The management of elderly patients with brain metastases from breast cancer
Introduction
Breast cancer is the most common type of malignancy diagnosed in women worldwide. An estimate of 271,270 new cases of breast carcinoma will be diagnosed in the United States in 2019 with 42,260 predicted deaths (1). Increasing age remains an important risk factor for the development of breast cancer, with an estimated incidence of 403.8 new patients per 100,000 women ≥65 years old versus only 82.2 per 100,000 women aged less than 65 years (2). Brain metastases (BM) represent an important cause of mortality in elderly population, where 40% of women older than 80 years old at diagnosis will die from breast cancer (3).
Statistics show that survival for breast carcinoma has improved through the years, which is attributed to better screening tests and treatment modalities (4). With an increase in survival, a higher incidence of metastatic breast cancer has been reported (5). Metastatic breast cancer to the brain is the second most common source of brain metastases, after metastases originating from the lung (6). Ten to 30% of women with breast cancer will develop brain metastases through the course of their disease, and as much as 5% of them will have presented with a brain metastatic lesion at diagnosis (7). Once diagnosed with metastatic disease, the prognosis can be limited, mostly due to the ultimate resistance of chemotherapy and radiotherapy, with an average survival of 12 months after the diagnosis of a brain metastasis (8).
Different therapeutic modalities have been directed towards prolonging the survival in these patients. These include surgical resection, radiotherapy and systemic therapies. For young patients who possess good functional statuses, there is a fairly clear standard of care. However, due to the underrepresentation of the elderly population in clinical trials, there exists a gap of knowledge regarding the best treatment for this group of patients, especially when dealing with radiotherapy. In this review, we aim to critically analyze the literature and describe the current treatment options for patients ≥65 years old with metastatic breast cancer to the brain.
Prognosis in breast cancer and breast cancer brain metastases
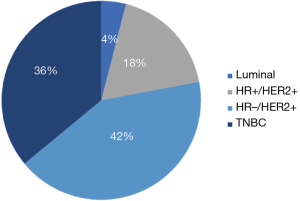
Breast cancer is the second most common malignancy that metastasizes to the brain. It is estimated that 10–30% of patients diagnosed with breast cancer will develop brain metastases during the course of their disease (9). Survival can range anywhere from 3–25 months according to the molecular subtype (4) and several other factors affecting survival. The propensity for breast cancer to metastasize to the brain has been related to the tumor subtype. Patients harboring a triple negative breast cancer (TNBC) have the highest probability of developing a BM, followed by human epidermal growth factor receptor 2 positive (HER2+) breast cancer patients and finally, patients with luminal breast cancers (5,7,10-16) (Table 1 and Figure 1). For instance, patients with HER2+ tumors have 2 to 4 times increased risk of developing a BM than patients with a HER2− tumor (17,18).
Table 1
| Molecular subtype | Frequency/prevalence of brain metastasis | Overall survival (months) (16) |
|---|---|---|
| Percentage of brain metastasis due to breast cancer | 10–30% (5,7) | |
| Luminal | 5% (11) | A: 23.1, B: 15.0 |
| HER2 positive | 15–29% (14) | 12.5 |
| TNBC | 22–46% (11,14-15) | 6.4 |
Overall survival measured from initial treatment of brain metastases to death. HER2, human epidermal growth factor receptor 2; TNBC, triple negative breast cancer.
After the occurrence of brain metastasis, patients with estrogen receptor (ER) and progesterone receptor (PR) positive tumors have been reported to live significantly longer when compared to hormone receptor negative tumors; similar survival advantage is observed with HER2+ tumors versus HER2− tumors (15). In HER2+ metastatic disease, trastuzumab plays an important role, prompting a median survival of 12.8 months versus only 4 months in patients without treatment (P=0.001) (15). The poor prognosis of TNBC patients typically occurs in the context of chemoresistant disease either from progressive extracranial disease, early recurrence of brain metastases, or both.
Age at diagnosis is another important factor when determining the prognosis of breast cancer patients. Studies have linked older age to a greater risk of developing brain metastases. Rotenberg et al. showed that the prevalence of metastatic disease significantly increases with older age from 3.9% to 23.4% (19). However; this increase in metastatic disease could potentially be related to the less aggressive therapeutic management offered to elderly patients. A large cohort study of 4,453 breast cancer patients showed that patients >80 years old had greater breast cancer specific mortality when compared to younger age groups (20). The increased mortality, found when stratifying patients according to age, can also be seen in the setting of metastatic disease. A retrospective analysis of the SEER database conducted by Chen et al. examined data regarding 4,932 patients with breast cancer metastases to sites including bone, lung liver and brain. The investigators concluded that when stratified by age, elderly patients (>69 years old) presented the worst overall survival (OS) (X2=121.9, P<0.001) and worst breast cancer specific survival (BCSS) (X2=69.8, P<0.001). Furthermore, after multivariable analysis, age at diagnosis was found to be associated with BCSS (P<0.05), defining age as an independent prognostic factor in distant metastatic breast cancer patients (21). While convincing, these results should be carefully analyzed as several non-evaluated factors could be playing a role in the reduced survival.
Prognosis of breast cancer patients can be evaluated through several prognostic indexes. These systems aid in determining and classifying patients with favorable or poor prognosis and ultimately, assist with decision-making. The most common prognostic scoring systems include the recursive partitioning analysis classification (RPA), the graded prognostic assessment (GPA) and disease-specific GPA (DS-GPA).
Patient selection: the role of prognostic scores in clinical decision making
Currently, there is controversy regarding the optimal management of patients with brain metastases. This is probably due to the heterogeneity of the data coming from a diverse group of patients, with different primary histologies, diverse genetic mutations, and other varied clinical features. This heterogeneous data has been generally taken together to drive conclusions about the universal standard of care for all BM patients (22-24). However, efforts have been made to combine these specific groups in order to obtain a more generalizable conclusion.
In the context where all patients with BM were considered a single group, Gaspar et al. published the first seminal paper evaluating the impact of patient selection on treatment outcomes (25). This study developed a RPA based on pretreatment variables such as tumor/patient characteristics as well as treatment variables. After analyzing a pool of 1,200 patients from three different Radiation Therapy Oncology Group (RTOG) trials conducted between 1979 and 1993, they found that pretreatment characteristics could define prognostic groups in BM patients. The group with best survival was integrated by patients with a Karnofsky performance status (KPS) ≥70, primary controlled disease, no extracranial metastases and age <65 years (RPA class 1: median survival of 7.1). The worst survival was found in the group of patients defined by a KPS <70. This study revealed the importance of the heterogeneity of patients with BM.
More than 20 years after the original work of Gaspar et al. (25) and upon the results of the RTOG-9508 trial, which supported the role of the number of BM as a potential prognostic factor (26), Sperduto et al. reevaluated the RTOG RPA classification system (27). The authors compared the RPA with a proposed updated version, the GPA, and found that the GPA was as good as RPA in regard to survival prognosis, but also the least subjective, most quantitative and easiest to use system. This new index did incorporate the number of BM into its four classification criteria: KPS, presence of extracranial disease, age (<50, 50–59, >60) and number of brain metastases (27).
After the acceptance of the GPA index (27), and with new data recommending the use of primary-specific prognostic systems (28), a subsequent study from the same group aimed to identify diagnosis-specific prognostic factors in order to develop a diagnosis-specific GPA (DS-GPA) (29). The authors found that patient stratification into different survival groups relies on different prognostic factors which vary according to specific primary sites. For instance, while classification of patients with brain metastatic lesions arising from non-small cell lung cancers (NSCLC) would depend on four prognostic factors (age, KPS, presence of extracranial disease, and number of BM), the DS-GPA classification for patients with brain metastases from breast cancer (breast-GPA) would solely depend on KPS score. Thus, as significant prognostic factors vary by diagnosis, DS-GPA indexes would also be particular for each primary site. The study also analyzed the effect of different therapeutic options on the survival of BM patients when classified according to these primary sites. Of note, there were different trends found in the analysis of potential treatments according to specific diagnoses. While WBRT alone presented as the best option for patients with BM from renal cell carcinoma, the same treatment suggested the less favorable outcomes for patients with BM arising from NSCLC or breast cancer. Overall, even with the retrospective nature of the study, the data suggested the urgent need to tailor BM patient’s treatment, taking into consideration patient-specific characteristics as well as disease-specific biological features and its potential implication in their therapeutic responses.
Efforts to refine these predictive tools continued in a steadfast fashion. In 2012, Sperduto et al. (30) published a specific RPA analysis of patients treated for newly diagnosed BM arisen from breast cancer. The study incorporated two new variables into the original breast-GPA; “tumor subtype” and “age at diagnosis” were found to be independent prognostic factors and consequently and were incorporated in the new breast-GPA. A follow-up study by Subbiah et al. (31) in a large population of patients with BM from breast cancer, identified the role of the “number of brain metastases” as another variable of a newly proposed, modified-breast GPA. However, in the same track that “age at diagnosis”, these two variables only have minor effects in the total score, whereas variables like KPS and tumor subtype were numerically the most important in defining the final GPA score (Table 2). The weight of these variables on the overall score was determined by the magnitude of their influence on survival. Furthermore, the RPA analysis showed that these two variables only define survival classes within subgroups of BM patients (32). For instance, “age at diagnosis” defined survival classes only in patients with KPS ≤50 but not in patients with higher KPS. These differences call for a holistic evaluation of each individual patient.
Table 2
| Variable | Score | ||||
|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | |
| RTOG Breast Graded Prognostic Assessment (30) | |||||
| KPS | ≤50 | 60 | 70–80 | 90–100 | – |
| Phenotype | TNBC | – | HR + BC | HER2HN | HER2HP |
| Age (years) | ≥60 | <60 | – | – | – |
| MDACC revalidation of RTOG Graded Prognostic Analysis (31) | |||||
| KPS | ≤50 | 60 | 70–80 | 90–100 | – |
| Phenotype | TNBC | HR+BC | HER2HN | HER2HP | – |
| Age (years) | >50 | ≤50 | – | – | – |
| Number | >3 | 1-3 | – | – | – |
RTOG, Radiation Therapy Oncology Group; KPS, Karnofsky performance status; MDACC, MD Anderson Cancer Center; HR, hormone receptors; HER2HN, HER2-positive, hormone negative; HER2HP, HER2-poistive, hormone-positive.
Overall, while prognostic indexes can be used as inclusion criteria for prospective studies or retrospectively to stratify patients for better group comparisons, all the previously described efforts have been made aiming to integrate simple but relevant patient characteristics to facilitate clinical decision making and individualize patient care. In this latter context, an important question arises: how accurate are these prognostic indexes for a specific patient? Although these prognostic indexes have been validated, it is clear that even the most accurate of them could potentially lead to overtreating patients who may actually have a really short survival, or even worse, undertreating patients because of an erroneously predicted unfavorable prognosis. As such, physician judgement should always be considered over any prognostic index alone.
Management of breast cancer brain metastases: a focus on elderly population geriatric assessment
Before the development of a treatment plan, all elderly individuals should undergo a comprehensive geriatric assessment. This should include the evaluation of functional, nutritional, and socioeconomic statuses, polypharmacy and comorbidities (33). Functional status can be assessed using the KPS scale, as it represents an effective proxy score for functional status in the elderly (34). When surgery represents a potential treatment option in elderly patients with BM, the Charlson comorbidity score could provide an insight about postsurgical outcomes such as risk of death, postoperative complication, and length of hospitalization stay, among others (35). Overall, this comprehensive evaluation provides important information used to evaluate the risk of future complications and death.
Healthy older adults who have no functional deficit and few comorbidities can be treated similarly to patients younger than 65 years old (33).
Treatment patterns in the elderly population
Local therapy has been the cornerstone in the management of patients harboring BM from breast cancer, where therapeutic options include surgical resection, whole brain radiation therapy (WBRT) or stereotactic radiosurgery (SRS); and more recently, the use of systemic agents. The selection of one or more of these treatments is based principally on the number and location of the lesions, symptoms, prognosis of systemic disease, and the performance status of the patient. We will discuss the relevance of each of these elements in the following sections.
Surgery
The first randomized controlled trial (RCT) presenting survival benefits from surgical intervention in the treatment of patients with single metastatic brain disease came from Patchell et al. (36). This phase III RCT randomized patients into “surgery + WBRT” versus “biopsy only + WBRT”; surgery was found to improve local control, preserve functional status and most importantly, improve OS compared to WBRT alone. Another phase III RCTs from Vecht et al. (37) confirmed the OS benefit of surgical resection. However; Mintz et al. (38) failed to prove this previously described benefit. This fact could be related to a higher percentage of patients with active extracranial disease (80% vs. 30–40%) and a lower KPS. An extension of the benefit from surgical intervention to patients with two to three metastatic brain lesions has been described for the those who are in good neurological condition and possess a well-controlled systemic disease (39). Furthermore, none of these RCTs were disease-specific studies and the ER/PR/HER2 status was not described in patients with BM from breast cancer (Table 3). Evidence supporting the benefit of surgery for metastatic breast cancer patients is an extrapolation from results obtained from a heterogenous population of BM patients.
Table 3
| Study | Randomization | n | Criteria | Primary end point | Breast cancer, n (%) | Elderly†, n (%) | Tumor control | Survival | |
|---|---|---|---|---|---|---|---|---|---|
| Local control | Distal control | ||||||||
| Evaluating the addition of surgery to WBRT | |||||||||
| Patchell et al. [1990] (36) | WBRT + surgery | 25 | 1 lesion | NR | 2 (8.0) | NR | 77% (1 year) | 80% | 36% (1 year) |
| WBRT + biopsy | 23 | No RT | 1 (4.3) | 14% (1 year) | 87% (P=0.52) | 5% (1 year)* | |||
| Vecht et al. [1993] (37) | WBRT + surgery | 32 | 1 lesion | Overall survival | 6 (18.8) | NR | NR | NR | 41% (1 year) |
| WBRT | 31 | 6 (19.4) | 23% (1 year)* | ||||||
| Mintz et al. [1996] (38) | WBRT + surgery | 41 | 1 lesion | Overall survival | 2 (4.9) | NR | NR | NR | 13% (1 year) |
| WBRT | 43 | 8 (18.6) | 31% (1 year) | ||||||
| Evaluating the addition WBRT to surgery | |||||||||
| Patchell et al. [1998] (40) | Surgery + WBRT | 49 | 1 lesion | Local control | 5 (10.2) | NR | 87% (1 year) | 93% (1 year) | NS |
| Surgery | 46 | 4 (8.7) | 37% (1 year) | 49% (1 year) | |||||
| Kocher et al. [2011]a (41) | Surgery + WBRT | 81 | 1 to 3 lesions | OS with FI | NR | NR | 75% (1 year) | 83% (1 year) | 46% (1 year) |
| Surgery | 79 | 46% (1 year)* | 62% (1 year)* | 46% (1 year)* | |||||
| Evaluating the addition of SRS to WBRT | |||||||||
| Kondziolka et al. [1999] (42) | WBRT + SRS | 13 | 2 to 4 lesions | Local control | 2 (15.4) | NR | 92% (1 year) | 34 m** | 46% (1 year) |
| WBRT | 14 | <2.5 cm | 2 (14.3) | 0% (1 year) | 5 m (P<0.002) | 20% (1 year) | |||
| Andrews et al. [2004] (26) | WBRT + SRS | 164 | 1 to 3 lesions | Overall survival | 15 (9.0) | 55 (34.0) | 82% | NR | 21% (1 year) |
| WBRT | 167 | <4 cm | 19 (11.0) | 66 (40.0) | 71% (P=0.01) | NR | 11% (1 year)*** | ||
| Evaluating the addition of WBRT to SRS | |||||||||
| Aoyama et al. [2006] (43) | SRS + WBRT | 65 | 1 to 4 lesions | Overall survival | 43 (66.0) | 33 (51.0) | 88.7% | 41.5% | 7.5 m |
| SRS | 67 | Each <3 cm | 45 (67.0) | 33 (49.0) | 72.5% (P=0.02) | 63.7% (P<0.003) | 8 m (P=0.42) | ||
| Kocher et al. [2011]b (41) | SRS + WBRT | 99 | 1 to 3 lesions | OS with FI | NR | NR | 81% | 67% | 10.7 |
| SRS | 100 | 69% (P=0.04) | 52% (P<0.02) | 10.9 (P=0.89) | |||||
| Chang et al. [2009] (44) | SRS + WBRT | 28 | 1 to 3 lesions | Cognitive outcomes | 4 (14.0) | NR | 100% | 73% | 63% |
| SRS | 30 | 4 (13.0) | 67% (P=0.012) | 45% (P=0.02) | 21% (P<0.003) | ||||
| Brown et al. [2016] (45) | SRS + WBRT | 102 | 1 to 3 lesions | Cognitive outcomes | 7 (6.9) | 58 (56.9)† | 90% | 92.3% | 34% (1 year) |
| SRS | 111 | <3 cm | 11 (9.9) | 58 (52.3)† | 73% (P<0.003) | 69.9% (P<0.001) | 37% (1 year)* | ||
*, values obtained indirectly from Kaplan-Meier curves using PlotDigitizer®; **, time to any brain failure; ***, survival time for patients with single metastasis; a,b are part of the same RCT (EORTC 22952-26001); †, elderly classified as >60 years old. WBRT, whole brain radiation therapy; SRS, stereotactic radiosurgery; Sx, surgery; NR, not reported; NS, not significant; LC, local control; OS, overall survival; FI, functional independence, CP, cognitive preservation.
Retrospective studies have highlighted the role of surgical resection in the treatment of breast cancer patients with CNS metastasis, pinpointing that surgical resection was associated with better outcomes when compared to WBRT alone (46). However, patients who undergo neurosurgical resection are usually individuals with a single brain metastasis, favorable KPS, and controlled extracranial disease, consistent with the aforementioned RCT. The role of resection in patients with multiple brain metastases is controversial.
Technical aspects of neurosurgical interventions have improved over time, and it is currently more feasible to perform maximal surgical resection with minimal morbidity. The use of intraoperative navigation, cortical mapping and minimal invasive approaches have increased the chances of achieving gross total resection and obtaining tumor tissue for pathology and molecular analysis (47-50). Patel et al. reported on the role of the surgical technique on patient’s outcomes. They found that en bloc resection of single metastatic lesions was associated with better local control when compared to piecemeal resection, without increase the rates of complications even in patients with large or eloquent-located lesions (51,52).
In general, resection should be considered in elderly patients with large tumors (generally >3 cm), particularly if they are causing edema and/or if neurologic symptoms are refractory to medical management. Surgical decompression remains the optimal approach to improve neurological function, reduce seizures and taper steroids. SRS or WBRT can be used as adjuvant therapies (25,26,53).
Whole brain radiotherapy
Whole brain radiotherapy has played a historical role in the management of BM patients for more than 60 years. While still the mainstay in the palliative treatment of patients with multiple symptomatic brain metastatic lesions not amenable to surgical resection or SRS or in cases with leptomeningeal involvement, its neurotoxicity profile has led to preferential selection of other radiation modalities such as SRS.
A second RCT from Patchell et al. (40) published in 1998, analyzed whether the addition of WBRT to surgery was beneficial in patients with single lesions. The study showed that the “surgery + WBRT” arm was superior to the “surgery + observation” arm in regard to local and distal intracranial tumor control as well as in the ability to decrease neurologic death rates; however, there was no improvement in OS. More than one decade later, Kocher et al. (41) published similar results adding WBRT to surgical treatment for patients with 1 to 3 lesions as part of the EORTC 22952-26001 study. This phase III RCT also evaluated the addition of WBRT to SRS, where the rates and grades of reported neurotoxicities were equivalent in both groups (surgery and SRS) (Table 3).
Short-term adverse effects of WBRT include headaches, nausea, vomiting, hair loss and increased fatigue, which can appear during treatment and last up to 2 to 6 months after therapy completion, especially in elderly patients. Long-term side effects, specifically cognitive decline has been associated with a consequent decreased quality of life (QoL). The most detrimental symptom in this setting is typically short-term memory decline (44,45,54,55).
The use of radiotherapy as a treatment of choice for breast cancer patients with CNS metastasis has increased through the years. This trend is evident in the study conducted by Leone et al. (56), where the investigators analyzed treatment patterns in patients >65 years old with CNS metastases from breast carcinoma. In this study, data was acquired and analyzed from 5,969 patients who received treatment for breast cancer metastases from 1992 to 2012. Findings revealed that 42.2% of patients did not receive any treatment; after palliative care, radiotherapy was the second most common treatment modality (27.1%). The authors demonstrated that the use of radiotherapy significantly increased over time (P=0.03), and when comparing radiotherapy to no treatment, the odds ratio (OR) of mortality was 0.83 (95% CI: 0.79–0.88). Furthermore, when therapeutic efforts were not limited in this elderly population, the administration of a combination of ≥2 therapies was associated with a significantly improved survival over time (P<0.05).
Frisk et al. analyzed a cohort of 241 patients with breast cancer BM receiving WBRT. For this cohort, the median OS for WBRT was 2.9 months. Patients over the age of 50 years old had an increased mortality; however, this association was no longer significant when adjusting for performance status, which at the same time was the strongest predictor of survival in the cohort. Interestingly, similar to the findings from Leone et al., mortality after WBRT was significantly better in those patients receiving transtuzumab or chemotherapy before radiation (57).
SRS in the definitive setting
SRS has challenged the role of WBRT as gold standard radiation modality in the treatment of BM patients. SRS involves the delivery of highly precise, ablative radiation to a well-defined area of disease while sparing normal brain parenchyma (58,59). Thus, SRS diminishes the risk of radiation-induced injury and safeguards patients from the detrimental effect of WBRT on neurocognition and QoL.
SRS has risen as one of the most effective therapeutic options for the management of metastatic brain disease. In general, SRS does not compromise patient survival and represents a lower risk of cognitive decline when compared to WBRT in patients with 1 to 4 brain metastases (45,60).
Choosing between surgery or SRS alone in cases of solitary or oligometastatic brain disease can still be controversial given the lack of solid RCT. In this setting, every patient should be evaluated on an individual basis, weighing all the aforementioned factors (like the need for symptomatic debulking) as well as the need for obtaining diagnostic tissue during decision making. Anecdotical reports like the study from Bindal et al., where SRS was compared to surgery alone in patients with lesions <3 cm treated between 1991 and 1994, support the superiority of surgery over SRS in terms of survival and local control. This led the authors to suggest that SRS should be reserved for lesions not amenable to surgery or for patients in poor medical conditions (61).
Muacevic et al. presented the results of a phase III RCT that was required to stop early due to poor accrual. The authors randomized 64 patients with single lesions <3 cm into “surgery + WBRT” or “SRS alone”. Similar median OS (9.5 vs. 10.8 months, P=0.8), local control (82% vs. 96%, P=0.06) and neurological death rates (29% vs. 11%, P=0.3) were reported (62). Furthermore, the addition of SRS to WBRT was studied in two RCTs from Kondziolka et al. and Andrews at al. The authors showed that SRS could add significant benefit in terms of LC and OS in patients with oligometastatic brain disease (26,42). Similarly, when the addition of WBRT to SRS was studied, combined therapy resulted in improved CNS endpoints like LC and DC when compared to SRS alone (41,43-45) (Table 3).
Studies have sought to compare SRS and WBRT as treatment modalities in the elderly. Minniti et al. evaluated the role of SRS in this population. Their findings suggest that initial management with SRS offer a good neurotoxicity profile while still associated with a survival benefit in patients older than 70 years old, with outcomes similar to those reported in historical series of SRS for younger patients (63).
Chen et al. (64) evaluated the toxicity associated with upfront SRS and WBRT in the elderly population with BM. This retrospective analysis included a cohort of elderly (70–79 years old) and very elderly (≥80 years old) patients with metastatic solid malignancies. The study accrued a total of 119 patients and found that WBRT was associated with worse OS [hazard ratio (HR) 3.7, 95% CI: 1.9–7.0, P<0.0001] and higher CNS toxicity profile when compared with SRS in multivariate analysis. However, even when patients undergoing urgent WBRT and WBRT for leptomeningeal disease were excluded from the analysis, the WBRT cohort still had worse KPS, higher tumor burden and more cases of uncontrolled systemic disease. Interestingly, being older than 80 years old was not associated with reduced OS nor higher risk of toxicities, which suggests that age alone could be a less important factor during treatment decisions. Finally, the authors advocate the consideration of SRS for the treatment of BM in the elderly or poor prognosis patients.
SRS in the pre or postoperative setting
The use of SRS to the surgical resection cavity has been studied in three different phase II and phase III RCTs in the general population (Table 4) (65-67). The findings support the benefit of boosting the post-surgical cavity with SRS in terms of local tumor control (66). When SRS was compared with WBRT as adjuvant treatment, the surgery + WBRT arm showed higher rates of LC and DC, but no differences in OS were described. Given SRS could improve local control with minimal complications, SRS was recommended over WBRT as a less toxic alternative in this group of patients (68).
Table 4
| Study | Randomization | n | Criteria | Primary end point | Breast Ca, N (%) | Elderly, N (%) | Tumor control | Survival | |
|---|---|---|---|---|---|---|---|---|---|
| Local control | Distal control | ||||||||
| Brennan et al., 2014, phase II (65) (MSKCC) | Surgery + SRS | 49 | 1 to 2 lesions | LC at 12 m | 9 (18) | NR | 85% | 44% | 14.7 m |
| >18 yo, PTV = cavity + 2 mm | 50% (P=0.08)* | ||||||||
| Mahajan et al., 2017, phase III (66) | Surgery + SRS | 63 | 1 to 3 lesions | LC | 9 (14.0) | 18 (29.0) | 72% | 42% | 17 m |
| Surgery + Obs | 65 | >3 yo, PTV = cavity + 1 mm | 14 (22.0) | 18 (28.0) | 43% (P=0.015) | 33% (P=0.35) | 18 m (P=0.24) | ||
| Brown et al. 2017, phase III (67) | Surgery + SRS | 98 | 1 lesion, >18 yo | OS and CDFS | NR | 59 (60.0)† | 61% | 64.70% | 12.2 |
| Surgery + WBRT | 96 | <5 cm, PTV = cavity + 2 mm | 59 (61.0)† | 81% (P<0.0007) | 89.2% (P<0.0005) | 11.6 (P=0.7) | |||
*, based on competing risk analysis including patients who completing postsurgical SRS and those who did not received SRS (n=40 and n=10 respectively); †, elderly classified as >60 years old. WBRT, whole brain radiation therapy; SRS, stereotactic radiosurgery; NR, not reported; LC, local control; OS, overall survival; CDFS, cognitive deterioration free survival; PTV, planning target volume.
Preoperative SRS is a novel modality of radiation delivery that has been gaining increased attention in the treatment of BM. It represents several advantages over postoperative SRS including decreased rates of radiation necrosis and leptomeningeal disease (69-71). Additionally, no compromise of local or distal control has been reported with this approach but several randomized trials are underway (70,72-74). Similar research is being conducted to determine the benefit of fractionated SRS (75).
There is a lack of studies specifically designed to evaluate these more recent radiation modalities in the elderly population. However, the previously described work from Chen et al. (64) included a high proportion of patients receiving SRS in the postoperative setting without describing any increased risk of toxicity. Forthcoming data from numerous RCTs will help to clarify the role SRS has in elderly population especially in the subgroup of patients harboring BM from breast cancer.
Overall, SRS remains an ideal treatment modality for the elderly population. It is an effective treatment option in BM from breast cancer and has a conservative toxicity profile for older patients, supporting its use in this patient population.
Systemic therapy
Prospective data to aid clinical decisions regarding the use of systemic agents are limited. This is due to the fact that patients with brain BM have been historically excluded from participating in clinical trials (76), probably because of concerns regarding blood-brain barrier (BBB) penetration, presence of transmembrane efflux pumps keeping the drug out of the CNS, drug interactions or intrinsic resistance to chemotherapy (77). This has led to missed opportunities in terms of collecting important data on CNS activity and potential efficacy endpoints. Nonetheless, there are some prospective and retrospective studies supporting the efficacy and/or activity of several agents in the brain. Relevant for treatment selection are the intriguing results showing differences in the expression of clinically-relevant genes in metastatic brain lesions when compared to their primary tumor, which could reflect the need for obtaining CNS tissue sample before treatment initiation (78).
The work from Leone et al. (56) showed that the use of a combination of ≥2 therapies was associated with a significant improvement in survival over time in patients >65 years old. Agents with proven effectivity in brain metastatic breast cancer include cytotoxic chemotherapies as well as novel targeted therapies. None of them should be excluded from the treatment of elderly population, geriatric assessment is warranted during the evaluation of these patients prior to the initiation of systemic treatment.
Different phase I and phase II trials have investigated the role of cytotoxic chemotherapy in the management of brain metastasis from breast cancer. However, to date, there are no FDA-approved systemic therapies for the treatment of CNS metastases originating from breast cancer (79). Table 5 summarizes clinical trials that have examined the role of various cytotoxic chemotherapies in this population (80-89). Out of all of this studies, anthracyclines and cisplatin seem to have the greatest objective response rate (62% and 40% respectively) (80,82); however, this data varies when compared to case series or retrospective cohorts. As observed the role of temozolomide for the treatment of CNS metastases from breast is limited if not futile, with most trials finding no CNS response. Only one multicentric phase II trial conducted by Siena et al. in 2010 examined the efficacy of dose-dense temozolomide, resulting in a poor a response rate of 4% and a progression- free survival of only 2 months (89).
Table 5
| Agent | Study | Regimen | Trial phase | Number of patients | CNS ORR |
|---|---|---|---|---|---|
| Platinum | Christodoulou et al. [2005] (80) | Cisplatin temozolomide | Phase II | 15 | 40% |
| Wu et al. [2015] (81) | Bevacizumab + etoposide + cisplatin | Pilot study | 8* | 60% | |
| Anthracycline | Caraglia et al. [2006] (82) | Pegylated liposomal doxorubicin | Phase II | 8 | 62% |
| Capecitabine | Rivera et al. [2006] (83) | Capecitabine + temozolomide | Phase I | 24 | 18% |
| Irinotecan | Anders et al. [2014] (84) | Irinotecan + iniparib | Phase II | 37 | 12% |
| Temozolomide | Christodoulou et al. [2001] (85) | Temozolomide | Phase II | 4 | 0% |
| Abrey et al. [2001] (86) | Temozolomide | Phase II | 10 | 0% | |
| Trudeau et al. [2006] (87) | Temozolomide | Phase II | 19 | 0% | |
| Iwamoto et al. [2008] (88) | Temozolomide | Phase II | 11 | 0% | |
| Siena et al. [2010] (89) | Temozolomide | Phase II | 51 | 4% |
*, leptomeningeal carcinomatosis. CNS, central nervous system; ORR, objective response rate.
Currently, the only biological target in metastatic breast cancer is HER2. Even though trastuzumab is considered as first-line systemic treatment in patients with HER2+ metastatic cancer (90,91) and retrospective studies have reported benefit in BM (92-95), trastuzumab has failed in preventing CNS relapse (96,97). The survival benefit reported in a patient with CNS metastatic disease in the context of HER2+ breast cancer could be linked to extracranial response rather that truly therapeutic CNS activity (92).
The small molecule tyrosine kinase inhibitor (TKI) lapatinib has been tested as a single agent (98,99) and in combination (99-101), with better results found in the last setting for previously treated and untreated patients. The LANSCAPE study, reported improved intracranial outcomes when the combination of lapatinib plus capecitabine was studied in previously untreated patients. The therapy was proven to lead to a 66% CNS response rate, as defined by a 50% or greater volumetric reduction in brain metastatic lesions (100). Neratinib is the next promising TKI, an irreversible inhibitor of both HER1 and HER2. The Translational Breast Cancer Research Consortium (TBCRC) has published the results from two phase II trials evaluating neratinib for patients with previously treated BM from breast cancer. Neratinib plus capecitabine seems to achieve better CNS response than Neratinib alone (Table 6) (98-104).
Table 6
| Study | Number of patients | Prior BM Therapy | Regimen details | ORR% (CR + PR) | Disease control% | PFS (months†) | Overall survival (months†) |
|---|---|---|---|---|---|---|---|
| Lin et al. (98), phase II [2008] | 39 | 100%; trastuzumab 95% WBRT and/or SRS | Lapatinib 750 mg BID | 2.6 (0+2.6) | 18* | 3.0 | NR |
| Lin et al. (99), phase II [2009] | 242 L, 50 L + Ca,b | 95% WBRT; 26% SRS | Lapatinib 750 mg BID; lapatinib 1,250 mg QD + capecitabine 1 g/m2 BID | 6 (0+6); 20 (0+20) | 43; 40** | 2.4; 3.6 | 6.4; NR |
| Lin et al. (101), phase II [2011] | 13 L + Ca, 9 L + Ta | 100% WBRT and trastuzumab; SRS in 1 case | Lapatinib + capecitabine; lapatinib + topotecan | 38 (0+38); 0 (0+0) | 84; 33 | NR | NR |
| Bachelot et al. (100), multicenter phase II (LANDSCAPE) [2013] | 44 | 0% WBRT or SRS | Lapatinib 1,250 mg ×21 d + capecitabine 2 g/m2 d1–d14; per cycle | 66 (0+66) | 93*** | 5.5 | 17.0 |
| Krop et al. (102), phase III (subgroup analysis of EMILIA) [2015] | 45 T-DM1, 50 C + L | 65% WBRT and/or local treatment; 70% WBRT and/or local treatment | T-DM1, capecitabine-lapatinib | NR | 78****; 84**** | 5.9; 5.7 | 26.8; 12.9 |
| Freedman et al. (103), multicentric phase II [2016] | 40 with BM after CNS directed therapy | 78% WBRT | Neratinib 240 mg QD | 8 (0+8) | NR | 1.9 | 8.7 |
| Freedman et al. (104), multicentric phase II [2019] | 49 total, 37 cohort 3A, 12 cohort 3B (prior lapatinib) | 59% WBRT; 41% SRS; 82% trastuzumab | Neratinib 240 mg QD + capecitabine 750 mg/m2; BID ×14 d then 7 d off | 49; 33 | NR | 5.5; 3.1 | 13.3; 15.1 |
†, incidence or median duration; *, free of any progression in both CNS and non-CNS lesions; **, % of patients with ≥20% reduction in volume CNS lesion; acombined therapy arm; bpatients came from lapatinib alone arm; ***, subgroup analysis of 42 patient using RECIST criteria; ****, 100-disease progression. BM, brain metastasis; WBRT, whole brain radiotherapy; SRS, stereotactic radiosurgery; L, lapatinib; C, capecitabine; ORR, objective response rate; CR, complete response; PR, partial response; SD, stable disease; disease control, CR + PR + SD; NR, not reported; CNS, central nervous system; HER-2, human epidermal growth factor receptor 2; KPS, Karnofsky performance score; PFS, progression free survival; BID, twice a day; QD, every day; T-DM1, ado-trastuzumab emtansine.
A retrospective analysis of the EMILIA trial randomized 991 patients with metastatic HER2+ breast cancer into trastuzumab-emtansine (T-DM1) or capecitabine plus lapatinib, and revealed similar rates of CNS progression in patients without brain metastatic disease at baseline (2% with trastuzumab-emtansine; 0.7% with capecitabine plus lapatinib). Additionally, for those with asymptomatic brain metastases at baseline, whom had received WBRT and/or local treatment in around 70% of cases in both arms, TTDM-1 was associated with significantly improved OS (102). As these biologic agents are increasingly utilized, there is a greater need to determine their safety profile among patients treated with concurrent brain directed therapies, like SRS (105).
Around 5% of patients with luminal breast cancer will develop BM during the course of the disease. It is also important to note that in almost 50% of these cases there is an overexpression of hormone receptors in the primary tumor while the metastatic lesion will not harbor receptor overexpression (106). Furthermore, even when receptor overexpression is still present, previous endocrine therapy could drive the emergence of mutations in the estrogen receptor gene 1 (ESR1) that confer constitutive expression, and potential resistance to hormonal therapy in metastatic lesions (107). In general, there is a good distribution of estrogen receptor antagonist within brain parenchyma and reports of prolonged survival and remission with tamoxifen, letrozole and megestrol acetate have been described in the literature (108).
Conclusions
Clinical decision making during the management of elderly patients with BM from breast cancer should be approached in a holistic way, particularly given the paucity of data among this patient population. Geriatric assessment including evaluation of comorbidities, functional status, and socioeconomic support should be warranted. Age, as an independent factor, does not seem to play a determinant role in treatment selection. The risks and benefits before initiation of any treatment should be weighed on an individual basis considering that the elderly represents a very heterogeneous population. Use of prognostic scores could aid in this endeavor; however, physician judgment is of utmost importance.
Acknowledgments
Funding: This publication was made possible through the support of the Eveleigh Family Career Development Award for Cancer Research at Mayo Clinic in Florida.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Vincent Vinh-Hung and Nam P Nguyen) for the series “Radiotherapy for Breast Cancer in Advanced Age” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.31). The series “Radiotherapy for Breast Cancer in Advanced Age” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Jolly T, Williams GR, Jones E, et al. Treatment of metastatic breast cancer in women aged 65 years and older. Womens Health (Lond) 2012;8:455-69; quiz 470-1.
- Rosso S, Gondos A, Zanetti R, et al. Up-to-date estimates of breast cancer survival for the years 2000-2004 in 11 European countries: the role of screening and a comparison with data from the United States. Eur J Cancer 2010;46:3351-7. [Crossref] [PubMed]
- Kotecki N, Lefranc F, Devriendt D, et al. Therapy of breast cancer brain metastases: challenges, emerging treatments and perspectives. Ther Adv Med Oncol 2018;10:1758835918780312. [Crossref] [PubMed]
- Custódio-Santos T, Videira M, Brito MA. Brain metastasization of breast cancer. Biochim Biophys Acta Rev Cancer 2017;1868:132-47. [Crossref] [PubMed]
- Leone JP, Leone BA. Breast cancer brain metastases: the last frontier. Exp Hematol Oncol 2015;4:33. [Crossref] [PubMed]
- Niikura N, Saji S, Tokuda Y, et al. Brain metastases in breast cancer. Jpn J Clin Oncol 2014;44:1133-40. [Crossref] [PubMed]
- Leone JP, Lee AV, Brufsky AM. Prognostic factors and survival of patients with brain metastasis from breast cancer who underwent craniotomy. Cancer Med 2015;4:989-94. [Crossref] [PubMed]
- Phillips C, Jeffree R, Khasraw M. Management of breast cancer brain metastases: A practical review. Breast 2017;31:90-8. [Crossref] [PubMed]
- Kim YJ, Kim JS, Kim IA. Molecular subtype predicts incidence and prognosis of brain metastasis from breast cancer in SEER database. J Cancer Res Clin Oncol 2018;144:1803-16. [Crossref] [PubMed]
- Arvold ND, Oh KS, Niemierko A, et al. Brain metastases after breast-conserving therapy and systemic therapy: incidence and characteristics by biologic subtype. Breast Cancer Res Treat 2012;136:153-60. [Crossref] [PubMed]
- Lin NU, Amiri-Kordestani L, Palmieri D, et al. CNS metastases in breast cancer: old challenge, new frontiers. Clin Cancer Res 2013;19:6404-18. [Crossref] [PubMed]
- Oehrlich NE, Spineli LM, Papendorf F, et al. Clinical outcome of brain metastases differs significantly among breast cancer subtypes. Oncol Lett 2017;14:194-200. [Crossref] [PubMed]
- Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol 2010;28:3271-7. [Crossref] [PubMed]
- Nam BH, Kim SY, Han HS, et al. Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res 2008;10:R20. [Crossref] [PubMed]
- Jeon W, Jang BS, Jeon SH, et al. Analysis of survival outcomes based on molecular subtypes in breast cancer brain metastases: A single institutional cohort. Breast J 2018;24:920-6. [Crossref] [PubMed]
- Leyland-Jones B. Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J Clin Oncol 2009;27:5278-86. [Crossref] [PubMed]
- Kaal EC, Vecht CJ. CNS complications of breast cancer: current and emerging treatment options. CNS Drugs 2007;21:559-79. [Crossref] [PubMed]
- Rottenberg Y, Naeim A, Uziely B, et al. Breast cancer among older women: The influence of age and cancer stage on survival. Arch Gerontol Geriatr 2018;76:60-4. [Crossref] [PubMed]
- Brandt J, Garne JP, Tengrup I, et al. Age at diagnosis in relation to survival following breast cancer: a cohort study. World Journal of Surgical Oncology 2015;13:33. [Crossref] [PubMed]
- Chen MT, Sun HF, Zhao Y, et al. Comparison of patterns and prognosis among distant metastatic breast cancer patients by age groups: a SEER population-based analysis. Sci Rep 2017;7:9254. [Crossref] [PubMed]
- Mampre D, Ehresman J, Alvarado-Estrada K, et al. Propensity for different vascular distributions and cerebral edema of intraparenchymal brain metastases from different primary cancers. J Neurooncol 2019;143:115-22. [Crossref] [PubMed]
- Chaichana KL, Acharya S, Flores M, et al. Identifying better surgical candidates among recursive partitioning analysis class 2 patients who underwent surgery for intracranial metastases. World Neurosurg 2014;82:e267-75. [Crossref] [PubMed]
- Chaichana KL, Gadkaree S, Rao K, et al. Patients undergoing surgery of intracranial metastases have different outcomes based on their primary pathology. Neurol Res 2013;35:1059-69. [Crossref] [PubMed]
- Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745-51. [Crossref] [PubMed]
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665-72. [Crossref] [PubMed]
- Sperduto PW, Berkey B, Gaspar LE, et al. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 2008;70:510-4. [Crossref] [PubMed]
- Golden DW, Lamborn KR, McDermott MW, et al. Prognostic factors and grading systems for overall survival in patients treated with radiosurgery for brain metastases: variation by primary site. J Neurosurg 2008;109:77-86. [Crossref] [PubMed]
- Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys 2010;77:655-61. [Crossref] [PubMed]
- Sperduto PW, Kased N, Roberge D, et al. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys 2012;82:2111-7. [Crossref] [PubMed]
- Subbiah IM, Lei X, Weinberg JS, et al. Validation and Development of a Modified Breast Graded Prognostic Assessment As a Tool for Survival in Patients With Breast Cancer and Brain Metastases. J Clin Oncol 2015;33:2239-45. [Crossref] [PubMed]
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419-25. [Crossref] [PubMed]
- Minniti G, Filippi AR, Osti MF, et al. Radiation therapy for older patients with brain tumors. Radiat Oncol 2017;12:101. [Crossref] [PubMed]
- Crooks V, Waller S, Smith T, et al. The use of the Karnofsky Performance Scale in determining outcomes and risk in geriatric outpatients. J Gerontol 1991;46:M139-44. [Crossref] [PubMed]
- Grossman R, Mukherjee D, Chang DC, et al. Predictors of inpatient death and complications among postoperative elderly patients with metastatic brain tumors. Ann Surg Oncol 2011;18:521-8. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990;322:494-500. [Crossref] [PubMed]
- Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol 1993;33:583-90. [Crossref] [PubMed]
- Mintz AH, Kestle J, Rathbone MP, et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer 1996;78:1470-6. [Crossref] [PubMed]
- Pollock BE, Brown PD, Foote RL, et al. Properly selected patients with multiple brain metastases may benefit from aggressive treatment of their intracranial disease. J Neurooncol 2003;61:73-80. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 1998;280:1485-9. [Crossref] [PubMed]
- Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 2011;29:134-41. [Crossref] [PubMed]
- Kondziolka D, Patel A, Lunsford LD, et al. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys 1999;45:427-34. [Crossref] [PubMed]
- Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 2006;295:2483-91. [Crossref] [PubMed]
- Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009;10:1037-44. [Crossref] [PubMed]
- Brown PD, Jaeckle K, Ballman KV, et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA 2016;316:401-9. [Crossref] [PubMed]
- Fokstuen T, Wilking N, Rutqvist LE, et al. Radiation therapy in the management of brain metastases from breast cancer. Breast Cancer Res Treat 2000;62:211-6. [Crossref] [PubMed]
- Gassie K, Alvarado-Estrada K, Bechtle P, et al. Surgical Management of Deep-Seated Metastatic Brain Tumors Using Minimally Invasive Approaches. J Neurol Surg A Cent Eur Neurosurg 2019;80:198-204. [Crossref] [PubMed]
- Ricciardi L, Chaichana KL, Cardia A, et al. The exoscope in neurosurgery: an innovative "point of view". A systematic review of the technical, surgical and educational aspects. World Neurosurg 2019; [Epub ahead of print].
- Mampre D, Bechtle A, Chaichana KL. Minimally Invasive Resection of Intra-axial Posterior Fossa Tumors Using Tubular Retractors. World Neurosurg 2018;119:e1016-20. [Crossref] [PubMed]
- Bakhsheshian J, Strickland BA, Jackson C, et al. Multicenter Investigation of Channel-Based Subcortical Trans-Sulcal Exoscopic Resection of Metastatic Brain Tumors: A Retrospective Case Series. Oper Neurosurg (Hagerstown) 2019;16:159-66. [Crossref] [PubMed]
- Patel AJ, Suki D, Hatiboglu MA, et al. Factors influencing the risk of local recurrence after resection of a single brain metastasis. J Neurosurg 2010;113:181-9. [Crossref] [PubMed]
- Patel AJ, Suki D, Hatiboglu MA, et al. Impact of surgical methodology on the complication rate and functional outcome of patients with a single brain metastasis. J Neurosurg 2015;122:1132-43. [Crossref] [PubMed]
- Trifiletti DM, Romano KD, Xu Z, et al. Leptomeningeal disease following stereotactic radiosurgery for brain metastases from breast cancer. J Neurooncol 2015;124:421-7. [Crossref] [PubMed]
- Pulenzas N, Khan L, Tsao M, et al. Fatigue scores in patients with brain metastases receiving whole brain radiotherapy. Support Care Cancer 2014;22:1757-63. [Crossref] [PubMed]
- Trifiletti DM, Lee CC, Schlesinger D, et al. Leukoencephalopathy After Stereotactic Radiosurgery for Brain Metastases. Int J Radiat Oncol Biol Phys 2015;93:870-8. [Crossref] [PubMed]
- Leone JP, Haraldsson B, Mott SL, et al. Treatment Patterns and Survival of Elderly Patients With Breast Cancer Brain Metastases. Am J Clin Oncol 2019;42:60-6. [Crossref] [PubMed]
- Frisk G, Tinge B, Ekberg S, et al. Survival and level of care among breast cancer patients with brain metastases treated with whole brain radiotherapy. Breast Cancer Res Treat 2017;166:887-96. [Crossref] [PubMed]
- Trifiletti DM, Lee CC, Kano H, et al. Stereotactic Radiosurgery for Brainstem Metastases: An International Cooperative Study to Define Response and Toxicity. Int J Radiat Oncol Biol Phys 2016;96:280-8. [Crossref] [PubMed]
- Emery A, Trifiletti DM, Romano KD, et al. More than Just the Number of Brain Metastases: Evaluating the Impact of Brain Metastasis Location and Relative Volume on Overall Survival After Stereotactic Radiosurgery. World Neurosurg 2017;99:111-7. [Crossref] [PubMed]
- Skeie BS, Eide GE, Flatebo M, et al. Quality of life is maintained using Gamma Knife radiosurgery: a prospective study of a brain metastases patient cohort. J Neurosurg 2017;126:708-25. [Crossref] [PubMed]
- Bindal AK, Bindal RK, Hess KR, et al. Surgery versus radiosurgery in the treatment of brain metastasis. J Neurosurg 1996;84:748-54. [Crossref] [PubMed]
- Muacevic A, Wowra B, Siefert A, et al. Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. J Neurooncol 2008;87:299-307. [Crossref] [PubMed]
- Minniti G, Esposito V, Clarke E, et al. Stereotactic radiosurgery in elderly patients with brain metastases. J Neurooncol 2013;111:319-25. [Crossref] [PubMed]
- Chen L, Shen C, Redmond KJ, et al. Use of Stereotactic Radiosurgery in Elderly and Very Elderly Patients With Brain Metastases to Limit Toxicity Associated With Whole Brain Radiation Therapy. Int J Radiat Oncol Biol Phys 2017;98:939-47. [Crossref] [PubMed]
- Brennan C, Yang TJ, Hilden P, et al. A phase 2 trial of stereotactic radiosurgery boost after surgical resection for brain metastases. Int J Radiat Oncol Biol Phys 2014;88:130-6. [Crossref] [PubMed]
- Mahajan A, Ahmed S, McAleer MF, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol 2017;18:1040-8. [Crossref] [PubMed]
- Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 2017;18:1049-60. [Crossref] [PubMed]
- Marchan EM, Peterson J, Sio TT, et al. Postoperative Cavity Stereotactic Radiosurgery for Brain Metastases. Front Oncol 2018;8:342. [Crossref] [PubMed]
- Routman DM, Yan E, Vora S, et al. Preoperative Stereotactic Radiosurgery for Brain Metastases. Front Neurol 2018;9:959. [Crossref] [PubMed]
- Patel KR, Burri SH, Asher AL, et al. Comparing Preoperative With Postoperative Stereotactic Radiosurgery for Resectable Brain Metastases: A Multi-institutional Analysis. Neurosurgery 2016;79:279-85. [Crossref] [PubMed]
- Prabhu RS, Miller KR, Asher AL, et al. Preoperative stereotactic radiosurgery before planned resection of brain metastases: updated analysis of efficacy and toxicity of a novel treatment paradigm. J Neurosurg 2018;1-8. [PubMed]
- Patel AR, Nedzi L, Lau S, et al. Neoadjuvant Stereotactic Radiosurgery Before Surgical Resection of Cerebral Metastases. World Neurosurg 2018;120:e480-7. [Crossref] [PubMed]
- Asher AL, Burri SH, Wiggins WF, et al. A new treatment paradigm: neoadjuvant radiosurgery before surgical resection of brain metastases with analysis of local tumor recurrence. Int J Radiat Oncol Biol Phys 2014;88:899-906. [Crossref] [PubMed]
- Patel KR, Burri SH, Boselli D, et al. Comparing pre-operative stereotactic radiosurgery (SRS) to post-operative whole brain radiation therapy (WBRT) for resectable brain metastases: a multi-institutional analysis. J Neurooncol 2017;131:611-8. [Crossref] [PubMed]
- Lehrer EJ, Peterson JL, Zaorsky NG, et al. Single versus Multifraction Stereotactic Radiosurgery for Large Brain Metastases: An International Meta-analysis of 24 Trials. Int J Radiat Oncol Biol Phys 2019;103:618-30. [Crossref] [PubMed]
- Camidge DR, Lee EQ, Lin NU, et al. Clinical trial design for systemic agents in patients with brain metastases from solid tumours: a guideline by the Response Assessment in Neuro-Oncology Brain Metastases working group. Lancet Oncol 2018;19:e20-32. [Crossref] [PubMed]
- Lin NU, Prowell T, Tan AR, et al. Modernizing Clinical Trial Eligibility Criteria: Recommendations of the American Society of Clinical Oncology-Friends of Cancer Research Brain Metastases Working Group. J Clin Oncol 2017;35:3760-73. [Crossref] [PubMed]
- Brastianos PK, Carter SL, Santagata S, et al. Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov 2015;5:1164-77. [Crossref] [PubMed]
- Lin NU. Breast cancer brain metastases: new directions in systemic therapy. Ecancermedicalscience 2013;7:307. [PubMed]
- Christodoulou C, Bafaloukos D, Linardou H, et al. Temozolomide (TMZ) combined with cisplatin (CDDP) in patients with brain metastases from solid tumors: a Hellenic Cooperative Oncology Group (HeCOG) Phase II study. J Neurooncol 2005;71:61-5. [Crossref] [PubMed]
- Wu PF, Lin CH, Kuo CH, et al. A pilot study of bevacizumab combined with etoposide and cisplatin in breast cancer patients with leptomeningeal carcinomatosis. BMC Cancer 2015;15:299. [Crossref] [PubMed]
- Caraglia M, Addeo R, Costanzo R, et al. Phase II study of temozolomide plus pegylated liposomal doxorubicin in the treatment of brain metastases from solid tumours. Cancer Chemother Pharmacol 2006;57:34-9. [Crossref] [PubMed]
- Rivera E, Meyers C, Groves M, et al. Phase I study of capecitabine in combination with temozolomide in the treatment of patients with brain metastases from breast carcinoma. Cancer 2006;107:1348-54. [Crossref] [PubMed]
- Anders C, Deal AM, Abramson V, et al. TBCRC 018: phase II study of iniparib in combination with irinotecan to treat progressive triple negative breast cancer brain metastases. Breast Cancer Res Treat 2014;146:557-66. [Crossref] [PubMed]
- Christodoulou C, Bafaloukos D, Kosmidis P, et al. Phase II study of temozolomide in heavily pretreated cancer patients with brain metastases. Ann Oncol 2001;12:249-54. [Crossref] [PubMed]
- Abrey LE, Olson JD, Raizer JJ, et al. A phase II trial of temozolomide for patients with recurrent or progressive brain metastases. J Neurooncol 2001;53:259-65. [Crossref] [PubMed]
- Trudeau ME, Crump M, Charpentier D, et al. Temozolomide in metastatic breast cancer (MBC): a phase II trial of the National Cancer Institute of Canada - Clinical Trials Group (NCIC-CTG). Ann Oncol 2006;17:952-6. [Crossref] [PubMed]
- Iwamoto FM, Omuro AM, Raizer JJ, et al. A phase II trial of vinorelbine and intensive temozolomide for patients with recurrent or progressive brain metastases. J Neurooncol 2008;87:85-90. [Crossref] [PubMed]
- Siena S, Crino L, Danova M, et al. Dose-dense temozolomide regimen for the treatment of brain metastases from melanoma, breast cancer, or lung cancer not amenable to surgery or radiosurgery: a multicenter phase II study. Ann Oncol 2010;21:655-61. [Crossref] [PubMed]
- Gradishar WJ, Anderson BO, Blair SL, et al. Breast cancer version 3.2014. J Natl Compr Canc Netw 2014;12:542-90. [Crossref] [PubMed]
- Giordano SH, Temin S, Chandarlapaty S, et al. Systemic Therapy for Patients With Advanced Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol 2018;36:2736-40. [Crossref] [PubMed]
- Bartsch R, Rottenfusser A, Wenzel C, et al. Trastuzumab prolongs overall survival in patients with brain metastases from Her2 positive breast cancer. J Neurooncol 2007;85:311-7. [Crossref] [PubMed]
- Park IH, Ro J, Lee KS, et al. Trastuzumab treatment beyond brain progression in HER2-positive metastatic breast cancer. Ann Oncol 2009;20:56-62. [Crossref] [PubMed]
- Church DN, Modgil R, Guglani S, et al. Extended survival in women with brain metastases from HER2 overexpressing breast cancer. Am J Clin Oncol 2008;31:250-4. [Crossref] [PubMed]
- Bartsch R, Berghoff A, Pluschnig U, et al. Impact of anti-HER2 therapy on overall survival in HER2-overexpressing breast cancer patients with brain metastases. Br J Cancer 2012;106:25-31. [Crossref] [PubMed]
- Swain SM, Kim SB, Cortes J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2013;14:461-71. [Crossref] [PubMed]
- Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015;372:724-34. [Crossref] [PubMed]
- Lin NU, Carey LA, Liu MC, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2008;26:1993-9. [Crossref] [PubMed]
- Lin NU, Dieras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res 2009;15:1452-9. [Crossref] [PubMed]
- Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol 2013;14:64-71. [Crossref] [PubMed]
- Lin NU, Eierman W, Greil R, et al. Randomized phase II study of lapatinib plus capecitabine or lapatinib plus topotecan for patients with HER2-positive breast cancer brain metastases. J Neurooncol 2011;105:613-20. [Crossref] [PubMed]
- Krop IE, Lin NU, Blackwell K, et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol 2015;26:113-9. [Crossref] [PubMed]
- Freedman RA, Gelman RS, Wefel JS, et al. Translational Breast Cancer Research Consortium (TBCRC) 022: A Phase II Trial of Neratinib for Patients With Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer and Brain Metastases. J Clin Oncol 2016;34:945-52. [Crossref] [PubMed]
- Freedman RA, Gelman RS, Anders CK, et al. TBCRC 022: A Phase II Trial of Neratinib and Capecitabine for Patients With Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer and Brain Metastases. J Clin Oncol 2019;37:1081-9. [Crossref] [PubMed]
- Lehrer EJ, Peterson J, Brown PD, et al. Treatment of brain metastases with stereotactic radiosurgery and immune checkpoint inhibitors: An international meta-analysis of individual patient data. Radiother Oncol 2019;130:104-12. [Crossref] [PubMed]
- Duchnowska R, Dziadziuszko R, Trojanowski T, et al. Conversion of epidermal growth factor receptor 2 and hormone receptor expression in breast cancer metastases to the brain. Breast Cancer Res 2012;14:R119. [Crossref] [PubMed]
- Jeselsohn R, Yelensky R, Buchwalter G, et al. Emergence of constitutively active estrogen receptor-alpha mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin Cancer Res 2014;20:1757-67. [Crossref] [PubMed]
- Chamberlain MC, Baik CS, Gadi VK, et al. Systemic therapy of brain metastases: non-small cell lung cancer, breast cancer, and melanoma. Neuro Oncol 2017;19:i1-i24. [Crossref] [PubMed]