
The role of prostaglandin E receptor 2 and epidermal growth factor receptor in esophageal squamous cell carcinoma patients with (pN+) regional lymph node metastasis
Introduction
Esophagus cancer (EC) is one of most common cancers in the world, among which esophageal squamous cell carcinoma (ESCC) is the most common histological type in China (1,2). Although the detailed examinations and treatment strategies have made great progress in recent years, it is still insufficiently studied. Recently, albeit a proportion of patients were taken operative treatment, most patients were marred by distant metastasis, local relapse (3,4). Given the high morbidity and mortality, especially for those patients with regional lymph node metastases, it is important to find the etiopathogenesis of the cancer and develop tools for early detection.
Prostaglandin E receptor 2 (EP2) is one of four kind prostaglandin E receptors (EP), and it plays an essential role in the regulation of inflammatory cytokine and chemokine expression in many different cell types, including tumor cells. In the recent years, researchers had found EP2 were play an important role in various human cancers, such as colon cancer (5), gastric cancer (6), breast cancer and non-small cell lung cancer (NSCLC), etc. The esophagus is often damaged by the excitant food, and manifested by persistent local inflammation response, which may induce the development and metastasis of the ESCC. However, some studies have demonstrated EP2 as a promising marker for EC (7-10), few were reported about the expression of EP2 in (pN+) ESCC and the way it worked. Epidermal growth factor receptor (EGFR), is a cell membrane tyrosine kinase (TK) receptor, is widely expressed on the surface of solid human malignancies (11), and it can be recognized as a target for the treatment of many cancer. Many studies reported overexpression of EGFR in ESCC, which may predict a trend of poor prognosis (12), and may predict a trend of poor prognosis. EP and EGFR signaling pathway was studied in many kinds of tumors, such as colon cancer, NSCLC, and hepatocellular carcinoma (HCC) (13-15). Some studies had also reported the prostaglandin E2 (PGE2) mediated the EGFR by the way of trans-activation, and this mechanism may relevant the way it works in ESCC, through the trans-activation of EGFR by EP2 and the relevant mechanism on works on ESCC cells (16). But there were few studies about the EP2 and EGFR in (pN+) ESCC.
In this study, we try to find the relationship between the expression of EP2 and EGFR in ESCC with local lymph node metastasis by IHC assay. We further analyze the correlation between the two markers expressions and clinical prognosis.
Methods
Patients
Between June 2004 and June 2012, 63 patients, who underwent R0 radical operation for ESCC in The First Affiliated Hospital of University of Science and Technology of China, were included in the study. Approaches for esophagectomy include tri-incisional esophagectomy, abdominothoracic esophagectomy (Ivor-Lewis procedure). The patients with serious postoperative complications were excluded. During the surgery, the number of lymph node dissection should be more than 15. All patients were diagnosed with limited disease without distance metastasis. Preoperative chemotherapy and/or radiotherapy were not permitted. But at least one local lymph node metastasis was presenting in postoperative pathology of all cases. That means the pTNM stage is pT14N13M0. None of these patients received previous anti-inflammatory treatment within one week, nor did they have previous or concomitant other cancer in preoperative assessments. The primary analysis data consisted of age, gender, smoking/drinking history, and tumor size/location/differentiation, postoperative treatment. Postoperative treatments include 2 cycles chemotherapy (platinum-based chemotherapy) at least and/or more than 30 Gy dose of radiotherapy. For the patients who received postoperative treatments, systemic examinations of computed tomography scanning every 2 treatments cycles were used to follow up. And for other patients it performed every 3 months for the first 2 years after surgery and thereafter every 6 months up to death or the end of the study for patients without death. American Joint Committee on Cancer (AJCC) staging system (7th edition, 2010) was used to classify disease progression. disease-free survival (DFS) was measured from the date of operation to the date of first evidence of relapse or death, whichever was observed first. For patients who had not relapsed or died, DFS was censored at the last date that the absence of relapse was confirmed. Overall survival (OS) was measured from the date of surgery to the date of death or last follow-up for surviving patients. And the ultimate time of follow-up was June 2017. The present study was authorized by the Ethics Committee of Anhui Provincial Hospital (The First Affiliated Hospital of University of Science and Technology of China West District) and all patients signed the informed consent.
IHC
Three paraffin-embedded blocks included tumor tissue, para-cancerous tissue, and the normal tissue in anastomosis were collected of each case. Expressions of EP2 and EGFR in the above-mentioned 3 tissues were detected by immunohistochemical method for each of the 63 cases. All the samples were fixed by formalin within 6–8 h after leaving the body. Paraffin embedded tissue sections (thickness, 4 µm) of ESCC were deparaffinized, heated in an oven for 2 h at 60 °C, natural cool completely 30 min. Steeped the slides in milk (whole milk: distilled water =1:6) for 15 min. Then immunohistochemically stained using IHC autostainer (Benchmark XT, Roche Pharmaceutical Ltd., Basel, Switzerland). Specific monoclonal rabbit antibody against EP2 (ab167171, Abcam Inc., USA) and Anti-EGFR rabbit monoclonal primary antibody (5B7, Roche Diagnostics Ltd., Switzerland) were used at a dilution of 1:500 and applied to tissue sections. All the secondary antibodies were purchased from Roche Diagnostics Ltd.
Immunohistochemical evaluation
Positive EP2 and EGFR staining were indicated by the presence of tan-colored particles in the cytoplasm and cell membranes, respectively. The immunohistochemical evaluation was independently performed by two pathologists who were blinded to the clinical data. Decision was made after the discussion of the two pathologists if the result disagreement among the experts. Each pathological slice was observed in 6 visual fields from different areas (×200), take the average value as the result of each glass evaluation of immunohistochemical result by immunoreactive score (IRS). IRS = staining intensity (SI) × percentage of positive cells (PP). SI was divided into four degrees: 0 is negative, 1 is weak, 2 is moderate, and 3 is strong. PP was defined as 0 is negative; 1 is 10% positive cells; 2 is 11–50% positive cells; 3 is 51–80% positive cells; and 4 is more than 80% positive cells. IRS >3 was defined as positive immunoreactive (17,18).
Statistical analysis
Statistical analysis was conducted with SPSS (SPSS 16.0, Inc., Chicago, IL, USA). Spearman test was used to analyze the correlation and difference of EP2 and EGFR in different tissues. The DFS and OS were calculated by the Kaplan-Meier method, and compared by log-rank test. Cox’s proportional hazards regression model were performed to evaluate the prognostic factors for DFS and OS. All statistical tests were two-sided, and P values <0.05 was considered statistically significant in all tests.
Results
In the present study, IHC showed that EP2 was expressed in ESCC cytoplasm with brown staining and the positive rate was 73.0% (Figure 1). The DFS and OS of EP2 positive group were shorter than the control group (Figure 2). The high expression of EP2 was closely associated with the poor differentiation (P=0.036) and younger patients (P=0.029). But sex, smoking, drinking, tumor size, tumor location, tumour stage showed no correlation with the expression of EP2 (P>0.05) (Table 1). EGFR staining was detected in the cell membrane in 54 of 67 cases (Figure 1). We found the smoker had a high rate of EGFR expression. The expression of EGFR was closely related to DFS and OS (P<0.05) (Figure 2). In multivariate analysis, the age (P=0.032), gender (P=0.026), EP2 (P=0.045), EGFR (P=0.001) and postoperative treatment (P=0.005) were the independent factors that could impact on the DFS (P<0.05). For the OS, we found the younger patients (P=0.016), EGFR positive (P=0.001) or without postoperative treatment (P=0.018) had a shorter OS, and the above factors are all the independent factors for OS (Tables 2,3).
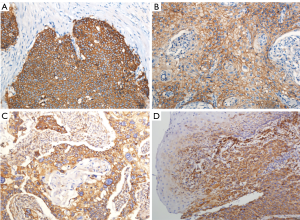
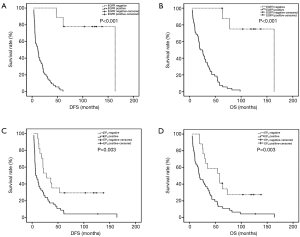
Table 1
| Clinical characteristics | Case | EP2 | EGFR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| + | − | χ2 | P | + | − | χ2 | P | |||
| Age (years) | ||||||||||
| ≤60 | 29 | 25 | 4 | 4.746 | 0.029 | 27 | 2 | 2.396 | 0.122 | |
| >60 | 34 | 21 | 13 | 27 | 7 | |||||
| Gender | ||||||||||
| Male | 52 | 36 | 16 | 2.166 | 0.141 | 44 | 8 | 0.294 | 0.588 | |
| Female | 11 | 10 | 1 | 10 | 1 | |||||
| Stage | ||||||||||
| II | 19 | 13 | 6 | 0.292 | 0.589 | 15 | 4 | 1.017 | 0.313 | |
| III | 44 | 33 | 11 | 39 | 5 | |||||
| Length of tumor (cm) | ||||||||||
| <3 | 9 | 6 | 3 | 0.215 | 0.643 | 9 | 0 | 1.750 | 0.186 | |
| ≥3 | 54 | 40 | 14 | 45 | 9 | |||||
| Location of tumor | ||||||||||
| Middle | 37 | 29 | 8 | 1.309 | 0.253 | 33 | 4 | 0.884 | 0.347 | |
| Low | 26 | 17 | 9 | 21 | 5 | |||||
| Differentiation | ||||||||||
| High or middle | 53 | 36 | 17 | 4.393 | 0.036 | 45 | 8 | 0.178 | 0.673 | |
| Poor | 10 | 10 | 0 | 9 | 1 | |||||
| Smoking | ||||||||||
| No | 35 | 26 | 9 | 0.064 | 0.800 | 27 | 8 | 4.725 | 0.030 | |
| Yes | 28 | 20 | 8 | 27 | 1 | |||||
| Drinking | ||||||||||
| No | 36 | 26 | 10 | 0.027 | 0.870 | 30 | 6 | 0.389 | 0.533 | |
| Yes | 27 | 20 | 10 | 24 | 3 | |||||
EP2, prostaglandin E receptor 2; EGFR, epidermal growth factor receptor; ESCC, esophageal squamous cell carcinoma.
Table 2
| Prognostic factor | DFS | ||
|---|---|---|---|
| Odds ratio | 95% CI | P value | |
| Age | 0.476 | 0.242–0.936 | 0.032 |
| Gender | 2.777 | 1.127–6.842 | 0.026 |
| EGFR | 46.713 | 5.241–416.340 | 0.001 |
| EP2 | 2.332 | 1.019–5.338 | 0.045 |
| Postoperative treatment | 0.359 | 0.175–0.736 | 0.005 |
| Stage | 0.949 | 0.470–1.918 | 0.884 |
| Length of tumor | 1.160 | 0.457–2.943 | 0.755 |
| Differentiation | 0.638 | 0.262–1.555 | 0.323 |
| Smoking | 0.901 | 0.369–2.201 | 0.819 |
| Drinking | 0.779 | 0.326–1.858 | 0.573 |
DFS, disease-free survival; ESCC, esophageal squamous cell carcinoma; EGFR, epidermal growth factor receptor; EP2, prostaglandin E receptor 2.
Table 3
| Prognostic factor | OS | ||
|---|---|---|---|
| Odds ratio | 95% CI | P value | |
| Age | 0.425 | 0.212–0.852 | 0.016 |
| Gender | 2.215 | 0.935–5.248 | 0.071 |
| EGFR | 16.572 | 3.229–85.053 | 0.001 |
| EP2 | 1.974 | 0.898–4.342 | 0.091 |
| Postoperative treatment | 0.452 | 0.234–0.871 | 0.018 |
| Stage | 1.437 | 0.702–2.942 | 0.321 |
| Length of tumor | 1.112 | 0.459–2.697 | 0.814 |
| Differentiation | 0.769 | 0.305–1.939 | 0.578 |
| Smoking | 1.090 | 0.408–2.911 | 0.863 |
| Drinking | 0.617 | 0.237–1.609 | 0.324 |
OS, overall survival; ESCC, esophageal squamous cell carcinoma; EGFR, epidermal growth factor receptor; EP2, prostaglandin E receptor 2.
Discussion
The most type of EC in China is squamous cell carcinoma. The most common and effective way to treat early esophageal cancer patients is operation. In the data reported by many studies, the survival decreased with presence of regional lymph node metastases (19), and the lymph nodes involvement has been shown to be a strong independent predictor of poor survival with surgery alone. The great majority of such patients are relapsed or metastasized in the short time after operation. So, these patients are therefore considered for induction therapy followed by surgery. But there had no precise set of indicators to predict the disease, so it is in bad need of finding indicator which can predict the prognosis.
EP2 was one of the four EP coupling receptors which mediate PGE2 signaling. Many tumors that intensely express COX-2 enzyme have also been found to contain high levels of PGE2 (20-22). Previous studies have well established that human ESCC frequently overexpress COX-2 and produce high levels of PGE2 (23,24). So that, it is indicated that EP2 plays a role in esophageal cancer. EP2 receptor performs its function by combining with the PGE2 that stimulates cell proliferation (25). Until recently, several studies have been focused on the association of EP2 expression with survival of patients with esophageal cancer. Kuo et al. used immuno-histochemical staining and Western blot to find that EP2 overexpression was associated with worse prognosis from 226 patients with ESCC. And they observed that EP2 overexpression was in 43.4% (98/226) of ESCC (7). Xu et al. also found EP2 positive expresses was more observed in ESCC than the control group (52.9% vs. 4.88%), with a significant difference (P<0.001). What’s more, overexpression of EP2 exhibited significant correlation with worse 5-year OS than those with negative result (10.9% vs. 34.1%, P<0.001) (8). Piazuelo et al. found EP2 may play important roles in the development of esophageal adenocarcinoma (EAC) induced by gastroduodenal reflux in the rat (9). In Barrett’s esophagus adenocarcinoma and EAC, they also found the EP2 was overexpressed, and they thought it might be used as a new method to treat Barrett's esophagus in the future (10). In a word, EP2 is an important marker in EC, But the role of EP2 in (pN+) ESCC is not available, and the way it works is still unknown.
In this study, we found EP2 was overexpression in (pN+) ESCC, and it was directly related to prognosis. DFS and OS were shorter in (pN+) ESCC patients with EP2 positive expression in tumor tissues than control group (6.8 vs. 27.7 months, P=0.003; 19.0 vs. 58.0 months, P=0.003). COX multivariate survival analysis also found that EP2 was an independent prognostic risk factor for DFS in (pN+) ESCC patients (P=0.045, 95% CI: 1.019–5.338). However, further analysis showed that the condition of EP2 did not affect OS. This may suggest that EP2 played an important role in recurrence of (pN+) ESCC. But the mechanism of EP2 was used to achieve its role in ESCC is no unified conclusion.
As we know, EP2 is an inflammatory marker. The study found that the activation of EGFR under inflammatory conditions might be positively attributed to the transformation of normal esophageal epithelia to squamous cell cancer (26). EGFR is a cell membrane TK receptor and is recognized as a target for the treatment of many cancers, including esophageal cancer. The role of EGFR in esophageal carcinoma had been studied for a long time. In the previous studies, the detection of the expression rate of EGFR in ESCC tissue was ranging from 40% to 80% (27). At the present time there are many research results concerning EGFR and esophageal cancer. As it was mentioned above in the part of results, most studies showed that over expression of EGFR was an independent adverse prognostic factor in esophageal cancer (8). In our study, EGFR was highly expressed in ESCC tumor tissues (54/63, 85.7%), and we can find that the 42 (66.7%) patients had EP2 and EGFR positive expresses at the same tissue. But the Pearson Chi-square test did not find there was a relationship between the express of EP2 and EGFR in the tissues of tumor (Pearson correlation coefficient =3.102, P=0.078). However, EP2 and EGFR expressions were both found in the same tissue from most patients. there is must be a relationship between the two markers. In our study, we did not find the statistical relationship between EP2 and EGFR, which might be due to the limited number of patients.
Therefore, we consider that there might be a connection between the EP2 and EGFR in ESCC. The exactly signaling pathway between the two markers was unavailable now. Of course, there might be more than one signaling pathway between them in ESCC. In the previous studies, we can find some clues. Donnini et al. found PGE2 as the selective stimulation of the EP2 receptor subtype, leading to EGFR transactivation via protein kinase (PK) A and c-Src activation in ESCC (28). Another path way between EP2 and EGFR was via PKC/extracellular signal regulated kinase pathway-dependent induction of c-Myc expression in human ESCC (29). What’s more, they might also exist EP2/EGFR/PI3 kinase-Akt signaling in cervical cancer (30). In addition to the above, Chun et al. found EP2 played a part in UVB-exposed mouse skin via an EGFR/STAT3 pathway (31). Cheng et al. reported that PGE2 could upregulate the expression level of Snail protein through the EP2/Src/EGFR/Akt/mTOR pathway in Huh-7 cells, which promotes HCC cell invasion and migration (32). EP a2nd EGFR may also work by immune escape. NSCLCs harboring EGFR mutations is associated with low overall response rate to PD-1/PD-L1 inhibitors, this immunosuppression phenomenon maybe caused by EP2-EGFR signal way. As you know the above mechanisms were in different cancers, they might exist in ESCC.
In this study, we found the DFS of (pN+) ESCC patients with EP2 or EGFR expresses was remarkably decreased as compared with the negative group. But for the OS, we did not find the EP2 was an independent of influential factors of prognosis. So, the EP2-EGFR signaling pathway may play a key role in the recurrence of the disease. The high-risk of relapse was found in (pN+) ESCC patients in the clinical practice. One possible reason may be the activating of this signaling pathways. So, we thought the EP2-EGFR may one reason cause the disease recurrence. Tumor recurrence may be delayed by inhibiting the single way. So, this may give us some inspiration for the treatment of postoperative therapy.
Some limitations should be interpreted in our study. First, the sample size is too small to cover these too many parameters. Second, this study did not go further in analysis the relation between EP2 and EGFR, the exact mechanisms involved are still mysterious. So, the further study is needed.
In conclusion, the expression of EP2 and EGFR were important independent prognostic factor for the DFS. So we proposed hypothesis that it exits a signal pathway about EP2-EGFR playing an important role in the occurrence and development of (pN+) ESCC. The limitation of this study is that we did not perform further work to confirm the signaling pathway between EP2 and EGFR in this study. This work was performed in the laboratory, and the full statistical results will be available soon.
Acknowledgments
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.06.51). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The present study was authorized by the Ethics Committee of Anhui Provincial Hospital (2018-16) (The First Affiliated Hospital of University of Science and Technology of China West District) and all patients signed the informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liang H, Fan JH, Qiao YL. Epidemiology, etiology, and prevention of esophageal squamous cell carcinoma in China. Cancer Biol Med 2017;14:33-41. [Crossref] [PubMed]
- Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med 2014;371:2499-509. [Crossref] [PubMed]
- Wang KL, Ling ZQ, Liu X. Clinicopathological Features of Resectable Esophageal Cancer Treated in Zhejiang Cancer Hospital from 2012 to 2016. China Cancer 2017;26:231-5.
- Huang X, Guan S, Wang J, et al. The effects of air pollution on mortality and clinicopathological features of esophageal cancer. Oncotarget 2017;8:58563-76. [PubMed]
- Aoki T, Narumiya S. Prostaglandin E2-EP2 signaling as a node of chronic inflammation in the colon tumor microenvironment. Inflamm Regen 2017;37:4. [Crossref] [PubMed]
- Lian S, Xia Y, Ung TT, et al. Prostaglandin E2 stimulates urokinase-type plasminogen activator receptor via EP2 receptor-dependent signaling pathways in human AGS gastric cancer cells. Mol Carcinog 2017;56:664-80. [Crossref] [PubMed]
- Kuo KT, Wang HW, Chou TY, et al. Prognostic role of PGE2 receptor EP2 in esophageal squamous cell carcinoma. Ann Surg Oncol 2009;16:352-60. [Crossref] [PubMed]
- Xu KK, Tian F, Chang D, et al. Clinical effect of E-series of prostaglandin receptor 2 and epidermal growth factor receptor signal pathways in the development of esophageal squamous cell carcinoma. Dis Esophagus 2014;27:388-95. [Crossref] [PubMed]
- Piazuelo E, Santander S, Cebrián C, et al. Characterization of the prostaglandin E2 pathway in a rat model of esophageal adenocarcinoma. Curr Cancer Drug Targets 2012;12:132-43. [Crossref] [PubMed]
- Jiménez P, Piazuelo E, Cebrian C, et al. Prostaglandin EP2 receptor expression is increased in Barrett's oesophagus and oesophageal adenocarcinoma. Aliment Pharmacol Ther 2010;31:440-51. [Crossref] [PubMed]
- Bukhalid R, Nielsen U, Werner S, et al. Monoclonal and oligoclonal anti-EGFR antibodies for use in the treatment of tumors expressing predominantly high affinity EGFR ligands or tumors expressing predominantly low affinity EGFR ligands. 2017. Google Patents. Available online: https://patents.google.com/patent/US20140170668A1/en
- Yoshioka M, Ohashi S, Ida T, et al. Distinct effects of EGFR inhibitors on epithelial- and mesenchymal-like esophageal squamous cell carcinoma cells. J Exp Clin Cancer Res 2017;36:101. [Crossref] [PubMed]
- Han C, Michalopoulos GK, Wu T. Prostaglandin E2 receptor EP1 transactivates EGFR/MET receptor tyrosine kinases and enhances invasiveness in human hepatocellular carcinoma cells. J Cell Physiol 2006;207:261-70. [Crossref] [PubMed]
- Al-Salihi MA, Ulmer SC, Doan T, et al. Cyclooxygenase-2 transactivates the epidermal growth factor receptor through specific E-prostanoid receptors and tumor necrosis factor-alpha converting enzyme. Cell Signal 2007;19:1956-63. [Crossref] [PubMed]
- Huang RY, Chen GG. Cigarette smoking, cyclooxygenase-2 pathway and cancer. Biochim Biophys Acta 2011;1815:158-69. [PubMed]
- Cui FB, Huang DF, Zhang FL, et al. Investigation on the regulatory effect of PGE2 on ESCC cells through the trans-activation of EGFR by EP2 and the relevant mechanism. Eur Rev Med Pharmacol Sci 2017;21:5668-76. [PubMed]
- Friedrichs K, Gluba S, Eidtmann H, et al. Overexpression of p53 and prognosis in breast cancer. Cancer 1993;72:3641-7. [Crossref] [PubMed]
- Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987;8:138-40. [PubMed]
- Chen YH, Lu HI, Wang YM, et al. The prognostic significance of celiac lymph node metastasis in patients with locally advanced esophageal squamous cell carcinoma receiving curative concurrent chemoradiotherapy. Oncotarget 2017;8:96190-202. [PubMed]
- Tong D, Liu Q, Liu G, et al. Metformin inhibits castration-induced EMT in prostate cancer by repressing COX2/PGE2/STAT3 axis. Cancer Lett 2017;389:23-32. [Crossref] [PubMed]
- Dong XF, Liu TQ, Zhi XT, et al. COX-2/PGE2 Axis Regulates HIF2α Activity to Promote Hepatocellular Carcinoma Hypoxic Response and Reduce the Sensitivity of Sorafenib Treatment. Clin Cancer Res 2018;24:3204-16. [Crossref] [PubMed]
- Ma NX, Sun W, Wu J, et al. Compound Wumei Powder inhibits the invasion and metastasis of gastric cancer via COX-2/PGE2 - PI3K/AKT/GSK-3β/β-catenin signaling pathway. Evid Based Complement Alternat Med 2017;2017:3039450. [Crossref] [PubMed]
- Luz CCF, Noguti J, Araújo L, et al. Expression of VEGF and Cox-2 in Patients with Esophageal Squamous Cell Carcinoma. Asian Pac J Cancer Prev 2018;19:171-7. [PubMed]
- Xiao HC, Zhang CF, Zhao RG, et al. Expression of neutrophil to lymphcyte ratio, cyclooxygenase-2 and nuclear factor kappa B in esophageal squamous carcinoma. Journal of Xinxiang Medical University 2017;34:184-9.
- Yu L, Wu WK, Li ZJ, et al. E series of prostaglandin receptor 2-mediated activation of extracellular signal-regulated kinase/activator protein-1 signaling is required for the mitogenic action of prostaglandin E2 in esophageal squamous-cell carcinoma. J Pharmacol Exp Ther 2008;327:258-67. [Crossref] [PubMed]
- Parthasarathy S, Dhayaparan D, Jayanthi V, et al. Aberrant expression of epidermal growth factor receptor and its interaction with protein kinase C δ in inflammation associated neoplastic transformation of human esophageal epithelium in high risk populations. J Gastroenterol Hepatol 2011;26:382-90. [Crossref] [PubMed]
- Li SY. Research Progress in EGFR Expression and Target Therapy in Esophageal Cancer. Journal of Oncology 2010;16:10-3.
- Donnini S, Finetti F, Solito R, et al. EP2 prostanoid receptor promotes squamous cell carcinoma growth through epidermal growth factor receptor transactivation and iNOS and ERK1/2 pathways. FASEB J 2007;21:2418-30. [Crossref] [PubMed]
- Yu L, Wu WK, Li ZJ, et al. Prostaglandin E promotes cell proliferation via protein kinase C/extracellular signal regulated kinase pathway-dependent induction of c-Myc expression in human esophageal squamous cell carcinoma cells. Int J Cancer 2009;125:2540-6. [Crossref] [PubMed]
- Adefuye AO, Sales KJ, Katz AA. Seminal plasma induces the expression of IL-1α in normal and neoplastic cervical cells via EP2/EGFR/PI3K/AKT pathway. J Mol Signal 2014;9:8. [Crossref] [PubMed]
- Chun KS, Langenbach R. The prostaglandin E2 receptor, EP2, regulates survivin expression via an EGFR/STAT3 pathway in UVB-exposed mouse skin. Mol Carcinog 2011;50:439-48. [Crossref] [PubMed]
- Cheng SY, Zhang H, Zhang M, et al. Prostaglandin E2 receptor EP2 mediates Snail expression in hepatocellular carcinoma cells. Oncol Rep 2014;31:2099-106. [Crossref] [PubMed]


