
Prognostic values of platelet-associated indicators in advanced breast cancer
Introduction
Breast cancer is the leading cause of cancer death among women (11.6% of the total cancer deaths). Approximately 2.1 million breast cancer patients will be newly diagnosed in 2018 (1). Advanced breast cancer (ABC) which includes locally advanced breast cancer (LABC) and metastatic breast cancer, is a serious disease with a 25% 5-year survival rate (2). LABC is defined as a type of ABC whose primary lesion is more than 5 cm in diameter, mostly associated with regional lymph node metastasis, and without overt evidence of distant metastatic disease (3). With LABC, locoregional recurrence and distant metastasis are extremely likely, so early diagnosis and access to therapy are critical (4). Metastatic breast cancer is an incurable disease with a very poor prognosis in the presence of distant metastases (5).
Platelet-related indicators include platelet count (PLT), plateletcrit (PCT), mean platelet volume (MPV), platelet distribution width (PDW), and platelet to lymphocyte ratio (PLR). Some reports have confirmed that platelets act in a positive role for tumor angiogenesis and metastatic dissemination (6,7). In recent reports, platelet-related indicators have been connected with the prognosis of some tumors, such as rectal cancer and pancreatic cancer (8,9). It has been confirmed that platelet contributes to breast cancer progression via cellular transformation, proliferation, or angiogenesis, and high PLT has been associated with poor prognosis of breast cancer (10). Platelet-related indicators representing the size, number, status, and activity of platelets were also proven to be related to a variety of tumors (6). PCT value, which is obtained by multiplying PLT by MPV, provides platelet activation and displays platelet status (11). MPV, an indicator representing platelet activation and the quantity of the average size of platelets, is related to poor prognosis of some malignant tumors (8,12,13). PDW describes the average change in platelet volume, and an elevated PLT will influence the value of PDW (14). PLR, which is obtained by dividing the PLT by the number of lymphocytes, is also associated with poor prognosis in breast cancer patients (15).
In this present study, we investigated whether platelet-related indicators could provide potential significant prognostic information for ABC patients.
Methods
Subjects and inclusion criteria
The study investigated the ABC patients that had been treated in the First Affiliated Hospital of Soochow University (Jiangsu, China) between November 2007 and July 2016. As a retrospective investigation, this study was approved by the Medical Ethics Committees of the First Affiliated Hospital of Soochow University. All the patients selected in the study had their clinical and pathological records inspected regularly. The inclusion criteria were as follows: (I) those with histologically or cytologically confirmed unresectable locally advanced or metastatic breast cancer; (II) age 18–70 years; (III) Karnofsky performance status score of ≥70; (IV) those with a predicted survival of ≥6 months; (V) those with either naive to anti-tumor treatment or with a postoperative adjuvant chemotherapy being performed ≥6 months after the last dose of chemotherapy; and (VI) those who met the laboratory criteria of white blood cell counts (WBCs) ≥3.5×109/L, absolute NE count ≥1.8×109/L, and PLT ≥100×109/L. Clinical and pathological records of all the patients participating in the study were reviewed periodically, the first follow-up was 3 months following adjuvant chemotherapy, and the last follow-up was conducted in July 2018.
In total, 94 breast cancer patients were recruited in this study. Patients had histologic or cytologic evidence of locally advanced or metastasis of the breast. Adjuvant chemotherapy for HER2-Negative ABC patients, which includes agents like anthracyclines or taxanes, is preferred to single-agent therapy. Anthracyclines, such as doxorubicin, are administered intravenously at a dose of 60–70 mg/m2 on day 1, cycled every 3 weeks. Taxanes, such as paclitaxel, are administered intravenously at a dose of 175 mg/m2 on day 1, cycled every 3 weeks. Adjuvant chemotherapy for HER2-Postive ABC patients is preferred to pertuzumab, trastuzumab, and docetaxel/paclitaxel combination regimens. Pertuzumab is administered intravenously at a dose of 840 mg on day 1, followed by 420 mg every next day, cycled every 3 weeks. Trastuzumab is administered intravenously at a dose of 8 mg/kg on day 1 followed by 6 mg/kg, cycled every 3 weeks. Docetaxel is administered intravenously at a dose of 75–100 mg/m2 on day 1, cycled every 3 weeks. Paclitaxel is administered intravenously at a dose of 175 mg/m2 on day 1, cycled every 3 weeks. Patient characteristics are detailed in Table 1. The median age of the 94 patients was 62 years (range, 26–70 years); the median BMI (kg/m2) of the 94 patients was 22.6 (range, 20.6–24.7) kg/m2; 62 patients had bone metastases; all patients were female. The prognostic analyses were performed in regards to overall survival (OS).
Table 1
| Clinicopathologic features | N | PLT | PCT | MPV | PDW | PLR | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (n) | High (n) | χ2 | P value | Low (n) | High (n) | χ2 | P value | Low (n) | High (n) | χ2 | P value | Low (n) | High (n) | χ2 | P value | Low (n) | High (n) | χ2 | P value | ||||||
| Gender | – | – | – | – | – | – | – | – | – | – | |||||||||||||||
| Male | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||
| Female | 94 | 47 | 47 | 48 | 46 | 49 | 45 | 48 | 46 | 47 | 47 | ||||||||||||||
| Age (years) | 2.725 | 0.099 | 9.556 | 0.002** | 4.229 | 0.040* | 0.378 | 0.539 | 4.257 | 0.039* | |||||||||||||||
| ≤62 | 48 | 28 | 20 | 32 | 16 | 30 | 18 | 26 | 22 | 19 | 29 | ||||||||||||||
| >62 | 46 | 19 | 27 | 16 | 30 | 19 | 27 | 22 | 24 | 28 | 18 | ||||||||||||||
| BMI (kg/m2) | 0.043 | 0.837 | 0.170 | 0.680 | 0.043 | 0.836 | 0 | 1 | 0.383 | 0.536 | |||||||||||||||
| ≤22.6 | 47 | 23 | 24 | 25 | 22 | 25 | 22 | 24 | 23 | 22 | 25 | ||||||||||||||
| >22.6 | 47 | 24 | 23 | 23 | 24 | 24 | 23 | 24 | 23 | 25 | 22 | ||||||||||||||
| Bone metastases | 0.190 | 0.663 | 1.039 | 0.308 | 1.021 | 0.312 | 2.539 | 0.111 | 0.758 | 0.384 | |||||||||||||||
| Yes | 62 | 32 | 30 | 34 | 28 | 30 | 32 | 28 | 34 | 29 | 33 | ||||||||||||||
| No | 32 | 15 | 17 | 14 | 18 | 19 | 13 | 20 | 12 | 18 | 14 | ||||||||||||||
*P<0.05; **P<0.01. PLT, platelet count; PCT, plateletcrit; MPV, mean platelet volume; PDW, platelet distribution width; PLR, platelet to lymphocyte ratio.
Blood samples
PLT-related indicators were analyzed within 30 min after collection using a hematology analyzer (Sysmex XE-2100; Sysmex, Kobe, Japan). The patients were divided into two groups according to the median value of PLT (low PLT, ≤179.500×109/L or high PLT, >179.500×109/L), PCT (low PCT, ≤0.180 L/L or high PCT, >0.180 L/L), MPV (low MPV, ≤10.400 fl or high MPV, >10.400 fl), PDW (low PDW, ≤15.680% or high PDW, >15.680%), or PLR (low PLR, ≤132.711 or high PLR, >132.711). The post-/pre-chemotherapy ratios were defined as the rate of pre-chemotherapy PLT-related indicators values and the corresponding rates obtained after chemotherapy.
Evaluation
Response for treatment of patients was assessed every 2 months by computed tomography (CT), and the related results were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 (16).
Follow-up
Partial response (PR), stable disease (SD), and progressive disease (PD) were recorded to infer different responses to chemotherapy. After first-line chemotherapy, PR was defined as lack of response to chemotherapy. In contrast, SD or PR was defined as a response to chemotherapy. Survival time generally means OS in oncology, which was measured from the date of diagnosis until death or last clinical evaluation. OS, which represents the time from the diagnosed date to death for any cause, was used as a prognostic analysis.
Statistical analysis
All statistical analyses were performed using SPSS 19.0 software (Chicago, IL, USA). The associations between blood parameter status and clinicopathologic features or chemotherapeutic efficacy were explored and assessed by the χ2 tests. For analysis of survival data, Kaplan-Meier curves were constructed, and statistical analysis was carried out using the log-rank test. A multivariate logistic regression model was employed to identify the independent risk factors associated with breast cancer. Numerical data are presented as the mean ± standard error. All values of P<0.05 were considered statistically significant.
Results
Baseline PCT level was correlated with outcomes of breast cancer patients
The Kaplan-Meier plots were used to determine the effect of PLT, PCT, MPV, PDW, and PLR status on OS (Figure 1). The median OS of the high baseline PLT group was 22 (95% CI, 11.924–32.076) months, while that of the low PLT group was 32 (95% CI, 25.283–38.717) months (P=0.257). The median OS of the high PCT group was 24 (95% CI, 16.411–31.589) months, while that of the low PCT group was 35 (95% CI, 27.079–42.921) months (P=0.040). The median OS of the high MPV group was 36 (95% CI, 28.129–43.871) months, while that of the low MPV group was 26 (95% CI, 15.045–36.955) months (P=0.869). The median OS of the high PDW group was 29 (95% CI, 20.454–37.546) months, while that of the low PDW group was 35 (95% CI, 18.026–51.974) months (P=0.146). The median OS of the high PLR group was 21 (95% CI, 16.970–25.030) months, while that of the low PLR group was 35 (95% CI, 28.283–41.717) months (P=0.292). Thus, patients who had a lower baseline PCT level were correlated with better OS. Whereas, baseline levels of PLT, MPV, PDW, or PLR had no significant effects on OS.
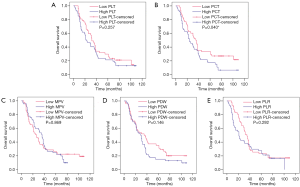
Relationship between baseline platelet-related indicator status and chemotherapeutic efficacy in breast cancer patients
To determine the association between baseline PLT, PCT, MPV, PDW, and PLR status and chemotherapeutic efficacy, the blood samples were obtained before chemotherapy, and CT evaluation was performed simultaneously after first-line chemotherapy. The relationships between baseline PLT-associated indicator levels and chemotherapeutic efficacy are presented in Table 2.
Table 2
| Indicators | PR + SD (n=76) | PD (n=18) | χ2 | P value |
|---|---|---|---|---|
| PLT | 4.398 | 0.036* | ||
| Low (n=47) | 42 | 5 | ||
| High (n=47) | 34 | 13 | ||
| PCT | 0.001 | 0.920 | ||
| Low (n=48) | 39 | 9 | ||
| High (n=46) | 37 | 9 | ||
| MPV | 0.040 | 0.841 | ||
| Low (n=49) | 40 | 9 | ||
| High (n=45) | 36 | 9 | ||
| PDW | 7.749 | 0.005** | ||
| Low (n=48) | 33 | 15 | ||
| High (n=46) | 43 | 3 | ||
| PLR | 2.471 | 0.116 | ||
| Low (n=47) | 41 | 6 | ||
| High (n=47) | 35 | 12 |
*P<0.05; **P<0.01. PLT, platelet count; PCT, plateletcrit; MPV, mean platelet volume; PDW, platelet distribution width; PLR, platelet to lymphocyte ratio; PR, partial response; SD, stable disease; PD, progressive disease.
Forty-seven patients had lower baseline PLT levels, including 42 PR or SD patients, and 5 PD patients, whereas 47 patients had higher baseline PLT levels, including 34 PR or SD patients and 13 PD patients (P=0.036).
Forty-eight patients had lower baseline PCT levels, including 39 PR or SD patients, and 9 PD patients, whereas 46 patients had higher baseline PCT levels, including 37 PR or SD patients and 9 PD patients (P=0.920).
Forty-nine patients had lower baseline MPV levels, including 40 PR or SD patients and 9 PD (progressive disease) patients, whereas 45 patients had higher baseline MPV levels, including 36 PR or SD patients and 9 PD patients (P=0.841).
Forty-eight patients had lower baseline PDW levels, including 33 PR or SD patients and 15 PD patients, whereas 46 patients had higher baseline PDW levels, including 43 PR or SD patients and 3 PD patients (P=0.005).
Forty-seven patients had lower baseline PLR levels, including 41 PR or SD patients and 6 PD patients, whereas 47 patients had higher baseline PLT levels, including 35 PR or SD patients and 12 PD patients (P=0.116).
Thus, a lower baseline PLT level and a higher baseline PDW level were related to better chemotherapeutic efficacy in breast cancer patients. Meanwhile, PCT, MPV, and PLR levels had no significant effects on chemotherapeutic efficacy.
Changes in PLT, PCT, MPV, PDW, and PLR levels of breast cancer patients
The Kaplan-Meier plots were used to determine the effect of changes in the PLT, PCT, MPV, PDW, and PLR status for OS (Figure 2). The median OS of patients whose PLT level increased following chemotherapy was 36 (95% CI, 29.370–42.630) months, while that of the not-increased group was 20 (95% CI, 15.590–24.410) months (P=0.056). The median OS of patients whose PCT level increased following chemotherapy was 32 (95% CI, 25.887–38.113) months, while that of the not-increased group was 22 (95% CI, 10.436–33.564) months (P=0.164). The median OS of patients whose MPV level increased following chemotherapy was 30 (95% CI, 17.507–42.493) months, while that of the not-increased group was 29 (95% CI, 20.919–37.081) months (P=0.176). The median OS of patients whose PDW level increased following chemotherapy was 26 (95% CI, 15.710–36.290) months, while that of the not-increased group was 30 (95% CI, 20.049–39.951) months (P=0.756). The median OS of patients whose PLR level increased following chemotherapy was 31 (95% CI, 20.432–41.568) months, while that of the not-increased group was 21 (95% CI, 9.082–32.918) months (P=0.091). Thus, changes in PLT, PCT, MPV, PDW, and PLR levels had no significant effects on OS.
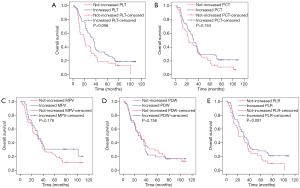
Prognostic factors for breast cancer
Univariate analyses demonstrated that a higher baseline PCT level [hazard ratio (HR) 1.577; 95% CI, 1.011–2.461; P=0.045) was a significant risk factor for poor prognosis (Table 3). In multivariate analysis, a higher baseline PCT level (HR 1.730; 95% CI, 1.092–2.741; P=0.019) was found to be independently associated with poor survival.
Table 3
| Risk factors | Overall survival (OS) | ||||
|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | ||||
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age (>62 or ≤62 years) | 1.042 (0.669–1.623) | 0.857 | – | – | |
| BMI (>22.6 or ≤22.6 kg/m2) | 1.037 (0.667–1.613) | 0.871 | – | – | |
| Bone metastases (yes or no) | 1.054 (0.659–1.687) | 0.826 | – | – | |
| Baseline PLT level (>179.500×109 or ≤179.500×109/L) | 1.286 (0.826–2.000) | 0.265 | – | – | |
| Baseline PCT level (>0.180 or ≤0.180 L/L) | 1.577 (1.011–2.461) | 0.045* | 1.730 (1.092–2.741) | 0.019* | |
| Baseline MPV level (>10.400 or ≤10.400 fl) | 1.038 (0.665–1.620) | 0.871 | – | – | |
| Baseline PDW level (>15.680 or ≤15.680%) | 1.384 (0.886–2.160) | 0.153 | – | – | |
| Baseline PLR level (>132.711 or ≤132.711) | 1.265 (0.811–1.972) | 0.300 | – | – | |
| Post-/pre-chemotherapy PLT ratio (>1 or ≤1) | 0.650 (0.414–1.021) | 0.061 | 0.745 (0.458–1.210) | 0.234 | |
| Post-/pre-chemotherapy PCT ratio (>1 or ≤1) | 0.734 (0.471–1.143) | 0.171 | – | – | |
| Post-/pre-chemotherapy MPV ratio (>1 or ≤1) | 0.704 (0.420–1.182) | 0.184 | – | – | |
| Post-/pre-chemotherapy PDW ratio (>1or ≤1) | 1.074 (0.681–1.693) | 0.760 | – | – | |
| Post-/pre-chemotherapy PLR ratio (>1or ≤1) | 0.684 (0.437–1.072) | 0.097 | 0.682 (0.415–1.120) | 0.130 | |
*P<0.05; **P<0.01. CI, confidence interval; PLT, platelet count; PCT, plateletcrit; MPV, mean platelet volume; PDW, platelet distribution width; PLR, platelet to lymphocyte ratio.
Discussion
Platelets have a crucial role in the progression and metastasis of cancer (6). Platelet-related indicators, such as PLT, PCT, MPV, PDW, and PLR, are associated with the prognosis of cancers according to many recent studies.
The majority of research so far has confirmed that PLT is a potential negative prognostic marker for colorectal cancer, endometrial cancer, and other diseases (8,17). Recent reports have verified that, apart from their action in regulating in hemostasis, and host immune and inflammatory responses, platelets have a role in cancer metastasis and angiogenesis. For example, platelets promote tumor angiogenesis through several biological factors, such as vascular endothelial growth factor (VEGF), serotonin, and endostatin (18,19). Also, platelets are a primary source of TGFβ1, which leads to cancer invasion and metastasis via promoting epithelial-mesenchymal transition (EMT) in cancer cells (20). Additionally, platelets adhere to cancer cells via P-selectin, which presents on the surface of platelets binding to CD24 ligand, thereby avoiding natural killer (NK) cell-mediated cytotoxicity and facilitating tumor growth (21,22). Furthermore, activated platelets release lysophosphatidic acid (LPA) and platelet factor 4 (PF4), which were both shown to play a role in tumor cell growth, migration, and invasion (23). In our present study, lower baseline PLT level was associated with better chemotherapeutic efficacy in breast cancer patients.
PCT value, which is obtained by multiplying PLT and MPV, is also associated with platelet activation and displays platelet status (11). The value of PCT varies between patients and healthy subjects of various types of cancer (24,25). Ma et al. determined that the value of PCT is higher in patients with epithelial ovarian cancer when compared to the healthy controls (24). However, Oncel et al. found that the value of PCT was lower in the patient group with lung cancer in comparison with the control group (25). Moreover, it has been established that the value of PCT in lung cancer patients with distant metastasis is higher than those without distant metastasis (25). In another recent study, elevated PCT level was shown to have a relationship with worse prognosis in pancreatic cancer (26). Therefore, PCT may be used as an indicator of diagnosis and prognosis of some cancers. Information about how PCT changes during the course of the advanced BC are still very limited. A previous study indicated a marked increase in PLT, PCT, and MPV in a 4T1 (mouse breast cancer cell strain type) mouse breast cancer metastasis model (27). Interestingly, another study discovered that the PCT in patients with breast cancer was lower in patients with metastasis when MPV did not change in the tumor stage (28). In our present study, we found that breast cancer patients with a lower baseline PCT level had better OS according to the Kaplan-Meier plots analysis, whereas baseline PCT level had no significant effect on chemotherapeutic efficacy. Moreover, univariate analysis and multivariate analysis both revealed that a higher baseline PCT level was an independent prognostic factor for OS.
MPV, an ordinary marker showing platelet function and activation, indicates the quantity of the average size of platelets (12,29). Recent studies have shown that a high MPV level is correlated with poor prognosis of various malignant tumors, such as breast cancer, endometrial cancer, and advanced gastric cancer (8,13,30). The impact of MPV on diagnosis, prognosis, and the chemotherapeutic effect in patients with breast cancer has been recently explored (30,31). Gu et al. found that high MPV can be useful in identifying patients with newly diagnosed breast cancer patients and is likely to present with distant metastasis in the future. An increased MPV has been consistently associated with larger tumors, higher stage, distant metastases, and a poorer prognosis in patients with breast cancer (30). Additionally, Mutlu et al. demonstrated that lower MPV was predictive of higher pathologic complete response after neoadjuvant chemotherapy in patients with ABC (31). In contrast, Gu et al. reported that there was no significant difference in survival between higher and lower MPV (30). Furthermore, other research has demonstrated that the value of MPV can be influenced by inflammation response (32,33). The elevated level of inflammatory factors can be detected by various kinds of tumors, including lung cancer and pancreatic carcinoma (34,35). Thus, changes in MPV status caused by cancer may be due to inflammatory stimuli. In this study, the MPV level had no relationship with the prognosis of breast cancer in patients.
PDW is an indicator reflecting the average change in platelet volume, whose relationship with prognosis of tumors is still controversial in the literature (8,36). Kurtoglu et al. confirmed that the PDW level of patients with endometrial cancer was significantly lower than that of healthy subjects (8). Conversely, Gunaldi et al. reported a high PDW level being positively related to metastasis of gastric cancer (36). Meanwhile, the value of PDW was shown not to be associated with the prognosis of esophageal cancer (14). In our study, a higher baseline PDW level was associated with better chemotherapeutic efficacy in breast cancer patients.
PLR, a value obtained by dividing PLT by lymphocyte count, has been proven to be a significant prognostic factor of some cancers, including non-small cell lung cancer, breast cancer, operable cervical cancer, and others (15,37). Li et al. conducted a meta-analysis confirming that elevated PLR level is associated with poor OS of pancreatic cancer patients (38). Also, Pietrzyk et al. found that PLR, as a blood parameter, was higher in patients with gastric cancer compared to the healthy control subjects (39). Furthermore, a recent meta-analysis, including 12,754 patients showed that a high PLR level was related to shorter OS in various solid tumors (40). In this study, the PLR level had no significant effect on the prognosis for breast cancer patients.
In conclusion, we have demonstrated that a lower baseline PCT level is correlated with better OS in ABC patients. This noninvasive, simple, and low-cost biomarker may be a prognostic indicator. However, there are some limitations to our study, which may include its retrospective design, the fact that all patients were from a single center, and an insufficient number of cases.
Conclusions
The lower baseline PCT level was related to better OS in ABC patients, and PCT level might thus be a potential prognosis factor for ABC.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.28). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was waived due to the retrospective nature of the study. As a retrospective investigation, this study was approved by the Medical Ethics Committees of the First Affiliated Hospital of Soochow University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Cardoso F, Costa A, Senkus E, et al. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Ann Oncol 2017;28:3111. [Crossref] [PubMed]
- Hashimoto M, Ohtsuka K, Suzuki Y, et al. A case of posterior ischemic optic neuropathy in a posterior-draining dural cavernous sinus fistula. J Neuroophthalmol 2005;25:176-9. [Crossref] [PubMed]
- Senkus E, Lacko A. Over-treatment in metastatic breast cancer. Breast 2017;31:309-17. [Crossref] [PubMed]
- Simos D, Clemons M, Ginsburg OM, et al. Definition and consequences of locally advanced breast cancer. Curr Opin Support Palliat Care 2014;8:33-8. [Crossref] [PubMed]
- Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost 2011;9:237-49. [Crossref] [PubMed]
- Kourelis TV, Wysokinska EM, Wang Y, et al. Early venous thromboembolic events are associated with worse prognosis in patients with lung cancer. Lung Cancer 2014;86:358-62. [Crossref] [PubMed]
- Kurtoglu E, Kokcu A, Celik H, et al. Platelet Indices May be Useful in Discrimination of Benign and Malign Endometrial Lesions, and Early and Advanced Stage Endometrial Cancer. Asian Pac J Cancer Prev 2015;16:5397-400. [Crossref] [PubMed]
- Wojtukiewicz MZ, Sierko E, Hempel D, et al. Platelets and cancer angiogenesis nexus. Cancer Metastasis Rev 2017;36:249-62. [Crossref] [PubMed]
- Lal I, Dittus K, Holmes CE. Platelets, coagulation and fibrinolysis in breast cancer progression. Breast Cancer Res 2013;15:207. [Crossref] [PubMed]
- Ifran A, Hasimi A, Kaptan K, et al. Evaluation of platelet parameters in healthy apheresis donors using the ADVIA 120. Transfus Apher Sci 2005;33:87-90. [Crossref] [PubMed]
- Bath PM, Butterworth RJ. Platelet size: measurement, physiology and vascular disease. Blood Coagul Fibrinolysis 1996;7:157-61. [Crossref] [PubMed]
- Matowicka-Karna J, Kamocki Z, Polinska B, et al. Platelets and inflammatory markers in patients with gastric cancer. Clin Dev Immunol 2013;2013:401623. [Crossref] [PubMed]
- Hirahara N, Matsubara T, Kawahara D, et al. Prognostic value of hematological parameters in patients undergoing esophagectomy for esophageal squamous cell carcinoma. Int J Clin Oncol 2016;21:909-19. [Crossref] [PubMed]
- Zhu Y, Si W, Sun Q, et al. Platelet-lymphocyte ratio acts as an indicator of poor prognosis in patients with breast cancer. Oncotarget 2017;8:1023-30. [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Rao XD, Zhang H, Xu ZS, et al. Poor prognostic role of the pretreatment platelet counts in colorectal cancer: A meta-analysis. Medicine (Baltimore) 2018;97:e10831. [Crossref] [PubMed]
- Da Prada M, Picotti GB. Content and subcellular localization of catecholamines and 5-hydroxytryptamine in human and animal blood platelets: monoamine distribution between platelets and plasma. Br J Pharmacol 1979;65:653-62. [Crossref] [PubMed]
- Zamani A, Qu Z. Serotonin activates angiogenic phosphorylation signaling in human endothelial cells. FEBS Lett 2012;586:2360-5. [Crossref] [PubMed]
- Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011;20:576-90. [Crossref] [PubMed]
- Chen M, Geng JG. P-selectin mediates adhesion of leukocytes, platelets, and cancer cells in inflammation, thrombosis, and cancer growth and metastasis. Arch Immunol Ther Exp (Warsz) 2006;54:75-84. [Crossref] [PubMed]
- Coupland LA, Chong BH, Parish CR. Platelets and P-selectin control tumor cell metastasis in an organ-specific manner and independently of NK cells. Cancer Res 2012;72:4662-71. [Crossref] [PubMed]
- Pucci F, Rickelt S, Newton AP, et al. PF4 Promotes Platelet Production and Lung Cancer Growth. Cell Rep 2016;17:1764-72. [Crossref] [PubMed]
- Ma X, Wang Y, Sheng H, et al. Prognostic significance of thrombocytosis, platelet parameters and aggregation rates in epithelial ovarian cancer. J Obstet Gynaecol Res 2014;40:178-83. [Crossref] [PubMed]
- Oncel M, Kiyici A, Oncel M, et al. Evaluation of Platelet Indices in Lung Cancer Patients. Asian Pac J Cancer Prev 2015;16:7599-602. [Crossref] [PubMed]
- Wang L, Sheng L, Liu P. The independent association of platelet parameters with overall survival in pancreatic adenocarcinoma receiving intensity-modulated radiation therapy. Int J Clin Exp Med 2015;8:21215-21. [PubMed]
- Wang C, Chen YG, Gao JL, et al. Low local blood perfusion, high white blood cell and high platelet count are associated with primary tumor growth and lung metastasis in a 4T1 mouse breast cancer metastasis model. Oncol Lett 2015;10:754-60. [Crossref] [PubMed]
- Okuturlar Y, Gunaldi M, Tiken EE, et al. Utility of peripheral blood parameters in predicting breast cancer risk. Asian Pac J Cancer Prev 2015;16:2409-12. [Crossref] [PubMed]
- Colkesen Y, Muderrisoglu H. The role of mean platelet volume in predicting thrombotic events. Clin Chem Lab Med 2012;50:631-4. [Crossref] [PubMed]
- Gu M, Zhai Z, Huang L, et al. Pre-treatment mean platelet volume associates with worse clinicopathologic features and prognosis of patients with invasive breast cancer. Breast Cancer 2016;23:752-60. [Crossref] [PubMed]
- Mutlu H, Eryilmaz MK, Musri FY, et al. Mean Platelet Volume as an Independent Predictive Marker for Pathologic Complete Response after Neoadjuvant Chemotherapy in Patients with Locally Advanced Breast Cancer. Asian Pac J Cancer Prev 2016;17:2089-92. [Crossref] [PubMed]
- Gasparyan AY, Ayvazyan L, Mikhailidis DP, et al. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des 2011;17:47-58. [Crossref] [PubMed]
- Kapsoritakis AN, Koukourakis MI, Sfiridaki A, et al. Mean platelet volume: a useful marker of inflammatory bowel disease activity. Am J Gastroenterol 2001;96:776-81. [Crossref] [PubMed]
- Weerasinghe P, Garcia GE, Zhu Q, et al. Inhibition of Stat3 activation and tumor growth suppression of non-small cell lung cancer by G-quartet oligonucleotides. Int J Oncol 2007;31:129-36. [PubMed]
- Raimondi S, Lowenfels AB, Morselli-Labate AM, et al. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol 2010;24:349-58. [Crossref] [PubMed]
- Gunaldi M, Erdem D, Goksu S, et al. Platelet Distribution Width as a Predictor of Metastasis in Gastric Cancer Patients. J Gastrointest Cancer 2017;48:341-6. [Crossref] [PubMed]
- Zheng RR, Huang M, Jin C, et al. Cervical cancer systemic inflammation score: a novel predictor of prognosis. Oncotarget 2016;7:15230-42. [Crossref] [PubMed]
- Li W, Tao L, Lu M, et al. Prognostic role of platelet to lymphocyte ratio in pancreatic cancers: A meta-analysis including 3028 patients. Medicine (Baltimore) 2018;97:e9616. [Crossref] [PubMed]
- Pietrzyk L, Plewa Z, Denisow-Pietrzyk M, et al. Diagnostic Power of Blood Parameters as Screening Markers in Gastric Cancer Patients. Asian Pac J Cancer Prev 2016;17:4433-7. [PubMed]
- Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2014;23:1204-12. [Crossref] [PubMed]


