
Adjuvant whole breast radiotherapy with simultaneous integrated boost to tumor bed with intensity modulated radiotherapy technique in elderly breast cancer patients
Introduction
Breast conserving surgery followed by adjuvant whole breast radiation is the standard of care for early breast cancer now. Adjuvant whole breast radiotherapy with or without tumor bed boost (1) reduces ipsilateral breast tumor recurrences (IBTR) and also contributes to improved overall survival (OS) (2). Currently, over 40% of early-staged breast cancer patients are aged ≥65-year-old in United States (3). With increasing life expectancy, advance of patient’s awareness, and ongoing screening programs, it can be expected that more early-staged breast cancer will be diagnosed in the elderly in the future. Since most of the clinical trials exclude senior patients, the actual benefit of radiotherapy in elderly patient group remains a matter of debate.
Although radiotherapy has no impact on OS in NSABP B-21 (4), PRIME II (5) and CALGB 9343 (6) trials, adding radiation therapy adjuvantly can still decrease the risk of IBTR. For example, in CALGB 9343 trial, 10-year locoregional recurrence is 2% in patients with tamoxifen plus radiation, and 10% in patients with tamoxifen alone (6). Thus, it is reasonable to provide radiotherapy as an option to elderly patients with good performance status and little comorbidity.
Intensity modulated radiotherapy (IMRT) has been utilized in patients of breast cancer in recent years. It is reported to improve the dose homogeneities and can spare more critical organs such as lungs and heart (7-9). It has also been demonstrated that IMRT can reduce the incidence of moist desquamation (31% vs. 48%, P=0.0019) (8), late telangiectasia (P=0.009) (10), and late toxicities (7-9,11) comparing to conventional radiotherapy. Since multiple co-morbidities, poor performance status and treatment related side effects are more common in elderly patients, IMRT can be an attractive option in this patient group because of its lower toxicity profile.
IMRT with simultaneous integrated boost (SIB) technique deliver higher dose to tumor bed and a lower dose level to the remaining breast. Compared to sequential boost, the potential advantages of SIB lie in several aspects. The first one is its shorter overall treatment duration, which can reduce the burden of daily life. The second one is that it can provide more homogeneous dose distribution compared to sequential boost. Besides, it can integrate irradiation of multiple subsites, obviating the needs of field junctions and the application of electron therapy.
The results of IMRT with SIB technique have been reported in several series (12-17). However, the median age in these studies ranged from 52 to 61 years old and the data on Asian population is sparse. As such, we present the long-term follow-up results of adjuvant IMRT with SIB technique in Asian breast cancer patients who are ≥65-year-old after partial mastectomy to evaluate its efficacy and safety in this patient group.
Methods
Eligibility criteria are breast cancer patients who were ≥65-year-old and received partial mastectomy followed by adjuvant whole breast IMRT with SIB technique for tumor bed boost. Patients were enrolled from January 2007 to January 2018. Exclusion criteria include patients with distant metastases at diagnosis; male breast cancer; patients with ductal carcinoma in situ; patients treated with total mastectomy, and patients had previous history of other malignancies or radiation therapy. Institutional Review Boards have approved the study protocols in two institutions and the Research Ethical Committee number is REC108-15 and CE19202A, respectively.
These patients received standard institutional staging protocols at diagnosis, including bilateral mammography and breast sonography, chest X-ray, liver sonography, and whole body Tc-99m bone scans. After completing adjuvant radiotherapy, regular follow-up was scheduled every 3 months and restaging workups were arranged every 6 months. The follow-up protocol is as follows: physical examination at each follow-up visit; breast sonography, liver sonography, and chest X-ray every 6 months; breast mammography every year. Whole body bone scans are arranged as clinically indicated.
Surgery
All of the 93 patients received partial mastectomy as primary surgery. Positive surgical margin was defined as the presence of tumor cells at the inked margin of the surgical specimen. In patients with involved surgical margin, re-excision was suggested and arranged with patient’s consent. Before 2011, axillary lymph node dissection (ALND) was performed in all patients who had positive sentinel lymph node biopsy (SLNB). After the publication of ACSOG-Z0011 trial (18), ALND was omitted in patients with cN0 status but ≤2 positive lymph nodes. Surgical clips were placed routinely at the center and the upper, lower, medial, and lateral border of surgical cavities to facilitate the delineation of primary tumor bed after 2015.
Molecular subtype
Immunohistochemistry stain was performed to assess the expression level of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). In patients with HER2 equivocal stain (score 2+), fluorescence in situ hybridization was performed to confirm the amplification of HER2 receptor. All patients with positive ER and/or PR without HER2 overexpression were classified as luminal subtype. Tumors with HER2 overexpression, no matter the ER/PR status, were classified as HER2 subtype. Patients with negative ER/PR and no HER2 overexpression were classified as triple negative subtype.
Systemic treatments
Systemic treatment strategies evolved over time. Before 2013, all patients were treated with primary surgery followed by adjuvant systemic treatment. No neoadjuvant chemotherapy was applied until 2013. Neoadjuvant hormonal therapy was introduced after 2015.
In patients with tumor ≥1 cm or positive lymph nodes, adjuvant chemotherapy was applied to medically fit patients. Trastuzumab were routinely introduced to patients with HER2 overexpression and involved lymph nodes after 2010. Target therapy was not administered simultaneously with epirubicin to avoid toxicities.
Adjuvant hormonal therapy, either with tamoxifen or aromatase inhibitors, was given to all patients with positive ER and/or PR after adjuvant radiotherapy.
Radiotherapy
In general, adjuvant whole breast radiotherapy with IMRT and SIB to tumor bed was delivered 4–6 weeks after partial mastectomy. In patients who received adjuvant chemotherapy, radiotherapy was given 3–4 weeks after the last dose of chemotherapy. In patients who received adjuvant trastuzumab, radiotherapy was administered simultaneously.
Computed tomography (CT) simulation was performed in supine position with both arms raised above head. All patients were immobilized with vacuum cushion molds. Radiopaque wires were placed on surgical scars, drain sites, and borders of palpable breast tissue. Slice thickness of the CT simulation was 5 mm before 2013 and 2.5 mm since 2014.
Contouring and treatment regimens
Clinical target volume irradiated to 50.4 Gy (CTV50.4) included ipsilateral whole breast in pN0 patients. In patients with positive axillary lymph nodes during SLNB or ALND, ipsilateral infraclavicular fossa (ICF) and supraclavicular fossa (SCF) were also included in CTV50.4. Axillary level I-II lymph nodes were included in CTV50.4 if positive lymph nodes were noticed at SLNB but no further ALND was delivered.
For those patients with involved axillary lymph nodes and tumor were located centrally or medially, internal mammary chain from 1st to 3rd intercostal space (IMC) was encompassed in CTV50.4. In patients who received neoadjuvant systemic treatment, the higher stage between clinical and pathological stage was applied to judge the treatment extent of CTV50.4. CTV50.4 was expanded isotropically by 0.5 cm to form planning target volume (PTV).
Clinical target volume of tumor bed boost (CTV_Boost) consisted of postoperative residual seroma and areas marked by surgical clips. CTV_Boost was expanded uniformly by 0.5 cm to generate the PTV. The contouring of CTV50.4, CTV_Boost, regional lymph nodes and organ at risk (OAR) closely followed the published RTOG guidelines (19).
The dose prescribed to CTV_Boost was 61.6, 62, or 66.4 Gy at the discretion of the radiation oncologist. The prescribed dose to CTV50.4 was 50.4 Gy in 28 fractions. The goals for optimization were as follows: 99% of the CTV (either CTV50.4 or CTV_Boost) should be covered with 100% of prescribed dose and 95% of the PTV should be covered by 100% of the prescribed dose. The volume of CTV50.4 receiving ≥110% of 50.4 Gy should be <40%.
Treatment planning
Treatment plans were designed with Varian Eclipse treatment planning system. All plans were designed with 6 MV photon beams from Varian Clinac series (Trilogy and iX) equipped with a Millennium Multileaf collimator (MLC) with 120 leaves.
Anisotropic analytical algorithm (AAA) was used and the calculation grid size was set to be 2.5 mm. All plans were delivered with dynamic sliding window method with fixed gantry beams. Four to six fields were applied to patients who only received whole breast irradiation. Fields were arranged basically following the tangential angles with field separation of 15–20 degrees. Seven to nine fields were applied to patients who received whole breast and regional lymph node radiotherapy. Among these fields, four to six fields were used to cover the whole breast and the other three fields in the Y-shape arrangement were utilized to irradiate the SCF area. To increase OAR sparing, collimator angles were adjusted to match the chest wall curvature.
The treatment plans were optimized according to Emami’s paper (20) and Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) (21,22) initially. After 2011, our institutional normal tissue constraints were modified according to the goals of RTOG 1005 protocol. The ideal goals for ipsilateral lung were set to V20 ≤15% and V5 ≤65%. The ideal goal for heart was mean dose ≤4 Gy. The mean dose of heart decreased significantly after new constraints were applied in 2011 (Median mean heart dose, after 2011 vs. before 2011, 397.2 vs. 565.7 cGy, P=0.042).
Toxicity evaluation
The treatment records and medical charts were reviewed to retrieve the grading of acute and late toxicities. The acute radiation toxicities were evaluated weekly following the Common Terminology Criteria of Adverse Events version 3.0 (CTCAE v3.0) from the beginning of radiotherapy until 1 month after the completion of radiotherapy. Acute toxicities were reported based on the highest-grade findings.
Statistical analysis
Follow-up period was defined from the date of the completion of radiotherapy to the date of failure, death, or last follow-up. Locoregional failure-free survival (LRFFS) was defined as the duration from the last day of radiotherapy to the date of IBTR or regional lymph node recurrence. OS was calculated to the date of death from any cause. Breast cancer specific survival (BCSS) was calculated to the date of death due to breast cancer itself or any treatment-related complications. Distant metastases-free survival (DMFS) was defined as the period from the last day of radiotherapy to the date of the occurrence of distant metastases. Disease-free survival (DFS) was defined as duration from the end of radiotherapy to any of the following events: death of any cause, locoregional recurrence, distant metastases, treatment-related death, or second primary cancer. Only the first site of recurrence or distant metastases was included in final statistical analysis. The primary endpoint was locoregional control. Secondary endpoints include: OS, BCSS, DMFS, DFS and acute and late toxicities.
The survival function was carried out with Kaplan-Meier estimates and the log-rank test was utilized to compare survival curves between different subgroups. Fisher’s exact test and Mann-Whitney U test were used to compare the differences of categorical variables and continuous variables, respectively. Candidate factors include: Age (≥70 vs. <70), ECOG (0 vs. 1-2), BMI (≥24 vs. < 24 kg/m2), tumor grade (grade 1-2 vs. grade 3), T-stage (T0-1 vs. T2-3), N-stage (N0 vs. N1-2), overall stage (stage I vs. stage II-III), lymphovascular invasion (LVSI, presence vs. absence), perineural invasion (PNI, presence vs. absence), extranodal extension (ENE, presence vs. absence), surgical margin (involved vs. free), chemotherapy exposure (Yes vs. No), and tumor molecular subtype (luminal vs. triple negative and HER2). Factors with P<0.25 in log-rank tests are assessed by Cox’s proportional hazard model to look for predictors of LRFFS, BCSS, DMFS, DFS and OS. A P value of less than 0.05 was considered to be statistically significant. The statistical analyses of the data were performed with MedCalc software, version 19.0.3.
Results
The median follow-up time of the 93 patients is 56.1 months. The median age is 68-year-old (range, 65–80 years) and 77.4% are on Eastern Cooperative Oncology Group (ECOG) performance status scale of 0. Among the 93 patients, there are no synchronous or metachronous breast cancer. There are also no contralateral breast tumor recurrences in these patients. The patient characteristics are summarized in Table 1.
Table 1
| Factors | N (%) or median (Inter-quartile range) |
|---|---|
| Follow-up time (months) | 56.1 (31.9 to 86.5) |
| Age (years) | 68.0 (66.0 to 70.8) |
| Height (cm) | 153.0 (150.0 to 157.0) |
| Weight (kg) | 59.0 (52.1 to 65.0) |
| Body Mass Index (kg/m2) | 24.7 (22.5 to 27.4) |
| Tumor size (cm) | 2.0 (1.5 to 2.5) |
| Gender | |
| Female | 93 (100.0) |
| Histology | |
| Invasive ductal carcinoma | 88 (94.6) |
| Invasive lobular carcinoma | 3 (3.2) |
| Others | 2 (2.2) |
| ECOG Performance Status | |
| 0 | 72 (77.4) |
| 1 | 20 (21.5) |
| 2 | 1 (1.1) |
| Laterality | |
| Left | 51 (54.8) |
| Right | 42 (45.2) |
| Location | |
| Upper outer quadrant | 40 (43.0) |
| Upper inner quadrant | 27 (29.0) |
| Central | 2 (2.2) |
| Lower outer quadrant | 17 (18.3) |
| Lower inner quadrant | 7 (7.5) |
| Molecular Subtype | |
| Luminal | 69 (72.2) |
| Triple positive | 9 (9.7) |
| HER2 overexpression | 6 (6.5) |
| Triple negative | 7 (7.5) |
| Unknown | 2 (2.2) |
| Surgery | |
| PM+SLNB | 62 (66.6) |
| PM+ALND | 31 (33.3) |
| Tumor grade | |
| Grade 1 | 12 (12.9) |
| Grade 2 | 51 (54.8) |
| Grade 3 | 22 (23.7) |
| Unknown | 8 (8.6) |
| T-stage | |
| Patients treated with primary surgery | |
| pT0 | 1 (1.1) |
| pT1a | 0 (0.0) |
| pT1b | 7 (7.5) |
| pT1c | 36 (38.7) |
| pT2 | 35 (37.6) |
| pT3 | 1 (1.1) |
| Patients treated with neoadjuvant therapy | |
| cT1b | 1 (1.1) |
| cT1c | 3 (3.3) |
| cT2 | 9 (9.9) |
| ypT0/Tis | 5 (5.4) |
| ypT1a | 1 (1.1) |
| ypT1b | 0 (0.0) |
| ypT1c | 6 (6.5) |
| ypT2 | 1 (1.1) |
| N-stage | |
| Patients treated with primary surgery | |
| pNx | 1 (1.1) |
| pN0 | 51 (54.8) |
| pN0(i+) | 4 (4.3) |
| pN1mi | 5 (5.4) |
| pN1a | 14 (15.1) |
| pN2a | 4 (4.3) |
| pN3a | 1 (1.1) |
| Patients treated with neoadjuvant therapy | |
| cN0 | 8 (8.6) |
| cN1mi | 2 (2.2) |
| cN1 | 3 (3.2) |
| ypNx | 3 (3.2) |
| ypN0 | 8 (8.6) |
| ypN1a | 0 (0.0) |
| ypN2a | 2 (2.2) |
| Pathological stage | |
| Patients treated with primary surgery | |
| pI | 35 (37.6) |
| pIIA | 28 (30.1) |
| pIIB | 12 (12.9) |
| pIIIA | 4 (4.3) |
| pIIIB | 0 (0.0) |
| pIIIC | 1 (1.1) |
| Patients treated with neoadjuvant therapy | |
| cI | 3 (3.3) |
| cIIA | 6 (6.5) |
| cIIB | 4 (4.3) |
| yp0/yis | 5 (5.4) |
| ypI | 4 (4.3) |
| ypIIA | 2 (2.2) |
| ypIIIA | 2 (2.2) |
| Lymphovascular space invasion | |
| No | 61 (65.6) |
| Yes | 26 (28.0) |
| Not specified | 6 (6.5) |
| Perineural invasion | |
| No | 58 (62.4) |
| Yes | 16 (17.2) |
| Not specified | 19 (20.4) |
| Extranodal extension | |
| No | 77 (82.8) |
| Yes | 6 (6.5) |
| Not specified | 10 (10.8) |
| Margins status | |
| Involved | 6 (6.5) |
| <1 mm | 19 (20.4) |
| ≥1 mm | 51 (54.8) |
| Not specified | 17 (18.3) |
| Systemic treatment | |
| Neoadjuvant chemotherapy | 5 (5.4) |
| Neoadjuvant target therapy | 4 (4.3) |
| Neoadjuvant hormonal therapy | 4 (4.3) |
| Adjuvant chemotherapy | 20 (21.5) |
| Adjuvant target therapy | 8 (8.6) |
| Adjuvant hormonal therapy | 81 (87.1) |
| Hormonal therapy category | |
| Aromatase inhibitor | 43 (46.2) |
| Tamoxifen | 38 (40.9) |
| None | 10 (10.8) |
| Unknown | 2 (2.2) |
ECOG, Eastern Cooperative Oncology Group; PM, partial mastectomy; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection.
Among all patients, infiltrating ductal carcinoma consists of 94.6% cases and 72.2% are classified as luminal subtype. The tumor locates most frequently in upper outer quadrant (43.0%) and 66.6% received partial mastectomy and SLNB as primary surgery. Thirteen patients receive neoadjuvant systemic treatments.
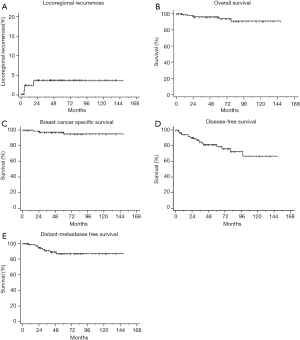
One (1.1%) patient had IBTR, 3 (3.2%) patients had regional lymph node recurrences and 9 (9.7%) patients had distant metastases during follow-up. Death occurred in 5 (5.4%) patients, of whom 3 (3.2%) patients died of breast cancer progression. Among those who had distant metastases, osseous metastases (n=7) were the most frequent metastatic site, followed by pulmonary metastases (n=2). The resultant 5-year LRRFS was 96.4% and 5-year OS was 96.3%. The 5-year BCSS, 5-year DMFS and 5-year DFS were 97.5%, 87.2% and 79.0%, respectively. The survival curves are shown in Figure 1.
Details about radiotherapy techniques, target volumes, prescribed doses and toxicities are provided in Table 2. Dosimetric parameters of the treatment plans are shown in Table 3. The median value of heart mean dose for all patients is 468.9 cGy. The median value of ipsilateral lung V20 and V5 is 17.1% and 57.2%, respectively. Finally, the median treatment duration is 39 days.
Table 2
| Factors | Number | Percentage |
|---|---|---|
| Radiotherapy duration (days) | 39 (median) | 37–41 (IQR) |
| Treatment fractions | 28 | 100.0 |
| Radiotherapy Technique | ||
| IMRT | 89 | 95.7 |
| RapidArc | 1 | 1.1 |
| Hybrid | 3 | 3.2 |
| Regional Target Volumes | ||
| None | 64 | 68.8 |
| SCF + ICF + IMC + ALN | 6 | 6.5 |
| SCF + ICF + IMC | 8 | 8.6 |
| SCF + ICF + ALN | 4 | 4.3 |
| SCF + ICF | 11 | 11.8 |
| Whole breast dose | ||
| 50.4 Gy/28 Fx | 93 | 100.0 |
| SIB dose | ||
| 61.6 Gy/28 Fx | 28 | 30.1 |
| 62.0 Gy/28 Fx | 25 | 26.9 |
| 66.4 Gy/28 Fx | 40 | 43.0 |
| Radiation dermatitis | ||
| Grade 1 | 76 | 81.7 |
| Grade 2 | 17 | 18.3 |
| Grade 3-4 | 0 | 0.0 |
| Radiation stomatitis | ||
| Grade 0 | 85 | 91.4 |
| Grade 1 | 8 | 8.6 |
| Grade 2 | 0 | 0.0 |
| Radiation pneumonitis | ||
| Grade 0 | 89 | 95.7 |
| Grade 1 | 4 | 4.3 |
| Symptomatic lymph edema | ||
| Present | 3 | 3.4 |
IQR, interquartile range; IMRT, intensity-modulated radiation therapy; SCF, supraclavicular fossa; ICF, infraclavicular fossa; IMC, internal mammary chain; ALN, axillary lymph nodes; SIB, simultaneous integrated boost.
Table 3
| Factors | Median | IQR |
|---|---|---|
| CTV50.4 Volume (cm3) | 650.2 | 540.1 to 932.3 |
| CTV_Boost Volume (cm3) | 24.4 | 14.8 to 37.7 |
| Heart mean dose, left side (cGy) | 740.5 | 468.7 to 1,006.0 |
| Heart mean dose, right side (cGy) | 193.7 | 109.5 to 450.0 |
| Ipsilateral lung mean dose (cGy) | 1,136.9 | 1,026.8 to 1,318.2 |
| Ipsilateral lung V20 (%) | 17.1 | 14.7 to 21.6 |
| Ipsilateral lung V5 (%) | 57.2 | 42.3 to 65.5 |
| Contralateral lung mean dose (cGy) | 183.7 | 49.2 to 311.6 |
| PTV_Boost coverage (%) | 100.0 | 98.4 to 100.0 |
| PTV50.4 coverage (%) | 96.7 | 95.0 to 98.0 |
IQR, interquartile range; CTV, clinical target volume; PTV, planning target volume.
Eighteen percent of patients have grade 2 radiation dermatitis during radiotherapy. Patients with CTV50.4 volume ≥700 cc have a greater chance of grade 2 dermatitis (CTV50.4 ≥700 vs. <700 cc, 28.6% vs. 9.8%, P=0.03). Higher SIB dose is not associated with higher risk of grade 2 radiation dermatitis (66.4 vs. 61.6–62 Gy, 15% vs. 20.8%, P=0.59). There are 4.3% of patients developing grade 1 radiation pneumonitis and 8.6% of patients have grade 1 stomatitis. No other acute grade 3 or 4 toxicities are observed. Three (3.2%) patients suffered from symptomatic forearm edema. Seven (7.5%) patients had secondary primary cancers. The type of secondary cancers and time elapsed from the end of radiotherapy are depicted in Table 4.
Table 4
| Type of secondary cancer | Time elapsed from end of radiotherapy (months) |
|---|---|
| Thyroid cancer | 4.1 |
| Colon cancer | 35.0 |
| Pancreatic cancer | 38.3 |
| Stomach cancer | 60.2 |
| Stomach cancer | 67.8 |
| Cervical carcinoma in situ | 80.2 |
| Lung cancer | 98.4 |
The results of Cox proportional hazard model analysis are shown in Table 5. No predictors of LRFFS and BCSS could be found. Patients with grade 3 tumors have the trend of worse OS (HR =5.88, P=0.05, 95% CI: 0.97–35.57). Patients with age ≥70 years old (HR =4.73, P=0.03) and stage II-III disease (HR =12.03, P=0.06, 95% CI: 0.90–159.74) have worse DMFS. Patients with perineural invasion (HR =3.71, P=0.02) have worse DFS and trend for worse OS (HR =4.75, P=0.10).
Table 5
| Predictors | HR | 95% CI | P |
|---|---|---|---|
| DFS | |||
| ENE (yes vs. no) | 3.85 | 0.81 to 18.30 | 0.09 |
| LVSI (yes vs. no) | 2.23 | 0.77 to 6.49 | 0.14 |
| N-stage (N1-2 vs. N0) | 0.50 | 0.12 to 2.09 | 0.35 |
| PNI (yes vs. no) | 3.71 | 1.29 to 10.65 | 0.02 |
| Stage (II-III vs. I) | 5.46 | 0.68 to 43.83 | 0.11 |
| T-stage (T2-3 vs. T0-1) | 0.58 | 0.11 to 3.06 | 0.53 |
| DMFS | |||
| Age | 4.73 | 1.13 to 19.80 | 0.03 |
| ENE (yes vs. no) | 2.24 | 0.37 to 13.50 | 0.38 |
| LVSI (yes vs. no) | 1.76 | 0.34 to 9.01 | 0.50 |
| Margin (involved vs. free) | 1.04 | 0.22 to 5.06 | 0.95 |
| PNI (yes vs. no) | 2.35 | 0.43 to 12.70 | 0.32 |
| Stage (II-III vs. I) | 12.03 | 0.90 to 159.74 | 0.06 |
| T-stage (T2-3 vs. T0-1) | 0.46 | 0.07 to 2.90 | 0.46 |
| OS | |||
| Grade (3 vs. 1-2) | 5.88 | 0.97 to 35.57 | 0.05 |
| PNI (yes vs. no) | 4.75 | 0.73 to 30.63 | 0.10 |
HR, hazard ratio; CI, confidence interval; DFS, disease-free survival; ENE, extranodal extension, LVSI, lymphovascular space invasion, PNI, perineural invasion, DMFS, distant metastases-free survival; OS, overall survival.
Discussion
To our knowledge, this is the study with the largest patient number and longest follow-up of elderly breast cancer patients treated with postoperative adjuvant whole breast IMRT with SIB technique. Our data demonstrates that IMRT with SIB technique is a well-tolerated and effective treatment choice for elderly breast cancer patients.
In other series with median age of 52–61 years-old, 2-year or 3-year locoregional control between 97.1–100% has been reported with IMRT with SIB technique (12,13,15,17). Fiorentino et al. also demonstrated that patients with age ≥70 years had excellent 3-year locoregional control of 97.2% after a median follow-up of 44 months (23). Our results confirm that IMRT with SIB technique can achieve 5-year locoregional control of 96.4% in elderly patients, which is comparable with younger patients.
Hamilton et al. reviewed the acute and late toxicities of IMRT with SIB technique in 17 different papers in 2016. Among those 5 trials using conventional fractionation, acute grade 2 dermatitis toxicities are seen in 43–71% patients (24). Comparing to earlier series (7,15,24), the incidence of grade 2 dermatitis in our study is lower. This may due to a smaller CTV50.4 volume (median volume =650.2 cm3) in our study. In Vicini’s trial, the CTV volume is greater than 975 cm3 in 71.5% of patients and the median volume of CTV is 732 cm3 in McDonald’s trial.
As shown in previous series by Fiorentino et al. (13,23), our data also demonstrated that patients with CTV50.4 volume ≥700 cc have significantly higher risk of developing grade 2 dermatitis.
No predictors of LRFFS or BCSS could be found due to few locoregional failure and breast-cancer death events. Nevertheless, patients with age ≥70-year-old have a higher risk of distant metastases and this may be attributed to less chemotherapy exposure (≥70 vs. <70, 14.8% vs. 37.9%, P=0.046). In addition, stage II-III patients also have higher risk of distant metastases.
Our results also revealed that secondary primary cancers and distant metastases have similar incidence rate. In our study, 7 patients had secondary primary cancers. Only one died of secondary cancer while the remaining 6 are rendered disease-free after curative treatments for secondary primary cancers, indicating that secondary cancers are highly curable even in elderly patients if it is treated promptly. So systemic therapy, close follow-up and enhanced awareness of possible secondary cancer are all crucial factors for improving outcome. The rate of secondary malignancy in our study is higher than previous reported 4% (25). In the paper published by Pignol et al. with 10-year follow-up, the rate of secondary malignancies is not reported (26).
Three patients suffered from symptomatic lymph edema in our study. No symptomatic fibrosis was recorded according to chart review. However, this is a retrospective study with inherent bias of incomplete data collection. Further prospective study is necessary to depict the full spectrum of late toxicities and cosmetic results. Moreover, as evidences from hypofractionated regimen emerges (27,28), hypofractionated whole breast radiotherapy with SIB will be the future research direction and it is being tested in RTOG 1005 (29) and HYPOSIB trial after a phase II study (14). As the results from these trials clear, the role of IMRT with SIB, either with conventional fractionation or hypofractionation, will be better clarified and defined.
Conclusions
Our data demonstrated that IMRT with SIB technique could achieve 5-year locoregional control of 96.4% if delivered adjuvantly after breast conserving surgery. Acute grade 2 dermatitis is seen in 18.3% of patients. IMRT with SIB technique in conventional fractionation is a safe and effective treatment in elderly breast cancer patients. Distant metastasis and secondary primary cancer occurs in 9.6% and 7.5% of patients, respectively. Further prospective studies are necessary to evaluate its true benefit in this patient subgroup and to fully depict the profile of toxicities.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Vincent Vinh-Hung and Nam P Nguyen) for the series “Radiotherapy for Breast Cancer in Advanced Age” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.19). The series “Radiotherapy for Breast Cancer in Advanced Age” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional Review Boards have approved the study protocols in two institutions and the Research Ethical Committee number is REC108-15 and CE19202A, respectively.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bartelink H, Maingon P, Poortmans P, et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol 2015;16:47-56. [Crossref] [PubMed]
- Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707-16. [Crossref] [PubMed]
- Kunkler I. Radiotherapy issues in elderly breast cancer patients. Breast Care (Basel) 2012;7:453-9. [Crossref] [PubMed]
- Fisher B, Bryant J, Dignam JJ, et al. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol 2002;20:4141-9. [Crossref] [PubMed]
- Kunkler IH, Williams LJ, Jack WJ, et al. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol 2015;16:266-73. [Crossref] [PubMed]
- Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol 2013;31:2382-7. [Crossref] [PubMed]
- Vicini FA, Sharpe M, Kestin L, et al. Optimizing breast cancer treatment efficacy with intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2002;54:1336-44. [Crossref] [PubMed]
- Pignol JP, Olivotto I, Rakovitch E, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol 2008;26:2085-92. [Crossref] [PubMed]
- Donovan E, Bleakley N, Denholm E, et al. Randomised trial of standard 2D radiotherapy (RT) versus intensity modulated radiotherapy (IMRT) in patients prescribed breast radiotherapy. Radiother Oncol 2007;82:254-64. [Crossref] [PubMed]
- Barnett GC, Wilkinson JS, Moody AM, et al. Randomized controlled trial of forward-planned intensity modulated radiotherapy for early breast cancer: interim results at 2 years. Int J Radiat Oncol Biol Phys 2012;82:715-23. [Crossref] [PubMed]
- Harsolia A, Kestin L, Grills I, et al. Intensity-modulated radiotherapy results in significant decrease in clinical toxicities compared with conventional wedge-based breast radiotherapy. Int J Radiat Oncol Biol Phys 2007;68:1375-80. [Crossref] [PubMed]
- Lee HH, Hou MF, Chuang HY, et al. Intensity modulated radiotherapy with simultaneous integrated boost vs. conventional radiotherapy with sequential boost for breast cancer - A preliminary result. Breast 2015;24:656-60. [Crossref] [PubMed]
- Fiorentino A, Mazzola R, Ricchetti F, et al. Intensity modulated radiation therapy with simultaneous integrated boost in early breast cancer irradiation. Report of feasibility and preliminary toxicity. Cancer Radiother 2015;19:289-94. [Crossref] [PubMed]
- Dellas K, Vonthein R, Zimmer J, et al. Hypofractionation with simultaneous integrated boost for early breast cancer: results of the German multicenter phase II trial (ARO-2010-01). Strahlenther Onkol 2014;190:646-53. [Crossref] [PubMed]
- McDonald MW, Godette KD, Whitaker DJ, et al. Three-year outcomes of breast intensity-modulated radiation therapy with simultaneous integrated boost. Int J Radiat Oncol Biol Phys 2010;77:523-30. [Crossref] [PubMed]
- Freedman GM, Anderson PR, Goldstein LJ, et al. Four-week course of radiation for breast cancer using hypofractionated intensity modulated radiation therapy with an incorporated boost. Int J Radiat Oncol Biol Phys 2007;68:347-53. [Crossref] [PubMed]
- Bantema-Joppe EJ, van der Laan HP, de Bock GH, et al. Three-dimensional conformal hypofractionated simultaneous integrated boost in breast conserving therapy: results on local control and survival. Radiother Oncol 2011;100:215-20. [Crossref] [PubMed]
- Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011;305:569-75. [Crossref] [PubMed]
- Gentile MS, Usman AA, Neuschler EI, et al. Contouring Guidelines for the Axillary Lymph Nodes for the Delivery of Radiation Therapy in Breast Cancer: Evaluation of the RTOG Breast Cancer Atlas. Int J Radiat Oncol Biol Phys 2015;93:257-65. [Crossref] [PubMed]
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109-22. [Crossref] [PubMed]
- Gagliardi G, Constine LS, Moiseenko V, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys 2010;76:S77-85. [Crossref] [PubMed]
- Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys 2010;76:S70-6. [Crossref] [PubMed]
- Fiorentino A, Mazzola R, Giaj Levra N, et al. Comorbidities and intensity-modulated radiotherapy with simultaneous integrated boost in elderly breast cancer patients. Aging Clin Exp Res 2018;30:533-8. [Crossref] [PubMed]
- Hamilton DG, Bale R, Jones C, et al. Impact of tumour bed boost integration on acute and late toxicity in patients with breast cancer: A systematic review. Breast 2016;27:126-35. [Crossref] [PubMed]
- McDonald MW, Godette KD, Butker EK, et al. Long-term outcomes of IMRT for breast cancer: a single-institution cohort analysis. Int J Radiat Oncol Biol Phys 2008;72:1031-40. [Crossref] [PubMed]
- Pignol JP, Truong P, Rakovitch E, et al. Ten years results of the Canadian breast intensity modulated radiation therapy (IMRT) randomized controlled trial. Radiother Oncol 2016;121:414-9. [Crossref] [PubMed]
- Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 2010;362:513-20. [Crossref] [PubMed]
- Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 2013;14:1086-94. [Crossref] [PubMed]
- Chen GP, Liu F, White J, et al. A planning comparison of 7 irradiation options allowed in RTOG 1005 for early-stage breast cancer. Med Dosim 2015;40:21-5. [Crossref] [PubMed]


