposures. Urol Oncol 2012;30:199-211. [Crossref] [PubMed]
- Zhong CY, Zhou YM, Douglas GC, et al. MAPK/AP-1 signal pathway in tobacco smoke-induced cell proliferation and squamous metaplasia in the lungs of rats. Carcinogenesis 2005;26:2187-95. [Crossref] [PubMed]
- Brait M, Munari E, LeBron C, et al. Genome-wide methylation profiling and the PI3K-AKT pathway analysis associated with smoking in urothelial cell carcinoma. Cell Cycle 2013;12:1058-70. [Crossref] [PubMed]
- Selvaraj N, Budka JA, Ferris MW, et al. Prostate cancer ETS rearrangements switch a cell migration gene expression program from RAS/ERK to PI3K/AKT regulation. Mol Cancer 2014;13:61. [Crossref] [PubMed]
- Chen Y, Sun Z, Qi M, et al. INPP4B restrains cell proliferation and metastasis via regulation of the PI3K/AKT/SGK pathway. J Cell Mol Med 2018;22:2935-43. [Crossref] [PubMed]
- Xu J, Cai J, Jin X, et al. PIG3 plays an oncogenic role in papillary thyroid cancer by activating the PI3K/AKT/PTEN pathway. Oncol Rep 2015;34:1424-30. [Crossref] [PubMed]
- Zhuo B, Li Y, Li Z, et al. PI3K/Akt signaling mediated Hexokinase-2 expression inhibits cell apoptosis and promotes tumor growth in pediatric osteosarcoma. Biochem Biophys Res Commun 2015;464:401-6. [Crossref] [PubMed]
- Li C, Xin P, Xiao H, et al. The dual PI3K/mTOR inhibitor NVP-BEZ235 inhibits proliferation and induces apoptosis of burkitt lymphoma cells. Cancer Cell Int 2015;15:65. [Crossref] [PubMed]
- Ma J, Cui B, Ding X, et al. Over-Expression of Cyclin D1 Promotes NSCs Proliferation and Induces the Differentiation into Astrocytes Via Jak-STAT3 Pathways. Neurochem Res 2015;40:1681-90. [Crossref] [PubMed]
- Song HM, Park GH, Eo HJ, et al. Anti-Proliferative Effect of Naringenin through p38-Dependent Downregulation of Cyclin D1 in Human Colorectal Cancer Cells. Biomol Ther (Seoul) 2015;23:339-44. [Crossref] [PubMed]
- Tsukahara T, Haniu H, Matsuda Y. Cyclic phosphatidic acid induces G0/G1 arrest, inhibits AKT phosphorylation, and downregulates cyclin D1 expression in colorectal cancer cells. Cell Mol Biol Lett 2015;20:38-47. [Crossref] [PubMed]
- Zhang J, Luo J, Ni J, et al. MMP-7 is upregulated by COX-2 and promotes proliferation and invasion of lung adenocarcinoma cells. Eur J Histochem 2014;58:2262. [Crossref] [PubMed]
- Thiele J, Zirbes TK, Lorenzen J, et al. Apoptosis and proliferation (PCNA labelling) in CML--a comparative immunohistological study on bone marrow biopsies following interferon and busulfan therapy. J Pathol 1997;181:316-22. [Crossref] [PubMed]
- Liao XH, Lu DL, Wang N, et al. Estrogen receptor α mediates proliferation of breast cancer MCF-7 cells via a p21/PCNA/E2F1-dependent pathway. FEBS J 2014;281:927-42. [Crossref] [PubMed]
- Jung H, Shin JH, Park YS, et al. Ankyrin repeat-rich membrane spanning (ARMS)/Kidins220 scaffold protein regulates neuroblastoma cell proliferation through p21. Mol Cells 2014;37:881-7. [Crossref] [PubMed]
- Shi Y, Liu Y, Wang J, et al. Downregulated Long Noncoding RNA BANCR Promotes the Proliferation of Colorectal Cancer Cells via Downregualtion of p21 Expression. PLoS One 2015;10:e0122679. [Crossref] [PubMed]


