
IL-10 promoter hypomethylation is associated with increased IL-10 expression and poor survival in hepatocellular carcinoma
Introduction
Liver cancer is the fifth most common cancer, the second most common cause of death from cancer worldwide, and is associated with a high recurrence rate. Reports from 2012 estimated 782,000 new cases and 745,000 liver cancer-related deaths, with an annual incidence of 5.6% (1).
Hepatocellular carcinoma (HCC) is the most common form of primary liver cancer (~90% of all cases) and is still associated with a poor survival rate after resection surgery. Many questions remain to be answered regarding HCC pathogenesis, which severely hampers our ability to develop novel and effective therapies for this dreadful disease (2).
The tumor microenvironment, basically comprises tumor, stromal, and immune cells, and is strongly influenced by the local balance of pro-inflammatory and anti-inflammatory cytokines (3). Inflammation plays a critical role in the genesis of HCC (4). Interleukin-10 (IL-10) is a cytokine mainly produced by B and T lymphocytes and macrophages that plays an essential role in limiting pro-inflammatory immune responses by inhibiting the effector function of Th1 cells (5). Deregulation of IL-10 expression is associated with allergic, autoimmune, and infectious disorders, along with cancer (6). Decreased expression of IL-10 typically leads to increased pro-inflammatory cytokine secretion and hinders anti-tumor immunity, thereby favoring tumor growth. As an anti-inflammatory and immunosuppressive cytokine, the IL-10 family of cytokines has vital functions closely related to immune tolerance in the tumor microenvironment, and its deficit can contribute to immune escape of neoplastic cells (7). Several studies have reported elevated IL-10 levels in HCC patients (8). Chan et al. found that hepatic injury in unresectable HCC patients could lead to higher serum IL-10 levels due to cirrhotic processes, rather than tumor load, and suggested the potential prognostic value of circulating IL-10 for these patients (9). Xue et al., on the other hand, proposed that the presence of a large number of IL-10+ B cells, observed in resected HCC samples, was responsible for restraining CD4+ cytotoxic T cell activity (10). Chau et al. found much higher serum levels of IL-10 in HCC patients than in healthy subjects and suggested that pre-surgical serum IL-10 levels are related to postoperative survival (11). A study by Hsia et al., meanwhile, proposed that serum IL-10 could serve as an HCC biomarker to help identify HCC cases with low levels of α-fetoprotein (AFP) (12).
Little is known about the tissue-specific regulation of IL-10 expression in HCC and other cancers. Gene methylation is a prominent epigenetic mechanism that regulates the expression of tumor-related genes in virtually all types of cancer. Previous studies suggested that promoter methylation in cytokine genes contributes to cancer pathogenesis by shaping inflammatory responses in the tumor microenvironment (13). Epigenetic analysis of the promoter of the IL-10 gene in B cells highlighted the importance of methylation and acetylation polymorphisms in inflammatory diseases such as periodontitis (14). Along these lines, hypomethylation of a proximal CpGs site within the IL-10 promoter was correlated with abnormal IL-10 mRNA and serum levels in rheumatoid arthritis patients (15). Another study showed that the methylation status of the IL-10 gene is reduced in patients with systemic lupus erythematosus, and suggested that detection of hypomethylated IL-10 promoter may be a clinical predictor in autoimmune diseases (16). On the other hand, IL-10 promoter methylation was found to be lower in breast cancer specimens compared with healthy breast tissues, leading the study authors to propose that hypomethylation of the IL-10 gene is involved in breast carcinogenesis (17).
Our previous studies analyzed promoter methylation patterns and highlighted the contribution of specific epigenetic events to HCC pathogenesis and progression (18). In the present study, we assessed the methylation status of the IL-10 promoter in matched HCC and non-tumor tissues to test the hypothesis that elevated IL-10 production correlates with hypomethylation of the IL-10 promoter in HCC. Our results indicate that IL-10 gene methylation could play a role in determining IL-10 mRNA expression and might be a useful prognostic indicator of outcome in HCC patients.
Methods
Patients and tissue samples
Matched tumor and surrounding non-tumor tissues were obtained from patients with a histologically confirmed diagnosis of HCC (n=120) that underwent surgical resection between 2007 and 2016 at Changzhou Cancer Hospital in China. The samples were frozen in liquid nitrogen right after surgical resection. Patients in this study were 96 men and 24 women, aged 39 to 85 years old [mean ± standard deviation (SD): 55.32±13.25 years]. We defined HCC classification by the 8th edition of AJCC for HCC [2017] (19). The study proposal and all ethical proceedings were approved by the committee on Human Experimentation of Changzhou Cancer Hospital. Written informed consent using a standardized questionnaire was obtained from all participants.
IL-10 promoter methylation analysis
Genomic DNA was isolated from frozen samples using the QIAamp DNA mini kit (QIAGEN, Hilden, Germany) according to the manufacturer’s protocol. DNA bisulfite modification was carried out with 1 µg of DNA by NaOH (3 M), sodium bisulfite (2.5 M) and hydroquinone (1 M) (18). We detected the IL-10 gene promoter methylation by methylation-specific polymerase chain reaction (MSP) assay. polymerase chain reaction (PCR) amplification with specific primers was performed to distinguish methylated from unmethylated DNA: methylated IL-10, forward primer 5'-GGGATTATAGGTATTTGTTATTATGT-3', reverse primer 5'-AAAAAAATCTACCTCCCTTATCAAA-3'. Unmethylated IL-10, forward primer 5'-GGGATTATAGGTATTTGTTATTACGT-3', reverse primer 5'-AAAAAATCTACCTCCCTTATCGAA-3' (20). PCR was carried out with 2 µL modified DNA samples in a total reaction volume of 50 µL in an Mx3000P thermal cycler (Stratagene, CA, USA) under the following conditions: 95 °C for 35 s, 50 °C for 60 s, and 72 °C for 45 s for 40 cycles, followed by a final 10 min extension. Unmethylated DNA products were 188 bp, and methylated DNA products were 187 bp. MSP products were separated by 2% agarose gel electrophoresis and visualized by Bio-Rad Gel Doc XR+ (Bio-Rad Laboratories, Inc., CA, USA). Gel intensity profile analyses were performed using Quantity One analysis software (Bio-Rad Laboratories, Inc.). Quantitative band comparison between samples was made in the same gel. We calculated the promoter methylation index (MI) of IL-10 gene using the following formula: 100× methylated reaction/(unmethylated reaction + methylated reaction). ΔMI was defined as MIHCC – MINon-tumor (21).
IL-10 mRNA analysis
Total RNA was extracted from HCC and adjacent non-tumor tissues using TRIzol reagent (SBS, China). cDNA was generated using a first-strand cDNA synthesis kit (Invitrogen, China) from 2 µg of total RNA in a final reaction volume of 20 µL (1× PCR buffer, 1 mmol/L dNTPs, 20 units AMV reverse transcriptase, 20 units RNA guard ribonuclease inhibitor, and 2.5 mmol/L random primers). Real-time PCR assays were performed to specifically quantitate the level of IL-10 mRNA transcripts in tissues. The forward primer of IL-10 mRNA for reverse transcription PCR (RT-PCR) reaction was 5'-TCAGGGTGGCGACTCTAT-3', and the reverse primer was 5'-TGGGCTTCTTTCTAAATCGTTC-3' (15). We carried out quantitative PCR (qPCR) in a total volume of 20 µL SYBR Green master mix using the Mx3000P qPCR System. Reaction mixtures for IL-10 were subjected to the following amplification conditions: 35 s at 95 °C, 40 s at 50 °C, and 45 s at 72 °C (40 cycles). β-actin mRNA served as an internal control for qPCR by comparing its average computed tomography (CT) values with those of IL-10 mRNA.
Statistical analysis
Statistical analysis was performed using SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA). Categorical variables were compared using the chi-squared test and Pearson’s correlation coefficient. Survival curves were based on Kaplan-Meier estimates. All P values presented were two-sided, and P<0.05 was regarded as statistically significant.
Results
IL-10 gene methylation status in HCC
The methylation status of the promoter region of the IL-10 gene was analyzed in matched HCC and non-tumor samples. The mean MI was 0.59 (95% CI, 0.55–0.62) in non-tumor samples, and 0.47 (95% CI, 0.43–0.51) in HCC specimens (P<0.0001) (Figure 1). Interestingly, relative to matched non-HCC specimens, 80 HCC cases (66.7%) showed decreased IL-10 promoter methylation (∆MI ≤0), while hypermethylation (∆MI >0) was seen in 40 cases (33.3%). Thus, while hypomethylation occurs in most cases, methylation of the IL-10 promoter appears to follow a biphasic distribution in our study population.

Correlation between IL-10 gene methylation status and clinicopathological features of HCC
HCC clinicopathological parameters and corresponding IL-10 promoter methylation status change (∆MI) are summarized in Table 1. Clinicopathological parameters included gender, age, hepatitis B virus (HBV) history, tumor differentiation, cirrhosis, tobacco, alcohol, lymph node status, metastasis classification (M0 vs. M1), and AFP level. HCC patients were classified according to ∆MI into hypomethylation (∆MI ≤0) and hypermethylation (∆MI >0) groups. While no correlation was observed between most clinicopathological variables and ∆MI, significant associations were detected for ΔMI ≤0 and both metastasis classification (i.e., M1 > M0) and serum AFP levels (P=0.001 and P=0.005, respectively).
Table 1
| Variable | Cases (120) | ∆MI ≤0 (N=80) | ∆MI >0 (N=40) | P* |
|---|---|---|---|---|
| Gender | 0.146 | |||
| Male | 96 | 67 | 29 | |
| Female | 24 | 13 | 11 | |
| Age (y) | 0.320 | |||
| ≥50 | 85 | 59 | 26 | |
| <50 | 35 | 21 | 14 | |
| HBV history | 0.760 | |||
| Yes | 92 | 62 | 30 | |
| No | 28 | 18 | 10 | |
| Differentiation | 0.429 | |||
| G1 | 48 | 30 | 18 | |
| G2-3 | 72 | 50 | 22 | |
| Cirrhosis | 0.781 | |||
| Yes | 82 | 54 | 28 | |
| No | 38 | 26 | 12 | |
| Tobacco | 0.135 | |||
| Yes | 76 | 49 | 37 | |
| No | 44 | 31 | 13 | |
| Alcohol | 0.358 | |||
| Yes | 71 | 45 | 26 | |
| No | 49 | 35 | 14 | |
| Lymph node status | 0.417 | |||
| N0 | 42 | 26 | 16 | |
| N1 | 78 | 54 | 24 | |
| Metastasis classification | 0.001 | |||
| M0 | 53 | 27 | 26 | |
| M1 | 67 | 53 | 14 | |
| AFP (µg/L) | 0.005 | |||
| ≥30 | 82 | 63 | 19 | |
| <30 | 38 | 17 | 21 |
*, statistical significance determined by Pearson’s Chi-squared test. IL-10, interleukin-10; HCC, hepatocellular carcinoma; MI, methylation index; HBV, hepatitis B virus; AFP, α-fetoprotein.
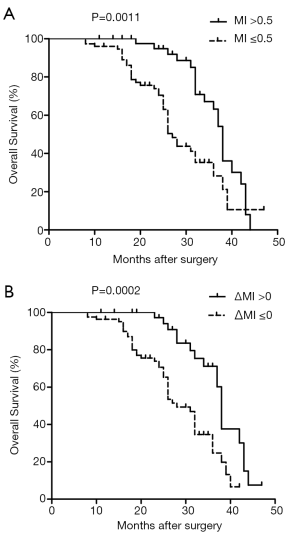
Next, we investigated the possible association between IL-10 gene methylation status and post-surgery survival in HCC patients (Figure 2). We found that there was a significantly shorter survival time (27 months) in HCC patients with IL-10 gene hypomethylation (MI ≤0.5), compared to HCC patients with hypermethylated IL-10 (MI >0.5), for which mean survival was 38 months (log-rank P=0.0011, HR: 0.401, 95% CI: 0.23–0.69). Meanwhile, a median cumulative survival of 28 months was calculated for HCC patients with ∆MI ≤0, compared with 38 months for those with ∆MI >0 (log-rank P=0.0002, HR: 0.347, 95% CI: 0.20–0.60). Our results suggest that IL-10 hypomethylation may serve as a clinical marker of poor prognosis in HCC.
IL-10 mRNA expression in HCC
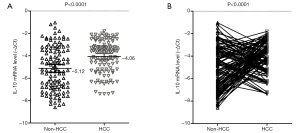
Relative IL-10 mRNA expression was assessed by real-time PCR in the 120 HCC samples and matched non-tumor tissues. We found that IL-10 mRNA levels were significantly higher in tumor samples (mean –∆Ct =–4.06; 95% CI, –4.31 to –3.80) compared with non-tumor specimens (mean –∆Ct =–5.12; 95% CI, –5.46 to –4.77) (P<0.0001; Figure 3). Specifically, IL-10 mRNA expression was elevated (–∆∆Ct >0) in 69 HCC cases (57.5%) and reduced (–∆∆Ct ≤0) in 51 (42.5%).
Correlation between IL-10 mRNA expression and clinicopathologic features of HCC
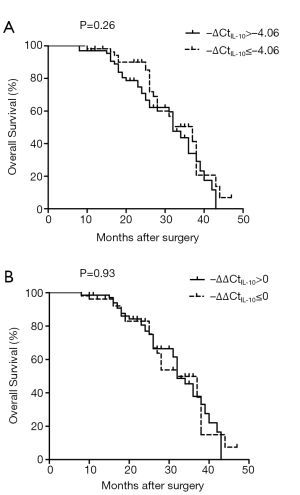
The relationship between IL-10 mRNA expression in the 120 matched HCC samples and clinical factors were examined next (Table 2). There was a significant, positive association between IL-10 mRNA levels and both metastasis classification (M1 > M0) and serum AFP (P<0.002 in both cases). The association between IL-10 mRNA expression and patient survival was assessed by the Kaplan-Meier method and the log-rank test, but no correlation was detected (Figure 4).
Table 2
| Variable | Cases (120) | –∆∆CtIL-10 ≤0 (N=51) | –∆∆CtIL-10 >0 (N=69) | P* |
|---|---|---|---|---|
| Gender | 0.140 | |||
| Male | 96 | 44 | 52 | |
| Female | 24 | 7 | 17 | |
| Age (y) | 0.243 | |||
| ≥50 | 85 | 39 | 46 | |
| <50 | 35 | 12 | 23 | |
| HBV history | 0.407 | |||
| Yes | 92 | 41 | 51 | |
| No | 28 | 10 | 18 | |
| Differentiation | 0.327 | |||
| G1 | 48 | 23 | 25 | |
| G2-3 | 72 | 28 | 44 | |
| Cirrhosis | 0.193 | |||
| Yes | 82 | 37 | 45 | |
| No | 38 | 22 | 16 | |
| Tobacco | 0.156 | |||
| Yes | 76 | 36 | 40 | |
| No | 44 | 15 | 29 | |
| Alcohol | 0.659 | |||
| Yes | 71 | 29 | 42 | |
| No | 49 | 22 | 27 | |
| Lymph node status | 0.405 | |||
| N0 | 42 | 20 | 22 | |
| N1 | 78 | 31 | 47 | |
| Metastasis classification | 0.002 | |||
| M0 | 53 | 31 | 22 | |
| M1 | 67 | 20 | 47 | |
| AFP (µg/L) | 0.002 | |||
| ≥30 | 82 | 27 | 55 | |
| <30 | 38 | 24 | 14 |
*, statistical significance determined by Pearson’s chi-squared test. IL-10, interleukin-10; HCC, hepatocellular carcinoma; MI, methylation index; HBV, hepatitis B virus; AFP, α-fetoprotein.
Elevated IL-10 mRNA is associated with IL-10 promoter hypomethylation in HCC
Methylation of genes within their promoter region is a common mechanism of transcriptional silencing. Accordingly, we found that IL-10 mRNA expression was lower (mean –∆∆Ct =–0.18; 95% CI, –1.04 to 0.68) in HCC cases with IL-10 promoter hypermethylation (∆MI >0), than in those showing hypomethylation of this gene (∆MI ≤0; mean –∆∆Ct =1.68; 95% CI, 1.13–2.22) (P=0.0002; Figure 5). Thus, IL-10 promoter hypomethylation and hypermethylation correlate, respectively, with increased and decreased IL-10 mRNA levels in HCC specimens (R2=0.095, P=0.001 and R2=0.063, P=0.006, respectively). In contrast, no association was detected between IL-10 gene methylation status and IL-10 mRNA expression in non-tumor tissues (R2=0.004, P=0.467). These results indicate that differential methylation of the IL-10 gene promoter could play an important role in determining IL-10 production in HCC. The results of the Cox regression analysis for survival factors in HCC are summarized in Table 3. Multivariate analysis revealed IL-10 MI and mRNA were associated with overall survival (OS) (P=0.009, P=0.014, respectively).
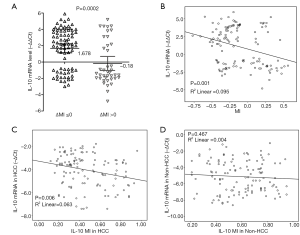
Table 3
| Factor | β | SE | Hazard ratio (95% CI) | P |
|---|---|---|---|---|
| AFP ≥30 µg/L | 0.021 | 0.036 | 1.021 (1.005, 1.084) | 0.032 |
| ∆MI ≤0 | 1.924 | 0.325 | 3.425 (2.126, 10.623) | 0.009 |
| –∆∆CtIL-10 >0 | 1.334 | 0.416 | 2.124 (1.162, 8.712) | 0.014 |
HCC, hepatocellular carcinoma; SE, standard error; CI, confidence interval; AFP, α-fetoprotein; MI, methylation index.
Discussion
Chronic inflammatory liver disease and liver cirrhosis are the main risk factors for the development of HCC, the most common form of primary liver cancer, which is now the second leading cause of cancer-related deaths worldwide (1). Tumor metastasis is the primary cause of death in HCC patients. The tumor microenvironment plays critical roles in hepatocarcinogenesis and is a main determinant of metastatic progression. Inflammatory cytokines promote chronic inflammatory liver disease and, as cancer eventually develops, sustain tumor progression through a complex interplay with both tumor cells and carcinoma-associated immune cells (22,23).
IL-10 is an anti-inflammatory cytokine that is highly expressed in HCC. While IL-10 overexpression correlates with tumor progression and has a predominantly immunosuppressive role, it is not clear whether an association between IL-10 expression and the risk for developing HCC exists (24). Thus, addressing the molecular mechanisms regulating IL-10 expression should lead to a better understanding of the pathophysiological involvement of this cytokine in HCC. The present study shows that hypomethylation of the IL-10 gene promoter correlates with increased IL-10 mRNA expression in clinical HCC samples, and is a predictor of poor outcome. Moreover, the IL-10 MI is related to tumor metastasis classification; i.e., hypomethylation is more prevalent in HCC patients classified as M1 rather than M0 (metastasis-free), and is also inversely related to serum AFP levels, a common diagnostic and prognostic biomarker of HCC. In addition, we detected a significantly shorter survival (27 months) in HCC patients with hypomethylated IL-10 genes compared with patients with IL-10 hypermethylation (38 months). HCC patients with IL-10 gene hypomethylation also had a shorter median cumulative survival of 28 months compared with 38 months for those with hypermethylated IL-10. These results suggest that IL-10 hypomethylation might be a useful prognostic factor in HCC.
Our study found that IL-10 mRNA levels were overall higher in HCC samples than in paired non-tumor specimens, and also correlated with metastatic status and elevated serum AFP. However, a relationship between IL-10 mRNA levels and HCC outcome could not be established. Our previous research investigated IL-10 gene expression in clinical HCC samples and indicated its key role in the pathogenesis of this disease. While DNA methylation exerts widespread control of gene transcription, several other regulatory mechanisms intervene at the mRNA and protein level. Thus, further studies addressing the regulation of IL-10 at the mRNA and protein levels in a more extensive population sampling are warranted to clarify this discrepancy.
The importance of epigenetic regulation of oncogene, tumor suppressor gene, and cytokine expression has received substantial support in recent years, as aberrant DNA promoter-specific hypermethylation or hypomethylation was shown to contribute to the development of human cancers (25,26). In a previous study, we performed a large-scale promoter methylation analysis of tumor-related genes in HCC and normal surrounding tissues and found various types of abnormal methylation patterns in HCC. Specifically, we reported that Line-1 gene hypomethylation was the most common molecular abnormality in HCC, and this event was associated with the overexpression of CD133 mRNA (18).
Hypermethylation of CpG dinucleotides in gene promoter regions is typically associated with transcription silencing, while hypomethylation usually leads to activation of gene expression (27). Accordingly, our study found that hypomethylation of the IL-10 promoter prevailed in HCC patients (66.7%) and correlated with higher IL-10 mRNA levels, while IL-10 mRNA expression was decreased in the remaining HCC patients (33.3%) with hypermethylated IL-10. In future research, it will be of interest to investigate the molecular bases of the biphasic distribution of IL-10 methylation profiles observed in our study; based on the present data, we speculate that IL-10 demethylation may represent a molecular switch indicative of metastatic progression in HCC (28).
In sum, the hypomethylation of the IL-10 gene promoter was significantly correlated with increased IL-10 mRNA expression in clinical HCC samples. But the results in our study have some limitations due to individual differences between tumor patients and data of recurrence cannot be obtained. A more thorough understanding of the mechanisms of epigenetic deregulation of the IL-10 gene and the role its transcript plays in the tumor microenvironment might lead to the development of novel targeted therapies for HCC.
Acknowledgments
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.33). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All of the patients gave written informed consent, and approval was given by the Ethics Committee of Changzhou Cancer Hospital of Soochow University (No. 2016006).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Niu ZS, Niu XJ, Wang WH. Genetic alterations in hepatocellular carcinoma: an update. World J Gastroenterol 2016;22:9069-95. [Crossref] [PubMed]
- Guo L, Zhang Y, Zhang L, et al. MicroRNAs, TGF-β signaling, and the inflammatory microenvironment in cancer. Tumour Biol 2016;37:115-25. [Crossref] [PubMed]
- Capece D, Fischietti M, Verzella D, et al. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int 2013;2013:187204. [Crossref] [PubMed]
- Szalmás A, Bánáti F, Koroknai A, et al. Lineage-specific silencing of human IL-10 gene expression by promoter methylation in cervical cancer cells. Eur J Cancer 2008;44:1030-8. [Crossref] [PubMed]
- Ouyang W, Rutz S, Crellin NK, et al. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 2011;29:71-109. [Crossref] [PubMed]
- Acuner-Ozbabacan ES, Engin BH, Guven-Maiorov E, et al. The structural network of Interleukin-10 and its implications in inflammation and cancer. BMC Genomics 2014;15:S2. [Crossref] [PubMed]
- Budhu A, Wang XW. The role of cytokines in hepatocellular carcinoma. J Leukoc Biol 2006;80:1197-213. [Crossref] [PubMed]
- Chan SL, Mo FK, Wong CS, et al. A study of circulating interleukin 10 in prognostication of unresectable hepatocellular carcinoma. Cancer 2012;118:3984-92. [Crossref] [PubMed]
- Xue H, Lin F, Tan H, et al. Overrepresentation of IL-10-expressing B cells suppresses cytotoxic CD4+ T cell activity in HBV-induced hepatocellular carcinoma. PLoS One 2016;11:e0154815. [Crossref] [PubMed]
- Chau GY, Wu CW, Lui WY, et al. Serum interleukin-10 but not interleukin-6 is related to clinical outcome in patients with resectable hepatocellular carcinoma. Ann Surg 2000;231:552-8. [Crossref] [PubMed]
- Hsia CY, Huo TI, Chiang SY, et al. Evaluation of interleukin-6, interleukin-10 and human hepatocyte growth factor as tumor markers for hepatocellular carcinoma. Eur J Surg Oncol 2007;33:208-12. [Crossref] [PubMed]
- Wilson AG. Epigenetic regulation of gene expression in the inflammatory response and relevance to common diseases. J Periodontol 2008;79:1514-9. [Crossref] [PubMed]
- Larsson L, Thorbert-Mros S, Rymo L, et al. Influence of epigenetic modifications of the interleukin-10 promoter on IL10 gene expression. Eur J Oral Sci 2012;120:14-20. [Crossref] [PubMed]
- Fu LH, Ma CL, Cong B, et al. Hypomethylation of proximal CpG motif of interleukin-10 promoter regulates its expression in human rheumatoid arthritis. Acta Pharmacol Sin 2011;32:1373-80. [Crossref] [PubMed]
- Lin SY, Hsieh SC, Lin YC, et al. A whole genome methylation analysis of systemic lupus erythematosus: hypomethylation of the IL10 and IL1R2 promoters is associated with disease activity. Genes Immun 2012;13:214-20. [Crossref] [PubMed]
- Son KS, Kang HS, Kim SJ, et al. Hypomethylation of the interleukin-10 gene in breast cancer tissues. Breast 2010;19:484-8. [Crossref] [PubMed]
- Zhang C, Xu Y, Zhao J, et al. Elevated expression of the stem cell marker CD133 associated with line-1 demethylation in hepatocellular carcinoma. Ann Surg Oncol 2011;18:2373-80. [Crossref] [PubMed]
- Kamarajah SK, Frankel TL, Sonnenday C, et al. Critical evaluation of the American joint commission on cancer (AJCC) 8th edition staging system for patients with hepatocellular carcinoma (HCC): a surveillance, epidemiology, end results (SEER) analysis. J Surg Oncol 2018;117:644-50.
- Fu LH, Cong B, Zhen YF, et al. Methylation status of the IL-10 gene promoter in the peripheral blood mononuclear cells of rheumatoid arthritis patients. Yi Chuan 2007;29:1357-61. [Crossref] [PubMed]
- Ling Y, Zhu J, Gao L, et al. The silence of MUC2 mRNA induced by promoter hypermethylation associated with HBV in hepatocellular carcinoma. BMC Med Genet 2013;14:14. [Crossref] [PubMed]
- Yang JD, Nakamura I, Roberts LR. The tumor microenvironment in hepatocellular carcinoma: current status and therapeutic targets. Semin Cancer Biol 2011;21:35-43. [Crossref] [PubMed]
- Wang H, Chen L. Tumor microenviroment and hepatocellular carcinoma metastasis. J Gastroenterol Hepatol 2013;28:43-8. [Crossref] [PubMed]
- Beckebaum S, Zhang X, Chen X, et al. Increased levels of interleukin-10 in serum from patients with hepatocellular carcinoma correlate with profound numerical deficiencies and immature phenotype of circulating dendritic cell subsets. Clin Cancer Res 2004;10:7260-9. [Crossref] [PubMed]
- Sato T, Issa JJ, Kropf P. DNA hypomethylating drugs in cancer therapy. Cold Spring Harb Perspect Med 2017;7: [Crossref] [PubMed]
- Perri F, Longo F, Giuliano M, et al. Epigenetic control of gene expression: Potential implications for cancer treatment. Crit Rev Oncol Hematol 2017;111:166-72. [Crossref] [PubMed]
- Kulis M, Heath S, Bibikova M, et al. Epigenomic analysis detects widespread gene-body DNA hypomethylation in chronic lymphocytic leukemia. Nat Genet 2012;44:1236-42. [Crossref] [PubMed]
- Oft M. IL-10: master switch from tumor-promoting inflammation to antitumor immunity. Cancer Immunol Res 2014;2:194-9. [Crossref] [PubMed]


