
Evidence behind use of orthovolt intraoperative radiotherapy and other techniques of IORT in recurrent colorectal cancer treatment
Introduction
There are only scarce literature data on the treatment of colorectal cancer (CRC) recurrence with the use of orthovolt intraoperative radiotherapy (IORT). IORT is a type of radiation treatment delivers a concentrated beam of radiation to tumor or tumour bed, as they are located during surgery. Depending on the type of radiation source following types of IORT are distinguished: intraoperative electron radiotherapy (IOERT) (delivering electron beams of high energies), high-dose rate intraoperative brachytherapy (HDR-IORT) (using high-dose rate source and remote afterloading technique) and orthovolt IORT (with low voltage X-ray beams). IORT, used as a component of combined therapy, allows to increase survival rates by about 15% (1). IORT is a safe and effective method of irradiation, significantly decreasing the risk of “geographical error”. IORT allows the administration of a single, high dose of radiation, applied during surgery under direct vision (2,3). The principle for the use of IORT is to eliminate the microscopic tumour foci by maximizing the radiobiological effects of a single dose of radiation and to optimize the treatment duration (4,5). At the time when the application of a sufficiently high dose of external beam radiation therapy (EBRT) is limited by tolerance of organs at risk (OAR), IORT is an excellent alternative allowing for safe dose escalation to cover the tumour while dose-limiting structures, such as the bowel, bladder or ureters, are safely shielded (1,6,7). Moreover during surgery, it is possible to release adhesions, moving normal tissues beyond the irradiation field, thereby protecting them while giving an appropriate dose to the precisely defined surgical bed area with a safe margin (8,9). IORT enables extra “sterilization” of surgical margins, otherwise the only chance to perform radical treatment is to extend the resection which is not always possible (2,3,10-12). According to Williams et al., IORT allows to limit the extent of the mutilating surgery, such as sacrectomy or exenteration (13). Most studies report a significant increase in survival rates in patients with recurrent CRC treated with IORT (5,14,15). The theoretical assumptions are promising, but the literature data on IORT in recurrent CRC come only from retrospective, single-institution studies.
IORT in recurrent colorectal cancer
Location of recurrent CRC in the pelvis is associated with a high risk of infiltration of the surrounding bony structures, which drastically reduces the chances of radical resection and usually involves the extended resections of multiple organs (16,17). The infiltration of side walls is associated with worse outcomes and obtaining radical resection is then significantly reduced (18,19). Surgical resection of recurrent CRC is frequently associated with a high risk of residual tumour tissue in the tumour bed and may have a negative impact on overall survival (OS) (20-22). The literature data also confirm better local control, with a lower risk of relapse at higher doses of radiation in the context of combination therapy with IORT (8,11). Improved local control and survival rates have been reported when IORT was used after neoadjuvant chemoradiotherapy for locally recurrent disease in radiation-naïve patients (23). Meta-analysis by Mirnezami et al. concerned the application of IORT in the treatment of recurrent CRC. The authors analyzed 29 studies, both prospective and retrospective, covering a total of 3,003 patients, of which 1,211 had recurrent CRC. IORT was used in patients with narrow or involved margins. The use of IORT was found to be associated with a significantly higher rate of wound healing complications without affecting the overall rate of complications and improved 5-year OS rates (P=0.001) (24). To date there are only two studies describing the use of orthovolt IORT in recurrent CRC, both published in 2012, by Guo et al. and Daly et al. (Table 1) (10,34). However, each of these publications describes the use of different systems for orthovolt IORT. In the study by Guo et al., the INTRABEAM® PRS 500 system was used whereas Day et al. used Phillips RT -250. Daly et al. analyzed a total of 61 cases, including 41 patients with recurrent colon (n=16) and rectal (n=25) cancer. All patients were treated with IORT. The 2-year survival rate was 52%. The median survival time was 22 months in all patients without distinguishing between primary CRC and recurrence (34). The study team from Cleveland analyzed a group of 42 patients, of whom 32 (76%) had recurrence of CRC. The median survival time in this subgroup was 32 months and the 3-year survival rate was 43% (10). Distribution of R0 and R1 resection rates was 52% and 45%, respectively. R2 resection was performed only in 2.4% of cases (10). Hashiguchi et al. analyzed 51 patients with recurrent rectal and sigmoid cancer, 27 patients were treated with IOERT at a dose of 15-30 Gy. The authors found significant effects of IOERT (P=0.0007) and a small volume of tumour tissue left on higher survival rates (P=0.0022). In this analysis, the use of EBRT had no effect on the late results (15). IOERT was an important prognostic factor, irrespective of the presence of distant metastases or radicality of resection. The median survival time in the IOERT group was 27 months. In the group without IOERT, 3- and 5-year survival rates were 5% and 0%, respectively. The authors have questioned the validity of the research in the arm without the IOERT scheme, proving the superiority of IOERT in terms of survival rates (15). Suzuki et al. found a significant difference in the 3- and 5-year survival rates between the IORT (+) and IORT (–) groups, 42%, 19% and 18%, 7%, respectively. None of the patients had distant metastases. All patients underwent non-radical resection and EBRT was performed in 41 of the 42 patients treated with IORT. In the IORT (+) group, patients with bulky, residual disease attained the 3-year survival rate of 44%. Moreover, the use of IORT improved local control and reduced the risk of re-recurrence. The 3-year re-recurrence rate in the IORT (+) group was 40% compared to 93% in the IORT (–) group (14). The literature is dominated mainly by studies describing the application of the IOERT technique for the treatment of CRC recurrence. The study by Nuyttens et al. analyzed 37 cases of patients with rectal cancer, including 19 who had local recurrence. HDR-IORT was used only in patients undergoing non-radical resection with a resection margin less than or equal to 2 mm. All patients had preoperative EBRT performed. The 3-year local re-recurrence rate was 57% (32). Summary of results depending on the IORT technique is presented in Table 1.
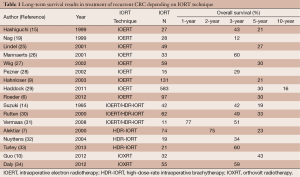
Full table
IORT treatment times and doses
In the study by Guo et al. the radiation dose administered was 5 Gy, at a distance of 1 cm from the applicator surface. The range of doses was 13.4-23.1 Gy with median 14.4 Gy (10). A dose in IOERT is given to the depth of 0.5-1 cm and reported on the surface (35). However, there is no uniformity in terms of the dose delivery reporting process. Generally, a dose is calculated to the surface or at a certain distance chosen by the study team (32). Lindel et al. differentiated the prescribed radiation dose ranges, depending on the radicality of resection. In the group of non-radical resection, the doses given ranged from 15 to 20 Gy, patients after radical resection received doses from 10 to 15 Gy (25). The median dose of IORT in the analysis by Eble et al. amounted to 13.6 Gy; however, in the R2 resection group the dose was higher –16 Gy (4). Haddock et al. determined the dose rate depending on two criteria: history of prior external field irradiation and radicality of resection. The patients previously irradiated received a dose of 12.5 Gy and those non-irradiated –17.5 Gy. Depending on the type of resection, the prescribed doses were as follows R0 –12.5 Gy, R1 –15 Gy and R2 –20 Gy. In this analysis, 98% of patients received a dose of not less than 20 Gy and the range of doses prescribed was 7.5-30 Gy (29). A similar IORT dose range was used by Hashiguchi et al. (15-30 Gy) and the median dose was 23 Gy (15). The dose of IORT is very important, because it allows in a measurable way to increase the total dose used in the treatment in order to eradicate the tumour. Doses exceeding 50 Gy allow to provide better control of symptoms; however, in cases of R1 resection the radiation doses should exceed 60 Gy to achieve a satisfactory treatment outcome (29,30). In some patients due to insufficient volume of a single field, it is essential to use the multiple radiation field technique (15). It is not the standard practice, but allows to cover a larger area, and in the case of IOERT it does not extend a total time of the entire treatment. The duration of IOERT irradiation is short (3-5 minutes) but preparations for the procedure with treatment usually take 30-45 minutes (19). In HDR-IORT, the operative time is extended by about 90 minutes due to the time of preparation and treatment (33). The multiple field technique in orthovolt IORT could significantly prolong the duration of surgery. IORT time prolongs with the increasing size of the applicator. The need to combine the radiation fields is often associated with the extensive areas of invasion, which means connecting the largest applicators, and thus the longest irradiation times. In Cleveland analysis, the median duration of IORT was 35 min (range, 14-39 min) (10). The size of applicators was selected based on the volume of recurrent tumour, so the surface of the applicator would adhere as closely as possible to the walls of the surgical bed. The median size of the applicator in Guo et al. analysis was 5 cm (10). Precise adherence between the tumour lodge and the applicator surface is extremely important. If the applicator is not fitted closely, the dose delivered to the lodge surface may vary markedly, resulting in the areas with insufficient dose coverage.
Re-irradiation for recurrent colorectal cancer
Dresden et al. analyzed 147 patients with non-metastatic relapse of rectal cancer. Their study confirmed an important role of combined therapy with the use of IORT in the treatment of local recurrence. The median OS time was 28 months. The 5-year OS rate was 31.5%. Patients re-irradiated or those who received a full course of radiotherapy before resection of recurrence had increased survival rates (P=0.043), longer times to local re-recurrence (P=0.038) and to distant metastases (P<0.001). The median time to re-recurrence was 13 months, and to disclose distant metastases 18 months (3). Safety of the second line of radiation is kept, when the dose does not exceed 30-40 Gy, the period between both lines of radiation is longer than 6 months and radiosensitive organs (i.e., small intestine) are moved from the irradiation field (3). The analysis by Koom et al. evaluated the toxicity profile of second line preoperative radiotherapy in patients with recurrent rectal cancer. The studied group consisted of 22 patients. Resection of recurrence was performed in 23% of patients. In the study acute and late toxicity rates, above the third degree, were 9% and 36%, respectively. Toxicity was mostly related to the digestive and urinary systems. The median dose of re-irradiation was 50 Gy (range, 30-66 Gy). The increased toxicity rate was significantly related to correlate with the central or anterior location of recurrence and the resection of tumour after re-irradiation (36). The doses prescribed in that study were higher than those conventionally used in the second line irradiation (30 Gy). Rutten et al. analyzed 62 patients with local recurrence of rectal cancer without distant metastases. The study proved that the total dose of radiation and R0 resection were significantly associated with improved survival. The study analyzed the use of two systems for IORT: IOERT and HDR-IORT in two major cancer centres in the Netherlands: Daniel den Hoed Cancer Center (DHCC), and Catharina Hospital Eindhoven. The basic assumption of the use of IORT was to supplement EBRT to achieve the highest possible dose of irradiation. The R0 resection rate was 48%. Forty-three percent of patients received prior EBRT, all patients previously non-irradiated and 10 of the 27 previously irradiated patients received the second line radiation to a dose 30 Gy or to a full dose. The survival results were found to be worse in patients who did not receive radiation before resection of recurrent tumour (P<0.05). The risk of death in patients not irradiated before resection of recurrence was 3-fold higher compared to patients undergoing radiation therapy (30). Vermaas et al. analyzed a small group of 11 patients with recurrent rectal cancer in whom re-irradiation with EBRT to a dose 30 Gy and resection with IORT were performed. IORT was performed using the IOERT or HDR- IORT technique. All patients were qualified as Tr4/5 according to the Wanebo classification and no cases of R0 resection were reported. Despite poor prognosis, the results achieved with this treatment schedule were similar to those obtained in other studies (Table 1). The median survival time without pain was 5 months (31). A group of researchers from Memorial Sloan-Kettering Cancer Center analyzed 100 patients who underwent resection with HDR-IORT for non-metastatic recurrent rectal cancer. Absence of angioinvasion and radical resection of the tumour were found to be independent predictors of longer survival (P<0.01 and P<0.05). The rate of re-recurrences after IORT was 60%, and the median time to re-recurrence was 15 months (37). Alektiar et al. analyzed 74 patients with recurrent rectal cancer undergoing surgery with HDR-IORT. The results confirmed the effectiveness of association of EBRT and IORT to prolong OS (P=0.04) (Table 1) (7). The analysis by Mannaerts et al. evaluated patients treated for recurrent CRC using three protocols. Patients were radiotherapy-naive, and had no evidence of distant metastases. The first group included patients who underwent only EBRT, the second one EBRT with resection and the third group EBRT with resection and IORT. The median dose of EBRT in each group was 50 Gy. R0 resection was performed in 37% and 64% cases, respectively. In group I (EBRT) the 3- and 5-year OS rates were 14% and 10%, respectively and the median survival time was 18 months. In group II (EBRT + resection) the 3-year survival was 11% (depending on the radicality of resection: R0, 29%; R1/2, 0%) and the median survival time was 19 months. In group III (EBRT + resection + IORT) the 3-year survival rate was 60% (R0, 63%; R1/2, 52%). The analysis demonstrated significantly higher survival rates in group III compared to group II after radical resection (26).
Radiation toxicity after IORT
Radiation-induced toxicity is extremely difficult to distinguish from surgical complications or symptoms of disease progression (38). Depending on the IORT technique, and thus the energy applied, the effects, both early and late, on the surrounding tissues are different. The main issue of research on the use of IORT using electrons is a high rate of complications (19). The incidence of postoperative complications varies from 15% to 68% (39). The most common types of early complications after treatment with IOERT are wound healing disturbances 3-46%, small bowel obstructions (14%) and formation of pelvic abscesses (12%), while in the group of patients who underwent surgical resection alone—pelvic abscesses (15%) (9,14,15,24,27,40). The percentage of serious postoperative complications ranges from 27% to 81% (15,19,41,42). Turley et al. reported 45% of postoperative complications without postoperative mortality (33). Wiig et al. and Hashiguchi et al. found no difference in the incidence of complications depending on the application of IORT (15,27). In the analysis by Williams et al., the most common acute complications associated with resection and IOERT were urinary tract infections, urinary incontinence and bladder dysfunction –13% (13). Dutch analysis by Dresen et al. reported 24% of acute complications of the urinary tract (3). Roeder et al. in the analysis of 97 patients with recurrent CRC, reported 59% rate of complications, including wound healing disorders, formation of abscesses, fistulas and disorders of micturition (6). An increased risk of complications in wound healing occurs in patients after preoperative radio and/or chemotherapy (4). In the analysis of the use of orthovolt IORT by Guo et al., the most frequent type of complications was hydronephrosis that occurred in 10 (24%) patients (10). Hydronephrosis and stricture of ureters in the case of high energy IOERT occur in about 2-12% of cases, however, it is difficult to compare these data with different types of IORT. Analysis by Daly et al. revealed 17 (31%) cases above the third degree of toxicity, including two cases of postoperative deaths. The most frequently reported complications were pelvic abscesses, small bowel obstruction, fistulas formation, ureteral stricture, and anastomotic leakage (34). The basic issue in the analysis of both Guo et al. and Daly et al. is that the incidence of complications was assessed in all patients, both in cases of recurrences and primary, advanced CRC. The toxicity profile in these groups of patients can vary greatly, mainly because patients with recurrent tumours have already undergone resection and radiation. Acute complications can also be caused by immobilization of bowel loops due to adhesions after primary treatment (14). The use of combined therapy (preoperative chemoradiotherapy, resection with IORT) in the analysis of researchers from the Mayo Clinic resulted in significantly higher rates of complications in patients with the grade of tumour immobilization above F2, according to the Suzuki-Gunderson classification (9). The most common complications were pelvic abscesses, intestinal obstruction and fistulas (9). Analysis of studies reporting complications after HDR-IORT in CRC recurrences shows that the types and rates of complications are similar to those reported for IOERT. Alektiar et al. reported that in patients with recurrent CRC undergoing surgery with HDR-IORT (followed by EBRT or otherwise) the rate of peripheral neuropathy was 16% and was similar to the data from other studies (7,32,37). Turley et al. found that surgical resection and HDR-IORT was associated with a high rate of both early (45%) and late (38%) complications. The most common early complications were postoperative wound infections (28%) and formation of intra-abdominal abscesses (14%). The prevalence of late complications such as neuropathy is reported to be 2% to 22% and is directly proportional to a dose of radiation (3,6,13,24,29,30). In the analysis by Nuyttens et al. abnormal wound healing occurred in 46% of patients, intra-abdominal abscesses in 16% and intestinal anastomotic leakage in 5% (32). To avoid the most common complications, it is necessary to perform the IORT procedure in sterile conditions and to shield the surrounding, healthy tissues, especially the ureters and the pelvic nerves. In cases at risk of ureter exposure to radiation, implantation of ureteral catheters or stents should be considered. The incidence of ureteral stricture requiring implantation of the catheter to prevent the development of hydronephrosis is as high as 23% (7,37). Despite the relatively high complication rates in patients undergoing resection with IORT, this treatment method is not less safe than surgery. In addition, IORT implementation can benefit in increased local control. Postoperative mortality depends, inter alia, on the scope of combination therapy, and the selection of patients for a particular treatment. Daly et al. reported two (3.6%), postoperative deaths unrelated to the use of IORT, whereas Guo et al. reported no deaths (10,34). Hahnloser et al. from the Mayo Clinic reported only one (0.3%) case of postoperative death (9). In the literature the 3-month postoperative mortality rate ranges from 0% to 8% (3,6,24,27,30,33). IORT is a technique that does not seem to increase the rates of complications or mortality (27). In the study by Guo et al., the median duration of postoperative hospital stay was 7 days (range, 2-59 days) (10). Some authors provide information about the duration of the entire hospital stay (8-19 days), which does not allow to make a meaningful comparison of results (3,9,27,31,33). The time of hospitalization in one of the studies, evaluating the use of IOERT in recurrences of rectal cancer, was shorter (13 days) in the group IOERT (+), compared to the IOERT (–) one (16 days) (27).
Conclusions
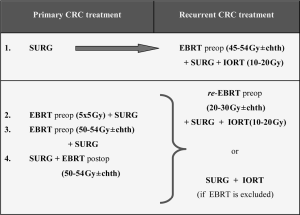
Analyzing the results of research on orthovolt IORT in recurrent CRC in the context of available literature, a number of limitations should be noted. Reports describing the use of orthovolt IORT in CRC recurrence do not constitute sufficient evidence, nor do they allow us to draw uniform conclusions, as these are single-centre studies. In both studies, a relatively small number of cases limit the possibility of statistical comparisons of certain parameters. The conclusions from these studies should be formulated with caution in relation to the known limitations of retrospective analyses in general, and in particular the possibility of selection bias. Unsatisfactory outcomes in patients treated with IORT arise mostly from inability to obtain a free resection margin (38). Particular emphasis should be placed on early detection of recurrence. Previous experience with IORT using low-energy photons highlights the need for better strategies of combined therapy and multidisciplinary care of patients with recurrent CRC. At the same time, the treatment of recurrence should be performed in referral centres where multidisciplinary treatment options are widely available. There is an absolute need for large randomized studies that will clearly assess the value of different treatment options in CRC recurrences, and thus create uniform rules to be observed in this disease. Based on the literature data available, following algorithms seems to be optimal to take full advantage of oncological treatment in patients with recurrent CRC (Figure 1).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Frederik Wenz and Elena Sperk) for the series “Intraoperative Radiotherapy” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.08.07). The series “Intraoperative Radiotherapy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bouchard P, Efron J. Management of recurrent rectal cancer. Ann Surg Oncol 2010;17:1343-56. [PubMed]
- Zhao J, Du CZ, Sun YS, et al. Patterns and prognosis of locally recurrent rectal cancer following multidisciplinary treatment. World J Gastroenterol 2012;18:7015-20. [PubMed]
- Dresen RC, Gosens MJ, Martijn H, et al. Radical resection after IORT-containing multimodality treatment is the most important determinant for outcome in patients treated for locally recurrent rectal cancer. Ann Surg Oncol 2008;15:1937-47. [PubMed]
- Eble MJ, Lehnert T, Treiber M, et al. Moderate dose intraoperative and external beam radiotherapy for locally recurrent rectal carcinoma. Radiother Oncol 1998;49:169-74. [PubMed]
- Calvo FA, Meirino RM, Orecchia R. Intraoperative radiation therapy first part: rationale and techniques. Crit Rev Oncol Hematol 2006;59:106-15. [PubMed]
- Roeder F, Goetz JM, Habl G, et al. Intraoperative Electron Radiation Therapy (IOERT) in the management of locally recurrent rectal cancer. BMC Cancer 2012;12:592. [PubMed]
- Alektiar KM, Zelefsky MJ, Paty PB, et al. High-dose-rate intraoperative brachytherapy for recurrent colorectal cancer. Int J Radiat Oncol Biol Phys 2000;48:219-26. [PubMed]
- Yeung JM, Ngan S, Lynch C, et al. Intraoperative radiotherapy and colorectal cancer. Minerva Chir 2010;65:161-71. [PubMed]
- Hahnloser D, Nelson H, Gunderson LL, et al. Curative potential of multimodality therapy for locally recurrent rectal cancer. Ann Surg 2003;237:502-8. [PubMed]
- Guo S, Reddy CA, Kolar M, et al. Intraoperative radiation therapy with the photon radiosurgery system in locally advanced and recurrent rectal cancer: retrospective review of the Cleveland clinic experience. Radiat Oncol 2012;7:110. [PubMed]
- Rodriguez-Bigas MA, Chang GJ, Skibber JM. Multidisciplinary approach to recurrent/unresectable rectal cancer: how to prepare for the extent of resection. Surg Oncol Clin N Am 2010;19:847-59. [PubMed]
- Merrick HW 3rd, Dobelbower RR Jr. Intraoperative radiation therapy in surgical oncology. Surg Oncol Clin N Am 2003;12:883-97. vii. [PubMed]
- Williams CP, Reynolds HL, Delaney CP, et al. Clinical results of intraoperative radiation therapy for patients with locally recurrent and advanced tumors having colorectal involvement. Am J Surg 2008;195:405-9. [PubMed]
- Suzuki K, Gunderson LL, Devine RM, et al. Intraoperative irradiation after palliative surgery for locally recurrent rectal cancer. Cancer 1995;75:939-52. [PubMed]
- Hashiguchi Y, Sekine T, Sakamoto H, et al. Intraoperative irradiation after surgery for locally recurrent rectal cancer. Dis Colon Rectum 1999;42:886-93; discussion 893-5. [PubMed]
- Dassanayake BK, Samita S, Deen RY, et al. Local recurrence of rectal cancer in patients not receiving neoadjuvant therapy - the importance of resection margins. Ceylon Med J 2011;56:159-61. [PubMed]
- Hellinger MD, Santiago CA. Reoperation for recurrent colorectal cancer. Clin Colon Rectal Surg 2006;19:228-36. [PubMed]
- Mirnezami AH, Sagar PM, Kavanagh D, et al. Clinical algorithms for the surgical management of locally recurrent rectal cancer. Dis Colon Rectum 2010;53:1248-57. [PubMed]
- Nag S, Martinez-Monge R, Martin EW. Intraoperative electron beam radiotherapy in recurrent colorectal carcinoma. J Surg Oncol 1999;72:66-71. [PubMed]
- Ferenschild FT, Vermaas M, Nuyttens JJ, et al. Value of intraoperative radiotherapy in locally advanced rectal cancer. Dis Colon Rectum 2006;49:1257-65. [PubMed]
- Vermaas M, Ferenschild FT, Nuyttens JJ, et al. Preoperative radiotherapy improves outcome in recurrent rectal cancer. Dis Colon Rectum 2005;48:918-28. [PubMed]
- Valentini V, Impiombato FA, De Paoli A, et al. The use of intraoperative radiation therapy according to evidence-based medicine. i supplementi di Tumori 2005;4:64-74.
- Willett CG, Czito BG, Tyler DS. Intraoperative radiation therapy. J Clin Oncol 2007;25:971-7. [PubMed]
- Mirnezami R, Chang GJ, Das P, et al. Intraoperative radiotherapy in colorectal cancer: systematic review and meta-analysis of techniques, long-term outcomes, and complications. Surg Oncol 2013;22:22-35. [PubMed]
- Lindel K, Willett CG, Shellito PC, et al. Intraoperative radiation therapy for locally advanced recurrent rectal or rectosigmoid cancer. Radiother Oncol 2001;58:83-7. [PubMed]
- Mannaerts GH, Rutten HJ, Martijn H, et al. Comparison of intraoperative radiation therapy-containing multimodality treatment with historical treatment modalities for locally recurrent rectal cancer. Dis Colon Rectum 2001;44:1749-58. [PubMed]
- Wiig JN, Tveit KM, Poulsen JP, et al. Preoperative irradiation and surgery for recurrent rectal cancer. Will intraoperative radiotherapy (IORT) be of additional benefit? A prospective study. Radiother Oncol 2002;62:207-13. [PubMed]
- Pezner RD, Chu DZ, Ellenhorn JD. Intraoperative radiation therapy for patients with recurrent rectal and sigmoid colon cancer in previously irradiated fields. Radiother Oncol 2002;64:47-52. [PubMed]
- Haddock MG, Miller RC, Nelson H, et al. Combined modality therapy including intraoperative electron irradiation for locally recurrent colorectal cancer. Int J Radiat Oncol Biol Phys 2011;79:143-50. [PubMed]
- Rutten HJ, Mannaerts GH, Martijn H, et al. Intraoperative radiotherapy for locally recurrent rectal cancer in The Netherlands. Eur J Surg Oncol 2000;26 Suppl A:S16-20.
- Vermaas M, Nuyttens JJ, Ferenschild FT, et al. Reirradiation, surgery and IORT for recurrent rectal cancer in previously irradiated patients. Radiother Oncol 2008;87:357-60. [PubMed]
- Nuyttens JJ, Kolkman-Deurloo IK, Vermaas M, et al. High-dose-rate intraoperative radiotherapy for close or positive margins in patients with locally advanced or recurrent rectal cancer. Int J Radiat Oncol Biol Phys 2004;58:106-12. [PubMed]
- Turley RS, Czito BG, Haney JC, et al. Intraoperative pelvic brachytherapy for treatment of locally advanced or recurrent colorectal cancer. Tech Coloproctol 2013;17:95-100. [PubMed]
- Daly ME, Kapp DS, Maxim PG, et al. Orthovoltage intraoperative radiotherapy for locally advanced and recurrent colorectal cancer. Dis Colon Rectum 2012;55:695-702. [PubMed]
- Wydmanski J, Miszczyk L, Suwiński R, et al. A new method of targeted intraoperative radiotherapy using the orthovoltage photon radiosurgery system. J Oncol 2005;55:320-3.
- Koom WS, Choi Y, Shim SJ, et al. Reirradiation to the pelvis for recurrent rectal cancer. J Surg Oncol 2012;105:637-42. [PubMed]
- Shoup M, Guillem JG, Alektiar KM, et al. Predictors of survival in recurrent rectal cancer after resection and intraoperative radiotherapy. Dis Colon Rectum 2002;45:585-92. [PubMed]
- Konski AA, Suh WW, Herman JM, et al. ACR Appropriateness Criteria®-Recurrent Rectal Cancer. Gastrointest Cancer Res 2012;5:3-12. [PubMed]
- Nielsen MB, Laurberg S, Holm T. Current management of locally recurrent rectal cancer. Colorectal Dis 2011;13:732-42. [PubMed]
- Bhangu A, Ali SM, Cunningham D, et al. Comparison of long-term survival outcome of operative vs nonoperative management of recurrent rectal cancer. Colorectal Dis 2013;15:156-63. [PubMed]
- Pezner RD, Chu DZ, Wagman LD, et al. Resection with external beam and intraoperative radiotherapy for recurrent colon cancer. Arch Surg 1999;134:63-7. [PubMed]
- Bhangu A, Ali SM, Darzi A, et al. Meta-analysis of survival based on resection margin status following surgery for recurrent rectal cancer. Colorectal Dis 2012;14:1457-66. [PubMed]


