
IL-35 inhibits CD8+ T cells activity by suppressing expression of costimulatory molecule CD28 and Th1 cytokine production
Introduction
As a novel cytokine, interleukin-35 (IL-35) exerts immune-suppressing effects (1). It comprised of two subunits, including the Epstein-Barr virus-induced gene 3 (EBI3) and IL-12p35 (2). It has been illustrated that secretion of IL-35 is exerted by Foxp3+CD4+CD25+ Tregs or a Foxp3− Tregs population (2,3). Some studies found that IL-35 inhibits the activity of Th1, Th2 and Th17, and expands Tregs (3,4). The deficiency of IL-35 can significantly reduce the regulatory activity of Tregs, resulting failure to alleviate inflammatory bowel disease in vivo (2). However, the effect of IL-35 in malignant disease is not investigated in detail.
Recently, reports have shown that IL-35 is highly expressed in serum of patients and tumor tissues with prostate cancer, breast cancer and non-small cell lung cancer (NSCLC) (5-7). Our previous study has demonstrated that IL-35 expression was obviously up-regulated in the tumor tissues of laryngeal squamous cell carcinoma (LSCC) compared with the adjacent normal tissues, while IL-35 expression was significantly reduced in patients’ peripheral blood after surgical resection (8). It has been illuminated that immune system involves in regulating progression of tumorigenesis (9). Among this system, the suppressive activities of regulatory T cells (Tregs) occur against tumor-specific T-cell responses (9,10). Furthermore, IL-35 can promote tumor angiogenesis and inhibit anti-tumor cytotoxic lymphocyte (CTL) response (7,11). In our previous study, we also found limited activity of anti-tumor CTL in a mice model with head and neck squamous cell carcinoma (12). However, there is little evidence about how IL-35 affects anti-tumor CTL response. Here, we aimed to investigate the potential mechanism of the function of IL-35 on anti-tumor CTL.
Methods
Cell line
The SCC VII cells which are a spontaneously arising HNSCC of C3H mice were kindly gifted by Prof. Shi-Xi Liu (Department of Otolaryngology, West China Hospital of Medical College of Sichuan University), and cultured in RPMI-1640 complete medium (Gibico, USA), which supplemented with 10% fetal bovine serum (FBS) (Gibco, USA) as well as 1% penicillin/streptomycin (Invitrogen, USA).
Mice
Male C3H mice, 6 to 8 weeks of age, were obtained from Zhejiang Chinese Medical University Laboratory Animal Research Center (Hangzhou, China).
Reagents
Purified recombinant murine granulocyte-macrophage colony-stimulating factor (rmGM-CSF) and IL-35 was provided by R&D Systems (Minneapolis, Minn., USA). PE-anti-CD28, PE-anti-FasL, FITC-anti-LFA-1 and APC-anti-CD2 antibody were obtained from Miltenyi Biotec (Germany). Anti-caspase 3/cleaved caspase 3 antibodies, anti-NF-κB p65 antibody and anti-β-actin were purchased from CST (USA).
Generation of murine bone marrow (BM)-derived dendritic cell (DC)
BM cells were harvested with a syringe and a 26-gauge needle from the tibias and femurs, which were obtained via cervical dislocation of the sacrificed mice under sterile conditions, followed by lysing the erythrocytes with the RBC lysis buffer and cultured the cells in RPMI-1640 complete medium containing rmGM-CSF (20 ng/mL), which was replaced with new medium every 2 days. At day 6, the cells were collected as DC for further use of the experiments.
Spleen-derived T cell
The spleen was teased to obtained the suspensions of the spleen cell (SC), followed by lysing the erythrocytes using RBC lysis buffer. After centrifuging for 5 min at 200 ×g, the SCs were placed in RPMI-1640 complete culture medium, which were used for CD8+ T cells isolation with magnetic CD8a (Ly-2) microbeads according to the instruction of the manufacturer (Miltenyi Biotec, Germany). The purity (>90%) of CD8+ T cell was assayed with staining of CD8a antibody (Miltenyi Biotec, Germany), followed by analyzing with a Becton-Dickinson FACS Calibur flow cytometer. A hemocytometer was used to count the purified CD8+ T cells and trypan blue was used to assess the cells viability, which exceeded 90%.
Flow cytometry
BM or SC (1×106/tube) were incubated with optimal concentrations of Abs for 30 min. Three controls were used: cell control, the cells were unstained; conjugate control, the procedur omitted the first step; mAb control, the conjugate antibody was replaced with an isotype-matched control mAb. The analysis was performed on the FACScan (Becton Dickinson, CA, USA). This experiment was performed on ice during the procedure.
Carboxyfluorescein Succinimidyl Ester (CFSE) staining
CD8+ T cells (2×106) were incubated in 1 mL PBS containing 1 µL CFSE (final concentration 2.5 µM) (Cell-Trace CFSE Proliferation Kit, Molecular Probes, Invitrogen, USA) for 15 min in the darkness, pipetting up and down every 5 min. After stopping the reaction with 2 mL PBS with 5% FCS, the cells were centrifugated and washed for 3 times. The stained cells were then placed in Transwell plates with SCC VII activated DCs for 5 d. The analysis was performed on a Becton FACScan (Mountain View, CA, USA).
Cytokine enzyme linked immunosorbent assay (ELISA)
Supernatant collected from the in vitro cultures was analyzed for IL-1β, IL-6, IL-10, IL-12p70, IFN-γ, IL-4 and TNF-α according to the manufacturer’s instruction (eBioscience).
Western blotting
The treated cells were lysed on ice using RIPA lysis buffer (Beyotime, Guangzhou, China) containing inhibitors of protease and phosphatase. BCA methods was used to quantify the concentrations of the proteins. After adding a quarter volume of 5× SDS-PAGE Sample Loading Buffer, the protein samples were boiled for 10 min and separated on an SDS-polyacrylamide gel using electrophoresis at 250 mA for 2.5 h, followed by transferring to polyvinylidene fluoride (PVDF) membrane (Millipore, Darmstadt, Germany). The membrane was then blocked with 3% skimmed milk for 1 h, incubated with a primary antibody at room temperature for 1 h, washed with TBST for 3 times. After incubating with a horseradish peroxidase-conjugated secondary antibody (1:3,000 dilution) for 1 h, the membrane was visualized with an ECL kit.
Statistical analysis
Data were processed as mean ± standard deviation (SD). For comparison between groups, the Student’s t-test was performed and the statistical difference was seen as significant when P≤0.05.
Results
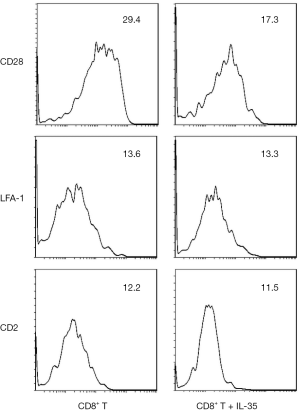
IL-35 does inhibit the expression of costimulatory molecule CD28 on CD8+ T cell surface
To investigate the impact of IL-35 on the expression of surface molecules on CD8+ T cells, the CD8+ T cells was purified from C3H SCs and cultured them with IL-35. The expression of costimulatory molecule CD28, adhesion molecules LFA-1 and CD2 on CD8+ T cell surface were detected by flow cytometry. We found that the expression of CD28 was significantly decreased on CD8+ T cells cultured with IL-35 compared with those cultured without IL-35. However, no statistical difference of the expression of LFA-1 or CD2 was observed between two groups (Figure 1).
IL-35 does not directly inhibit anti-tumor CTL proliferation nor enhance the expression of apoptosis-related proteins of anti-tumor CTL
After being incubated with SCC VII cells, pulsed-DCs were used to activate CD8+ cytotoxic T cells by co-culturing with or without IL-35 for 5 days. The CFSE-labelled activated CTL was processed by flow cytometry, the results of which showed no statistical differences with or without IL-35 (data not show).
To determine if IL-35 enhances the expression of apoptosis-related proteins of anti-tumor CTL, the activated CD8+ cytotoxic T cells cultured with IL-35 were harvested and the protein levels of nuclear transcription factor NF-kappa B (NF-κB) and apoptotic protein caspase-3 were determined by Western blot. As a result, no statistical differences were detected in the expression of both NF-κB and caspase-3 of CTL with or without IL-35 (Figures 2,3).
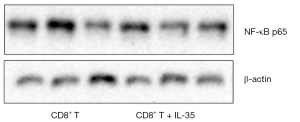
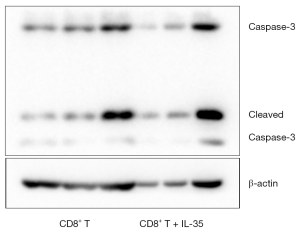
IL-35 does suppress Th1 cytokine production
To determine whether IL-35 affects Th1 or Th2 cytokine production, we harvested the culture supernatant of activated CD8+ cytotoxic T cells cultured with or without IL-35. The production of cytokines IL-1β, IL-6, IL-10, IL-12p70, IFN-γ, IL-4 and TNF-α were analyzed by ELISA. As shown in Table 1, we found that all cytokines production was decreased in presence of IL-35. While the production of IL-1β and IL-12p70 reached a statistically significant reduction. It indicated that IL-35 can inhibit Th1 cytokine production and do not promote Th2 cytokine production.
Table 1
| Cytokines | PBS | CD8+T | CD8+T + IL-35 | t | P |
|---|---|---|---|---|---|
| IL-1β | 0.021±0.002 | 3.678±0.541 | 2.730±0.173 | −2.220 | 0.037 |
| IL-6 | 0.031±0.004 | 25.337±7.113 | 11.687±1.598 | 1.872 | 0.077 |
| IL-10 | 0.013±0.001 | 25.193±6.271 | 7.143±1.508 | 1.633 | 0.117 |
| IL-12p70 | 0.041±0.003 | 1357.776±200.494 | 1006.806±64.37 | −2.220 | 0.037 |
| IFN-γ | 0.022±0.002 | 230.796±52.678 | 117.833±60.951 | 1.147 | 0.264 |
| IL-4 | 0.009±0.001 | 16.324±3.477 | 16.204±2.197 | −0.051 | 0.962 |
| TNF-α | 0.016±0.002 | 33.324±4.17 | 31.537±5.011 | −0.475 | 0.660 |
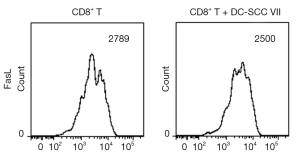
IL-35 does not directly inhibit the expression of Fas ligand (FasL) on CTL surface
To determine if IL-35 inhibits the expression of FasL on CTL surface, we harvested the activated CD8+ cytotoxic T cells and analyzed the FasL expression by flow cytometry. As shown in Figure 4, in presence of IL-35, the expression of FasL on CTL surface did not decrease significantly.
Discussion
IL-35 has been considered as new member of the IL-12 family (2). It has been illustrated that IL-35 inhibits T cells proliferation and transforms T cells into suppressive inducible Tregs (3). Recently, some studies have demonstrated the expression of IL-35 is correlated with tumor progression, including breast cancer, NSCLC and prostate cancer (5,6,13). Moreover, our previous study found that IL-35 is expressed highly in the peripheral blood and tumor tissues of patients with LSCC (8). Turnis et al. using an IL-35 reporter mouse find that the tumor growth was suppressed in the condition of IL-35 specific antibody mediated neutralization or Treg cell-restricted deletion of IL-35 production of various murine models of human cancer (14). These findings revealed that IL-35 exerted its effects in suppressing anti-tumor immunity. However, the suppressive mechanism of IL-35 on anti-tumor immune responses is very limited.
Here, the effect of IL-35 on anti-tumor CTL was investigated. CTLs are considered as critical effectors of anti-tumor immune responses (15). In previous study, we elicited anti-LSCC CTLs were proved to have a strong anti-LSCC effect in vitro. But, in vivo, the activity of anti-LSCC immunotherapy is limited (12). Based on previous findings, we hypothesized that IL-35 could inhibit anti-tumor CTL responses. To test this hypothesis, we first explored the effect of IL-35 on the surface molecular expression of naïve CD8+ T cells. We observed that IL-35 decreased the costimulatory molecule CD28 but not adhesion molecules. As a key T cell costimulatory molecule, CD28 binds B7 molecules on the surface of professional APC (16,17). Professional APCs, such as DCs, upregulate B7 molecules upon pathogen uptake and can thus efficiently induce responses in CD4+ and CD8+ T cells that harbor the primary costimulatory receptor CD28 (18,19). It has been found that CD28− T cells lack CD28/B7 costimulatory pathway, which may contribute to functional impairment and a low proliferative capacity of this T cell subset (20). Our findings indicated that IL-35 may inhibit the differentiation of naïve CD8+ T cells into anti-tumor CTL by decreasing the costimulatory CD28, which was contrary to other IL-12 family cytokines such as IL-12, which can upregulate CD28 expression (21).
The effects of IL-35 were further investigated on anti-tumor CTL proliferation and apoptosis. As a result, we did not observe that IL-35 could directly inhibit CTL proliferation. Moreover, IL-35 did not impact on the expression of apoptosis-related proteins NF-κB and caspase-3 in anti-tumor CTL. NF-κB is an apoptosis inhibitor, which plays a critical role in the anti-apoptosis regulation of tumors (22). Caspase-3 is the effector of the caspase enzyme system (23). Furthermore, multiple cell apoptosis elements exert their function via the caspase enzyme system (24). Wang et al. also demonstrated IL-35 cannot suppress tumor antigen specific CTL proliferation directly. But they did not report the impact of IL-35 on the apoptosis of the anti-tumor CTL (11). Shao et al. investigated IL-35 function in infection of chronic hepatitis B virus and found IL-35 elevated apoptosis in peripheral blood mononuclear cells (25). While the study by van Bergen et al. illustrated that IL-12-induced suppression of CD8+ CTL clones did not result in apoptosis (26).
We further demonstrated that IL-35 significantly inhibits Th1 cytokine production. Th1 cells are one of the two main Th cells and Th1 differentiation is IL-12 dependent (27). The main effector cells of Th1 immunity are CD8+ T cells. It has been found that Th1 cells activate anti-tumor cytotoxic CD8+ T cells by activation the APCs which activate the CTL via antigen presentation (28). Th2 suppresses Th1 differentiation and function of DCs (29). The polarization of Th2 is associated with tumor proliferation (30). Thus, the reduction of Th1 cytokine production may partly inhibits activation of anti-tumor CTLs. Otherwise, CD28/B7 pathway is crucial for activation of Th1 lymphocytes (31). In this study, we found IL-35 inhibited CD28 expression on naïve CD8+ T cells, which consequently also inhibited activation of Th1 cells and anti-tumor CTLs. Similar to our findings, recent study illustrated IL-35 suppresses the activity of Th1 (3). Zhang et al. reported that IL-35 inhibits Th1 cytokine secretion under normal conditions in mice (32). Moreover, Choi et al. identified that IL-12p35 could inhibit the expansion of Th1 cells (33).
Finally, we found that IL-35 does not suppress the level of FasL on CTL surface. FasL, belonging to the tumor necrosis factor family, can bind to its receptor Fas, thus leading the apoptosis induction of Fas-expressing cells (34). FasL, predominantly expressed in activated T cells, can down-regulate T cell-mediated cytotoxicity (34). It has been revealed that FasL-dependent CTL effector pathway is vital for optimal tumor regression (35). Our findings indicated that IL-35 failed to directly suppress tumor antigen-specific CTL effector function that mediated by FasL-Fas pathway. Other study also illustrated that IL-35 does not suppress CTL cytotoxicity directly (11). But IL-35 mediated expression of FasL on anti-tumor CTL surface was not reported in their study.
Conclusions
Taken together, our investigation revealed that IL-35 did not directly suppress anti-tumor of CTL or promoted anti-tumor apoptosis of CTL, and not suppress tumor antigen-specific CTL effector function. But, IL-35 may suppress the differentiation of naïve CD8+ T cells into differentiate into anti-tumor CTL by decreasing the costimulatory molecule CD28 expression on naïve CD8+ T cell surface, and inhibit anti-tumor CTL activation by inhibiting activation of Th1 cells which may partly be attributed to CD28 expression decreasing. These results may help to better understand the function of IL-35 in tumor immune escape.
Acknowledgments
Funding: This work was supported by grants from
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.30). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Banchereau J, Pascual V, O'Garra A. From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nat Immunol 2012;13:925-31. [Crossref] [PubMed]
- Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 2007;450:566-9. [Crossref] [PubMed]
- Collison LW, Chaturvedi V, Henderson AL, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol 2010;11:1093-101. [Crossref] [PubMed]
- Niedbala W, Wei XQ, Cai B, et al. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol 2007;37:3021-9. [Crossref] [PubMed]
- Hao S, Chen X, Wang F, et al. Breast cancer cells-derived IL-35 promotes tumor progression via induction of IL-35-producing induced regulatory T cells. Carcinogenesis 2018;39:1488-96. [Crossref] [PubMed]
- Chatrabnous N, Ghaderi A, Ariafar A, et al. Serum concentration of interleukin-35 and its association with tumor stages and FOXP3 gene polymorphism in patients with prostate cancer. Cytokine 2019;113:221-7. [Crossref] [PubMed]
- Wang HM, Zhang XH, Feng MM, et al. Interleukin-35 Suppresses the Antitumor Activity of T Cells in Patients with Non-Small Cell Lung Cancer. Cell Physiol Biochem 2018;47:2407-19. [Crossref] [PubMed]
- Wu W, Jiang H, Li Y, et al. IL-35 expression is increased in laryngeal squamous cell carcinoma and in the peripheral blood of patients. Oncol Lett 2017;13:3303-8. [Crossref] [PubMed]
- Alhamarneh O, Amarnath SM, Stafford ND, et al. Regulatory T cells: what role do they play in antitumor immunity in patients with head and neck cancer? Head Neck 2008;30:251-61. [Crossref] [PubMed]
- Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 2006;6:295-307. [Crossref] [PubMed]
- Wang Z, Liu JQ, Liu Z, et al. Tumor-derived IL-35 promotes tumor growth by enhancing myeloid cell accumulation and angiogenesis. J Immunol 2013;190:2415-23. [Crossref] [PubMed]
- Yang BB, Jiang H, Chen J, et al. Dendritic cells pulsed with GST-EGFR fusion protein: effect in antitumor immunity against head and neck squamous cell carcinoma. Head Neck 2010;32:626-35. [PubMed]
- Sun M, Zheng X, Meng Q, et al. Interleukin-35 Expression in Non-Small Cell Lung Cancer is Associated with Tumor Progression. Cell Physiol Biochem 2018;51:1839-51. [Crossref] [PubMed]
- Turnis ME, Sawant DV, Szymczak-Workman AL, et al. Interleukin-35 Limits Anti-Tumor Immunity. Immunity 2016;44:316-29. [Crossref] [PubMed]
- Melief CJ. Tumor eradication by adoptive transfer of cytotoxic T lymphocytes. Adv Cancer Res 1992;58:143-75. [Crossref] [PubMed]
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol 2005;23:515-48. [Crossref] [PubMed]
- Linsley PS, Brady W, Grosmaire L, et al. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med 1991;173:721-30. [Crossref] [PubMed]
- Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol 2002;2:116-26. [Crossref] [PubMed]
- Harding FA, McArthur JG, Gross JA, et al. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature 1992;356:607-9. [Crossref] [PubMed]
- Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1). Blood 2002;100:3698-3702. [Crossref] [PubMed]
- Warrington KJ, Vallejo AN, Weyand CM, et al. CD28 loss in senescent CD4+ T cells: reversal by interleukin-12 stimulation. Blood 2003;101:3543-9. [Crossref] [PubMed]
- Ohshima K, Sugihara M, Haraoka S, et al. Possible immortalization of Hodgkin and Reed-Sternberg cells: telomerase expression, lengthening of telomere, and inhibition of apoptosis by NF-kappaB expression. Leuk Lymphoma 2001;41:367-76. [Crossref] [PubMed]
- Fernandes-Alnemri T, Litwack G, Alnemri ES. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem 1994;269:30761-4. [PubMed]
- Yang LQ, Fang DC, Wang RQ, et al. Effect of NF-kappaB, survivin, Bcl-2 and Caspase3 on apoptosis of gastric cancer cells induced by tumor necrosis factor related apoptosis inducing ligand. World J Gastroenterol 2004;10:22-5. [Crossref] [PubMed]
- Shao X, Ma J, Jia S, et al. Interleukin-35 Suppresses Antiviral Immune Response in Chronic Hepatitis B Virus Infection. Front Cell Infect Microbiol 2017;7:472. [Crossref] [PubMed]
- van Bergen CA, Smit WM, van Sluijters DA, et al. Interleukin-10, interleukin-12, and tumor necrosis factor-alpha differentially influence the proliferation of human CD8+ and CD4+ T-cell clones. Ann Hematol 1996;72:245-252. [Crossref] [PubMed]
- Akdis M, Burgler S, Crameri R, et al. Interleukins, from 1 to 37, and interferon-γ: receptors, functions, and roles in diseases. J Allergy Clin Immunol 2011;127:701-21.e1. [Crossref] [PubMed]
- Mailliard RB, Egawa S, Cai Q, et al. Complementary dendritic cell-activating function of CD8+ and CD4+ T cells: helper role of CD8+ T cells in the development of T helper type 1 responses. J Exp Med 2002;195:473-83. [Crossref] [PubMed]
- Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood 2008;112:1557-1569. [Crossref] [PubMed]
- Kim HJ, Cantor H. CD4 T-cell subsets and tumor immunity: the helpful and the not-so-helpful. Cancer Immunol Res 2014;2:91-8. [Crossref] [PubMed]
- Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol 2009;27:393-422. [Crossref] [PubMed]
- Zhang X, Zhang Z, He Z, et al. Interleukin 35 induced Th2 and Tregs bias under normal conditions in mice. PeerJ 2018;6:e5638. [Crossref] [PubMed]
- Choi JK, Dambuza IM, He C, et al. IL-12p35 Inhibits Neuroinflammation and Ameliorates Autoimmune Encephalomyelitis. Front Immunol 2017;8:1258. [Crossref] [PubMed]
- Nagata S, Golstein P. The fas death factor. Science 1995;267:1449-1456. [Crossref] [PubMed]
- Caldwell SA, Ryan MH, McDuffie E, et al. The Fas/Fas ligand pathway is important for optimal tumor regression in a mouse model of CTL adoptive immunotherapy of experimental CMS4 lung metastases. J Immunol 2003;171:2402-12. [Crossref] [PubMed]


