Stereotactic body radiation therapy or surgery for stage I–II non-small cell lung cancer treatment?—outcomes of a meta-analysis
Introduction
Lung cancer is the most common type of cancer in China, with an annual incidence of approximately 7,810,001 new cases (1), and it is also the main cause of cancer death in the United States (2). In 2018, 154,050 deaths are estimated to be related to lung cancer (3). Only 18% of all patients with lung cancer are alive 5 years or more after diagnosis (4). Advanced diagnosis has been the main obstacle to the improvement of lung cancer survival rates (5,6). The National Lung Screening Trial (ACRIN Protocol A6654) showed that screening individuals with high-risk factors using low-dose CT decreased the mortality rate from lung cancer by 20% (7). Thus, early diagnosis and treatment of lung cancer are extremely important. Lung cancer is the leading cause of cancer-related deaths worldwide. NSCLC constitutes approximately 80% of lung cancer cases. For early-stage NSCLC, lobectomy with mediastinal node dissection or sampling remains the standard therapy for operable patients (8). However, some patients cannot undergo surgical treatment because of considerable complications or advanced age. Recently, SBRT has been increasingly used for the treatment of early NSCLC. Chang et al. (9) reported the results of two phase III clinical trials (STARS and ROSEL) for operable stage I NSCLC, which showed that the 3-year survival rate following treatment with stereotactic ablation radiotherapy (SABR) was higher than that following treatment with surgery (P=0.037). However, Samson et al. (10) performed a clinical study focusing on the same inclusion criteria as the STARS and ROSEL trials but with different sample sizes and confirmed that the survival results of small sample studies were highly variable and unreliable. The results of retrospective studies on SBRT and surgery for I/II NSCLC are inconsistent (11,12). Therefore, we carried out a meta-analysis with the aim of comparing the efficacy of SBRT and surgery for stage I/II NSCLC.
Methods
Search strategy
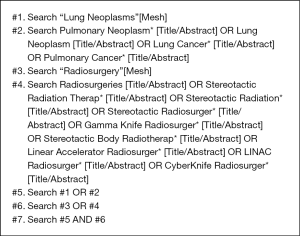
The electronic databases searched included PubMed, The Cochrane Library, EMBASE and the Chinese Biomedical Literature Database from their inception to March 14, 2018. All searches used a combination of advanced retrieval and topic retrieval. References of relevant studies were hand-searched to identify additional relevant publications. The search strategy for PubMed is shown in Box 1, and the search strategies for other databases can be found in Appendix 1.
Inclusion and exclusion criteria
Studies were included if they met the following criteria: (I) Published in Chinese or English; (II) early-stage NSCLC strictly limited to stage I and II; (III) the type of intervention was stereotactic body radiation therapy, equivalent to SABR and stereotactic radiosurgery. The control was surgical procedures that could be either full anatomical resections, including lobectomy, bilobectomy, and pneumonectomy, or limited lung resection, including sublobar resection, segmentectomy, and wedge resection; (IV) retrospective study design; and (V) outcomes of interest included overall survival (OS), cause-specific survival (CSS), freedom from progression (FFP), recurrence-free survival (RFS), disease-free survival (DFS), local control rate (LCR), regional control rate (RCR), loco-regional control rate (L-RCR) and/or distant control rate (DCR).
Exclusion criteria: (I) For republished literature, if more than one study reported the same measurement for the same clinical trial, only the broader study was selected; if the measurement indicators were different, the corresponding measurement indicators were all included in the analysis; (II) studies with incomplete data or missing information, such as case reports, reviews, notes, letters, commentaries and errata; and (III) studies that included other treatment measures.
Study selection and data collection
Two investigators (L Shao and Y Liao) independently screened the titles and abstracts of potentially relevant studies. We retrieved the full text of relevant studies for further review by the same two reviewers. A third senior investigator (Q Zhang) resolved any discrepancies between the reviewers. The same paired reviewers extracted study details independently. A third investigator (Q Zhang) reviewed all data entries. We extracted the following data: author, study design, study period, patient characteristics (sex, age, case number, tumour size, stage), interventions (radiation dose and fractionation schedule), sample size, length of follow-up, and outcomes of interest [hazard ratios (HR) with corresponding 95% confidence interval (95% CI) or relevant data for HR and 95% CI calculation].
Quality assessment
We used the Newcastle-Ottawa Scale (NOS) to assess the quality of the included studies (13). This scale judged a study based on three broad perspectives: the selection of the study groups, the comparability of the groups, and the ascertainment of either the exposure or outcome of interest for case-control or cohort studies, respectively.
Statistical analysis
This meta-analysis was performed with STATA 12.0 software. The endpoint outcomes were considered as a weighted average of individual estimates of the HR in each included study, using the inverse variance method. In a meta-analysis, it is usually required that the corresponding sample statistic of the effect size approximately obey a normal distribution. When the effect indicator of the endpoints of interest is the hazard ratio, the effect size is the logarithm of HR. The lnHR were considered to obey a normal distribution. If the HR and the corresponding 95% CI were reported, the lnHR and the corresponding lnLL and lnUL were used as data points in the pooled analysis. If the HR and 95% CI for surgical treatment to stereotactic radiotherapy were provided, the HR and 95% CI for stereotactic radiotherapy to surgical treatment were calculated using the method described by Tierney et al. (14). If the HR or 95% CI was not provided and when the K-M curves were available, survival data were extracted from amplified K-M curves using an open digitizing programme (GetData Graph Digitizer), and the estimates of HR and 95% CI were calculated according to the method described by Tierney et al. (14).
A sensitivity analysis was conducted for each study to rule out its predominant influence on the pooled results. Heterogeneity was assessed by the χ2 test according to the Cochrane systematic review handbook and was investigated using the I2 statistic. Studies with an I2 of 25 to 50%, 50% to 75%, or >75% were considered to have low, moderate, or high heterogeneity, respectively. The pooled HRs were first calculated using the fixed-effects model. If there was high heterogeneity among studies, the randomized-effects model was used. A P value less than 0.05 was considered statistically significant.
Results
Overview of literature search and study characteristics
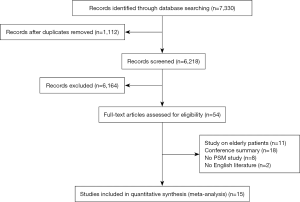
A total of 7,330 studies were identified from the databases, among which 54 were included in the full-text evaluation. Fifteen retrospective studies were included in this meta-analysis (11,12,15-27) (Figure 1). All the included studies were of moderate quality at least. Table 1 shows the basic characteristics of the 15 studies. Among them, 6 studies compared SBRT with lobectomy.
Table 1
| Study | Research year range | Treatment type | Number of cases | Gender (M/F) |
Age [mean] range or SD | Tumour size [cm] | Stage T1/T2 | Follow-up time (months) | Dose range (Gy) | The main outcome of interest | NOS | Matching characteristics |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ye et al. 2018 | Feb 2010–Jun 2016 | Surgery | 66 | 46/20 | 69 | 2.44±0.89 | 44/22 | 27 | OS, L-RCR, DCR, PFS | 7 | abcde*fghi | |
| SBRT | 66 | 42/24 | 71 | 2.49±0.92 | 45/21 | 26.5 | 50 Gy/5 F, 60 Gy/10F | |||||
| Varlotto et al. 2013 | 2000–2008 | SBRT | 77 | 18.8 | 60 [48–60] Gy/3 [3–5] F | OS, DFS | 8 | NA | ||||
| 1999–2008 | Surgery | 77 | 35 | |||||||||
| Verstegen et al. 2013 | 2007– | VATS lobectomy | 64 | 36/28 | 67.95±8.84 | 2.86±1.24 | 39/24 | 16 | OS, L-RCR, DCR, PFS | 7 | abcdjkefl | |
| Nov 2003– | SABR | 64 | 37/27 | 70.53±9.91 | 2.88±1.287 | 39/25 | 30 | 54–60 Gy/3, 5, 8, 12 F | ||||
| Rosen et al. 2016 | 2008–2012 | Lobectomy | 1,781 | 777/1,004 | 74.8 | 2.37 | 1,374/407 | 31.6 | OS | 6 | a*bcde*fm*nzαo | |
| SBRT | 1,781 | 767/1,014 | 75.5 | 2.38 | 1,371/410 | 28.6 | BED100–200 Gy/3–5 F | |||||
| Robinson et al. 2013 | Jan 2004–Jan 2008 | Lobar resection | 76 | 37/39 | 65 [40–87] | 2 [0.8–5.8] | 59/17 | 51.3 | OS, LCR, RCR, DCR, CSS | 7 | a*bcdj*k* ep*q*r*zh | |
| SBRT | 76 | 42/34 | 76 [31–93] | 2 [1.1–6] | 56/20 | 50.3 | 45–54 Gy/3–5 F | |||||
| Puri et al. 2012 | Jan 1, 2000–Dec 21, 2006 | Surgery | 57 | 34/23 | 71.54±7.9 | 40/17 | OS, CSS | 7 | ab*dk*p*q | |||
| Feb 1, 2004–May 5, 2007 | SBRT | 57 | 23/34 | 71.79±10.6 | 39/18 | 54 Gy/3 F | ||||||
| Puri et al. 2015 | 1998–2010 | Surgery | 5,355 | 2,382/2,973 | 74.2±8.4 | 2.33±1.03 | 4,099/1,256 | 27.5 | OS | 6 | abcdjnzβs* | |
| 2003–2010 | SBRT | 5,355 | 2,407/2,948 | 74.3±8.5 | 2.34±0.95 | 4,063/1,292 | 16.6 | 54 Gy | ||||
| Mokhles et al. 2015 | Jan 2003–Jan 2012 | Surgery | 73 | 44/29 | 67 [39–83] | 2.4 [1–6.6] | 49 | OS, L-RCR, DCR, PFS | 7 | abcdjkeftl | ||
| SABR | 73 | 42/31 | 67 [47–89] | 2.5 [0.8–7] | 28 | 54–60 Gy/3, 5, 8, 12 F | ||||||
| Matsuo et al. 2014 | Jan 2003–Dec 2009 | Sublobar resection | 53 | 37/16 | 76 [50–88] | 2 [0.6–5] | 63.6 | OS, LCR, RCR, DCR, CSS | 7 | abcjke*l | ||
| SBRT | 53 | 42/11 | 76 [58–86] | 2.2 [1–3.7] | 80.4 | 48, 56, 60 Gy/4, 8 F | ||||||
| Kastelijn et al. 2015 | 2008–2011 | Surgery | 175 | 31.8 | OS, L-RCR, DCR, PFS | 7 | NA | |||||
| SBRT | 53 | 41.5 | 18 Gy/3 F 12 Gy/5 F 7.5 Gy/8 F |
|||||||||
| Hamaji et al. 2015 | 2003–2009 | VATS lobectomy | 41 | 32/9 | 74 [61–86] | 2.5 [1.2–4.5] | 27/14 | 54 | OS, LCR, RCR, DCR, CSS, RFS | 7 | abcdjkefguvγw | |
| SBRT | 41 | 31/10 | 73 [58–85] | 2.5 [1.4–4.5] | 29/12 | 40.7 | 48, 56, 60 Gy/4, 8F | |||||
| Eba et al. 2016 | 2002–2004 | Lobectomy | 21 | 8/13 | 73 [67–74] | 2.1 [1.8–2.4] | 21/0 | OS | 7 | abcx | ||
| 2004–2007 | SBRT | 21 | 11/10 | 75 [68–78] | 2.3 [1.9–2.6] | 21/0 | 48 Gy/4 F/4–8 D | |||||
| Crabtree et al. 2014 | Jun 2004–Dec 2010 | Surgery | 56 | 32/24 | 70.0±8.1 | 3.0±1.6 | 32/24 | 34 | OS, LCR, RCR, DCR, DFS | 7 | abcdjk ftgzδp* | |
| SBRT | 56 | 29/27 | 70.7±10.6 | 2.5±1.1 | 40/16 | 23.4 | 45–60 Gy/3–6 F | |||||
| Cornwell et al. 2018 | Jul 1, 2009–Dec 31, 2014 | VATS lobectomy | 37 | 36/1 | 68 [63–73] | 2.3 [1.7–3.0] | 43.2 | OS, LCR, RCR, DCR, CSS, RFS | 8 | abcdjketgy* | ||
| SBRT | 37 | 36/1 | 66 [63–72] | 2.2 [1.6–2.7] | 44.4 | 56 [50–56] Gy/4 [4–5] F | ||||||
| Yerokunet al. 2017 | 2008–2011 | Wedge resection | 1,584 | 622/962 | 73 [67–79] | 1.5 [1.3–1.9] | 1,584/0 | OS | 7 | abcjefnε | ||
| SBRT | 1,584 | 654/930 | 73 [67–79] | 1.5 [1.3–1.8] | 1,584/0 |
*The characteristics are significantly different between SBRT and surgery (P<0.05). NA, not applicable; SD, Standard deviation; NOS, Newcastle-Ottawa Scale; OS, overall survival; CSS, cause-specific survival; FFP, freedom from progression; RFS, recurrence-free survival; DFS, disease-free survival; LCR, local control rate; RCR, regional control rate; L-RCR, loco-regional control rate; DCR, distant control rate; SABR, stereotactic ablation radiotherapy; SBRT, stereotactic body radiotherapy. a, age; b, male/female; c, tumour size; d, clinical staging; e, pathological cell type; f, tumour location; g, smoking status; h, SUVmax; i, COPD; j, CCI; k, FEV1; l, WHO performance score; m, pathological grade; n, facility type; o, facility location; p, DLCO; q, ACE score; r, FVC; s, chemotherapy; t, hypertension; u, comorbidities; v, serum CEA, SCC; w, follow-up period; x, C/T ratio; y, mediastinal staging via EBUS; z, race; α, Spanish Hispanic, origin primary payer, median income, high school degree, urban/rural; β, urban location, income >$35,000/year; γ, mortality within 30 days of treatment; δ, weight (lb); ε, insurance status distance to hospital (Table S1). There are two studies that did not match any characteristics (16,22) in Table 1 because the two studies only listed the matching characteristics in the text. However, there is no specific table description, we do not know the specific P value of the comparison of matched characteristics between the SBRT and surgery groups in the propensity-matched patients. The data could not be further analysed, so we expressed the results as NA.
Meta-analysis results
OS
Fifteen studies reported OS (11,12,15-27). The pooled HR showed that surgery was associated with a significantly higher OS than SBRT (HR =1.81; 95% CI, 1.72–1.90; P=0.000; Figure 2). The sensitivity analysis demonstrated that the result of OS was relatively stable and credible (Table S2). However, the matched baseline characteristics in each study were not consistent (Table S1). We restricted studies to the same matched and comparable characteristics, and the results are shown in Table 2. The effect estimates of SBRT versus surgery for each of the subgroups were as follows: matched on six characteristics (11,20,23,25,26) (HR =1.769; 95% CI, 1.223–2.559; P=0.002); matched on seven characteristics (11,20,23,25) (HR =1.650; 95% CI, 1.112–2.447, P=0.013); matched on eight characteristics (11,20,23) (HR =1.623; 95% CI, 0.848–3.106; P=0.144); and matched on nine characteristics (11,20) (HR =1.156; 95% CI, 0.623–2.146; P=0.646). The sensitivity analysis demonstrated that some of the results of OS for studies that were restricted to the same matching and comparable characteristics were not stable (Table S3).
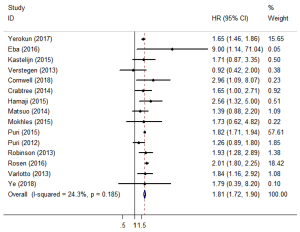
Table 2
| Matching and comparable basic features | Study number | Surgery N |
SBRT N | Heterogeneity | Meta-analysis results | ||||
|---|---|---|---|---|---|---|---|---|---|
| I2 (%) | P | HR (95% CI) | P | Z | |||||
| Age/sex/tumour size/stage/CCI/FEV1 | 5 | 271 | 271 | 20.4 | 0.285 | 1.769 (1.223–2.559) | 0.002 | 3.03 | |
| Age/sex/tumour size/stage/CCI/FEV1/tumour site | 4 | 234 | 234 | 22.5 | 0.276 | 1.650 (1.112–2.447) | 0.013 | 2.49 | |
| Age/sex/tumour size/stage/CCI/FEV1/tumour site/pathology | 3 | 178 | 178 | 48.3 | 0.144 | 1.623 (0.848–3.106) | 0.144 | 1.46 | |
| Age/sex/tumour size/stage/CCI/FEV1/tumour site/pathology/WHO performance score | 2 | 137 | 137 | 0 | 0.332 | 1.156 (0.623–2.146) | 0.646 | 0.46 | |
OS, overall survival; SBRT, stereotactic body radiotherapy; CCI, Charlson Comorbidity Index; FEV1, forced expiratory volume in 1 second.
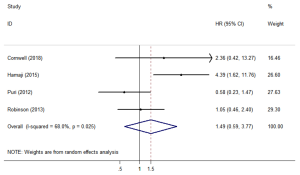
CSS
Four studies (12,18,23,26) assessed CSS. The forest plot is shown in Figure 3. The CSS (HR =1.49; 95% CI, 0.59–3.77; P=0.401) was similar between SBRT and surgery treatments. The sensitivity analysis excluding Hamaji’s research (Table S2) showed that the HR =0.919; 95% CI, 0.50–1.70, the CSS still similar between SBRT and surgery treatments.
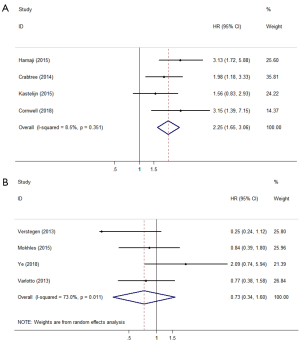
FFP, DFS, or RFS
There were 8 studies that reported FFP, DFS, or RFS according to the definitions in the literature. Four studies defined FFP or DFS as the time from the start of treatment until tumour recurrence or death (22,23,25,26). Surgery showed significantly better outcomes compared with SBRT (HR =2.25; 95% CI, 1.65–3.06; P=0.000; Figure 4A). The other four studies (11,15,16,20) defined RFS as freedom from any tumour recurrence, and the pooled results showed that there was no significant difference between surgery and SBRT (HR =0.73; 95% CI, 0.34–1.60; P=0.434; Figure 4B). According to the results of the sensitivity analysis, the pooled results are relatively stable and credible (Table S2).
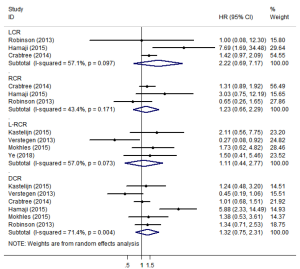
LCR, RCR, L-RCR, or DCR
Three studies (12,23,25) reported data on LCR and RCR (Figure 5). The pooled analysis showed that SBRT and surgery had similar LCR/RCR, with pooled HRs of 2.22 (95% CI, 0.69–7.17; P=0.184) and 1.23 (95% CI, 0.66–2.29; P=0.517), respectively. Furthermore, four studies reported data on L-RCR (11,15,20,22), six studies (11,12,20,22,23,25) reported data on DCR, and the pooled analysis showed that the differences were not statistically significant, with pooled HRs of 1.11 (95% CI, 0.44–2.77; P=0.830) and 1.32 (95% CI, 0.75–2.31; P=0.341), respectively (Figure 5). According to the results of the sensitivity analysis, the pooled results are relatively stable (Table S2).
OS comparison between SBRT and lobectomy
Six of the included studies (11,17,20,23,24,26) performed a comparative study of lobectomy and SBRT for stage I/II NSCLC. A pooled analysis of these 6 studies showed that lobectomy had a better survival benefit over SBRT (HR =2.00; 95% CI, 1.45–2.74; P=0.000; Figure 6), and the sensitivity analysis also showed similar results (Table S2). The pooled results from analyses restricting studies to those with comparable characteristics are shown in Table 3, and the effect estimates of SBRT to lobectomy for each subgroup were as follows: matched on three characteristics (11,20,23,24,26) (HR =2.044; 95% CI, 1.150–3.634; P=0.015); matched on six characteristics (11,20,23,26) (HR =1.837; 95% CI, 1.068–3.158; P=0.028); matched on eight characteristics (11,20,23) (HR =1.623; 95% CI, 0.848–3.106; P=0.144); and matched on nine characteristics (11,20) (HR =1.156; 95% CI, 0.623–2.146; P=0.646). The sensitivity analysis of studies that were restricted to the same matched and comparable characteristics showed that the results were not very stable (Table S3).
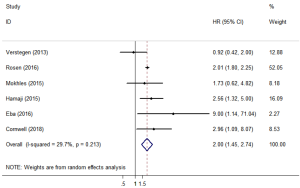
Table 3
| Matching and comparable basic features | Study number | Lobectomy N | SBRT N | Heterogeneity | Meta-analysis results | ||||
|---|---|---|---|---|---|---|---|---|---|
| I2 (%) | P | HR (95% CI) | P | Z | |||||
| Age/sex/tumour size | 5 | 236 | 236 | 43.6 | 0.131 | 2.044 (1.150–3.634) | 0.015 | 2.44 | |
| Age/sex/tumour size/stage/CCI/FEV1 | 4 | 215 | 215 | 39 | 0.178 | 1.837 (1.068–3.158) | 0.028 | 2.2 | |
| Age/sex/tumour size/stage/CCI/FEV1/tumour site/pathology | 3 | 178 | 178 | 48.3 | 0.144 | 1.623 (0.848–3.106) | 0.144 | 1.46 | |
| Age/sex/tumour size/stage/CCI/FEV1/tumour site/pathology/WHO performance score | 2 | 137 | 137 | 0 | 0.332 | 1.156 (0.623, 2.146) | 0.646 | 0.46 | |
OS, overall survival; SBRT, stereotactic body radiotherapy; CCI, Charlson Comorbidity Index; FEV1, forced expiratory volume in 1 second.
Publication bias
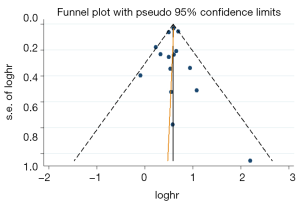
A funnel plot was generated for OS to evaluate publication bias (Figure 7). Egger’s test (P=0.773) indicated that there was no obvious publication bias.
Discussion
Lung cancer is the world’s leading cause of cancer-related death (28). The prevalence of early-stage NSCLC is expected to increase given the current trends in the widespread implementation of computed tomography (CT) screening (7,29). Although lobectomy remains the treatment of choice for early-stage NSCLC, some patients with early-stage NSCLC are not considered candidates for lobar resection because of concomitant severe medical comorbidities or patient preference. SBRT is a non-invasive treatment that delivers precisely targeted ablative doses of radiation using the principles of stereotaxis, rigorous patient immobilization and/or tumour tracking, and modern radiotherapy treatment planning. SBRT was initially introduced as an alternative to conventionally fractionated radiation therapy for medically inoperable patients with early-stage NSCLC. SBRT in medically operable patients was first reported in Japan (30), where higher 3-year rates of local control (94%) and OS (86%) were documented in patients refusing surgery. Outcomes from SBRT are so promising that there are increasing numbers of studies on the effect of surgery and SBRT for the treatment of early-stage NSCLC. Three randomized clinical trials were carried out, but they were all terminated because of poor accrual (31-33). Retrospective studies have shown that the survival rate of early-stage NSCLC patients treated with SBRT may be worse, better, or not different compared with that of patients treated with surgery (11,17,20).
We included fifteen retrospective studies in this meta-analysis. The baseline characteristics of patients in the surgical treatment group were better than those of patients in the SBRT group; therefore, propensity matching analysis was used to compensate for significant baseline differences between the two groups to achieve an objective analysis of the association between treatment and outcomes. Based on the pooled analysis of these PSM studies, we found that the OS of SBRT for stage I/II NSCLC was inferior to that of surgery (P=0.000), but there were no significant differences in LCR (P=0.184), RCR (P=0.517), L-RCR (P=0.830) or DCR (P=0.341). In addition, the pooled results showed that surgery yielded lower rates of tumour recurrence or death (P=0.000), but there was no significant difference in the rate of absence of tumour recurrence between surgery and SBRT (P=0.434). This further confirmed that surgical treatment of NSCLC was associated with a better survival advantage over SBRT, but there is no difference in recurrence. It is noteworthy that there was no significant difference between surgery and SBRT in the CSS (P=0.401), indicating that patients who undergo SBRT have the same risk of dying from cancer as those undergoing surgery, even though the OS is worse than that associated with surgical treatment. Therefore, compared with surgical treatment, SBRT patients are unhealthier and die more often from non-cancer causes. In the study of Eba et al. (24), multivariate analysis of OS showed that age and C/T ratio had a significant impact on OS. In the study by Robinson et al. (12), a univariate analysis revealed that ACE-27, CCI, sex, age and FEV1 had significant effects on survival, and a multivariate analysis showed that CCI and age had a significant impact on OS. Research conducted by Varlotto et al. (16) showed that OS was significantly correlated with histology, Charlson Comorbidity Index, tumour size, aspirin use, and SBRT/SABR based on a univariate analysis, while a multivariate analysis without propensity score (PS) correction correlated better OS with surgery, lower Charlson Comorbidity Index score, and adenocarcinoma histology. After adjusting for propensity scores, OS correlated only with the Charlson Comorbidity Index. The study by Ye et al. (15) showed that COPD (yes/no), sex (male vs. female), site (central vs. peripheral), age, tumour size, SUVmax, histology, T status, treatment (SBRT vs. surgery) and smoking status were related to OS through the univariate analysis; the multivariate analysis showed that OS was only correlated with tumour size and SUVmax. Based on this finding, and although propensity score matching (PSM) was conducted in each of the included studies, there were significant differences in the matching baseline characteristics (Table S1); therefore, we further analysed OS results according to the match of the basic characteristics of the patients in each PSM study. The results are shown in Table 2 and show that with an increase in matching and comparable basic characteristics between the SBRT and surgical treatment groups, the difference in survival between the two groups gradually decreased, and there was eventually no significant difference. In addition, a separate meta-analysis of 6 studies that compared lobectomy with SBRT for stage I/II NSCLC also yielded similar OS results (Table 3). However, according to the sensitivity analysis (Table S3), some of the above results changed after deleting a study, indicating that the results were not stable. However, it is worth noting that the number of studies restricted to the same matching and comparable characteristics for analysis was small, and there may be many potential factors affecting the pooled results, more studies need to be involved in the research to validate the results in the future. We further conducted an OS pooled analysis for studies restricted to a single matching and comparable characteristic (Tables S4,S5). The results show that the pooled HR based on age, pathology, FEV1 and especially WHO performance score (P=0.16) was reduced compared with the pooled HR (1.809) for the entire study. According to the sensitivity analysis, the pooled results are relatively stable and credible (Tables S4,S5). Therefore, age, pathology, FEV1 and WHO performance score may have significant effects on survival. In the current study, only partial adjustment factors were included in the PSM; however, some of the unmeasured characteristics may be confounders that could affect the results of OS. Chang et al. (9) reported the results of a phase III randomized clinical study that balanced the basic characteristics of patients in the surgery and SBRT groups, and the results showed that SBRT had a survival advantage over surgery. In view of the above findings, although SBRT is commonly used to treat medically inoperable patients with early-stage NSCLC, in patients with stage I/II NSCLC, who usually choose surgical treatment, and with better baseline characteristics, such as a better WHO performance score, higher FEV1 and lower CCI, SBRT may be an effective alternative treatment and is worthy of further study.
Compared with SBRT, surgical treatment of stage I/II NSCLC can include the performance of mediastinal lymph node sampling/dissection, can reveal occult nodal disease, and then corresponding patients will receive radiotherapy or chemotherapy to reduce recurrence and distant metastasis. However, in our meta-analysis, we did not find differences in LCR, RCR, L-RCR, or DCR between surgery and SBRT. Several theories have been postulated to explain this phenomenon, including the possible improvement of function of the immune system by radiation that is mediated by T-cell regulation (34,35). The high radiation doses used in SABR may also have resulted in low-dose spillage to the regional nodes, possibly eliminating microscopic disease (36). Surgery-induced oxidative stress may potentiate tumour growth through the local release of cytokines, and growth factors may stimulate tumour growth (37).
The present study has some limitations. Most importantly, this study was based on retrospective trials. To date, three phase 3 random trials have been initiated to compare SBRT with surgery in patients with early-stage NSCLC, but all of them were closed early because of slow accrual. New randomized trials, such as randomized phase III studies of sublobar resection (SR) versus SABR in high-risk patients with stage I NSCLC (STABLe-mates; CT01622621, formerly American College of Surgeons Oncology Group Z4099) and SABRTooth (ISRCTN13029788) (38), are now ongoing, and it is likely to be several years before the results are reported. Second, although all the included studies performed PSM, the matching characteristics of each study were not the same. In addition, propensity matching, although technically feasible, is essentially infeasible because medically inoperable patients who received SBRT have no true counterpart in the surgery cohort. Third, different surgical methods and radiation doses may have different efficacies in the treatment of early NSCLC. Although the surgical treatments and stereotactic radiotherapy doses vary among the studies included in this report, the data provided by each study are limited, making it difficult to conduct further analysis. Fourth, because the results of most studies included in our meta-analysis show that surgery has a significant survival advantage over SBRT, our findings may have potential bias.
Conclusions
In conclusion, compared with SBRT, surgery was associated with more favourable survival for stage I/II NSCLC, but when increasing numbers of comparable characteristics between surgery and SBRT were matched, the differences in survival gradually decreased until they were no longer significant. There were also no significant differences in CSS and recurrence (local, regional, or disseminated). Therefore, SBRT has the potential to be an alternative to surgical treatment in patients with stage I/II NSCLC, but these findings need to be confirmed by large-sample, long-term follow-up randomized clinical studies.
Search strategies of all databases besides PubMed
EMBASE
Cochrane Library
CBM
Table S1
| Study | Matching characteristics |
|---|---|
| Ye et al. 2018 | Age, male/female, tumour size, clinical staging, pathologic cell type*, tumour location, smoking status, SUVmax, COPD |
| Varlotto et al. 2013 | NA |
| Verstegen et al. 2013 | Age, male/female, tumour size, clinical staging, CCI, FEV1, pathologic cell type, tumour location, WHO performance score |
| Rosen et al. 2016 | Age*, male/female, tumour size, clinical staging, pathologic cell type*, tumour location, grade*, facility type, race, spanish hispanic origin, primary payer, median income, high school degree, urban/rural, facility location |
| Robinson et al. 2013 | Age*, male/female, tumour size, clinical staging, CCI*, FEV1*, pathologic cell type, DLCO*, ACE score*, FVC*, race, SUVmax |
| Puri et al. 2012 | Age, male/female*, clinical staging, FEV1*, DLCO*, ACE score |
| Puri et al. 2015 | Age, male/female, tumour size, clinical staging, CCI, facility type, race, urban location, income >$35,000/year, chemotherapy*, median survival* |
| Mokhles et al. 2015 | Age, male/female, tumour size, clinical staging, CCI, FEV1, pathologic cell type, tumour location, hypertension, WHO performance score |
| Matsuo et al. 2014 | Age, male/female, tumour size, CCI, FEV1, pathologic cell type*, performance status (0:1) |
| Kastelijn et al. 2015 | NA |
| Hamaji et al. 2015 | Age, male/female, tumour size, clinical staging, CCI, FEV1, pathologic cell type, tumour location, smoking status, comorbidities, serum CEA, serum SCC antigen, mortality within 30 days of treatment, follow-up period |
| Eba et al. 2016 | Age, male/female, tumour size, C/T ratio |
| Crabtree et al. 2014 | Age, male/female, tumour size, clinical staging, CCI, FEV1, tumour location, hypertension, smoking status, race, weight (lb), DLCO* |
| Cornwell et al. 2018 | Age, male/female, tumour size, clinical staging, CCI, FEV1, pathologic cell type, hypertension, smoking status, mediastinal staging via EBUS* |
| Yerokun et al. 2017 | Age, male/female, tumour size, CCI, pathologic cell type, tumour location, facility type, insurance status, distance to hospital |
*, the characteristics have significant differences between SBRT and surgery (P<0.05). NA, not applicable; FEV1, forced expiratory volume in one second; CEA, carcinoembryonic antigen; DLCO, diffusing capacity of lung for carbon monoxide; ACE, adult comorbidity evaluation; FVC, forced vital capacity; SUVmax, maximum standardized uptake value; CCI, Charlson Comorbidity Index; SCC, squamous cell carcinoma.
Table S2
| Study omitted | hr | ul | ll |
|---|---|---|---|
| OS | |||
| Yerokun | 1.8400707 | 1.7465854 | 1.9385599 |
| Eba | 1.8073877 | 1.7228516 | 1.8960718 |
| Kastelijn | 1.8094635 | 1.7246432 | 1.8984553 |
| Verstegen | 1.8136526 | 1.7286876 | 1.9027938 |
| Cornwell | 1.8069048 | 1.7223189 | 1.8956448 |
| Crabtree | 1.8104978 | 1.7254543 | 1.8997327 |
| Hamaji | 1.8056992 | 1.7210512 | 1.8945105 |
| Matsuo | 1.8142195 | 1.7289299 | 1.9037163 |
| Mokhles | 1.8091191 | 1.724434 | 1.897963 |
| Puri | 1.7940363 | 1.6668215 | 1.9309602 |
| Puri | 1.8213148 | 1.7353703 | 1.9115158 |
| Robinson | 1.8073045 | 1.722218 | 1.8965948 |
| Rosen | 1.7663994 | 1.6751817 | 1.862584 |
| Varlotto | 1.8086122 | 1.7235931 | 1.8978251 |
| Ye | 1.808966 | 1.7243375 | 1.8977481 |
| Combined | 1.8089472 | 1.7243604 | 1.8976834 |
| CSS | |||
| Cornwell | 1.3648237 | 0.44740593 | 4.1634312 |
| Hamaji | 0.91862035 | 0.49507394 | 1.7045199 |
| Puri | 2.1175666 | 0.78394288 | 5.7199168 |
| Robinson | 1.75502 | 0.43099326 | 7.1465039 |
| Combined | 1.4895626 | 0.58800853 | 3.7734091 |
| RFS and DFS | |||
| Hamaji | 2.0039792 | 1.3983246 | 2.8719602 |
| Crabtree | 2.408608 | 1.6349978 | 3.5482571 |
| Kastelijn | 2.5226545 | 1.7660397 | 3.6034217 |
| Cornwell | 2.1214278 | 1.5168952 | 2.9668865 |
| Combined | 2.2454038 | 1.6462356 | 3.0626468 |
| RFS | |||
| Verstegen | 0.99619269 | 0.5796833 | 1.7119689 |
| Mokhles | 0.71148819 | 0.23445702 | 2.159097 |
| Ye | 0.55184406 | 0.26303679 | 1.1577539 |
| Varlotto | 0.73461908 | 0.23294972 | 2.3166597 |
| Combined | 0.73248023 | 0.33585692 | 1.5974877 |
| LRC | |||
| Robinson | 2.8066239 | 0.55296022 | 14.245398 |
| Hamaji | 1.4087356 | 0.96393794 | 2.05878 |
| Crabtree | 3.5956235 | 0.5200488 | 24.860184 |
| Combined | 2.2168428 | 0.68552249 | 7.1688269 |
| RCR | |||
| Crabtree | 1.28017 | 0.28629088 | 5.72437 |
| Hamaji | 1.0520202 | 0.55643898 | 1.9889807 |
| Robinson | 1.5083785 | 0.81575012 | 2.7890964 |
| Combined | 1.2287541 | 0.65902543 | 2.2910143 |
| L-RCR | |||
| Kastelijn | 0.90567803 | 0.28358454 | 2.8924453 |
| Verstegen | 1.754505 | 0.88387251 | 3.4827282 |
| Mokhles | 0.93215638 | 0.26229578 | 3.3127315 |
| Ye | 1.0030768 | 0.28693643 | 3.5065713 |
| Combined | 1.1059231 | 0.44077615 | 2.7748005 |
| DCR | |||
| Kastelijn | 1.3352301 | 0.68837976 | 2.5899065 |
| Verstegen | 1.5862026 | 0.90612304 | 2.7767076 |
| Crabtree | 1.4209255 | 0.66007811 | 3.0587735 |
| Hamaji | 1.0237905 | 0.73714757 | 1.4218956 |
| Mokhles | 1.3104142 | 0.67754769 | 2.5344126 |
| Robinson | 1.3211111 | 0.64130294 | 2.7215447 |
| Combined | 1.3150553 | 0.7484743 | 2.3105276 |
| Lobectomy vs. SBRT OS | |||
| Verstegen | 2.0371864 | 1.8275077 | 2.2709227 |
| Rosen | 2.0443203 | 1.1500962 | 3.6338234 |
| Mokhles | 2.0352147 | 1.3764541 | 3.0092535 |
| Hamaji | 1.8959923 | 1.2384275 | 2.9027023 |
| Eba | 1.9469334 | 1.4940101 | 2.5371647 |
| Cornwell | 1.9151517 | 1.3214743 | 2.7755408 |
| Combined | 1.9953141 | 1.452325 | 2.7413137 |
hr, hazard ratio; ul, upper CI limit; ll, lower CI limit; OS, overall survival; CSS, cause-specific survival; FFP, freedom from progression; RFS, recurrence-free survival; DFS, disease-free survival; LCR, local control rate; RCR, regional control rate; L-RCR, loco-regional control rate; DCR, distant control rate; SABR, stereotactic ablation radiotherapy.
Table S3
| Study omitted | hr | ll | ul |
|---|---|---|---|
| SBRT vs. surgery | |||
| Age/sex/tumour size/stage/CCI/FEV1 | |||
| Cornwell | 1.6495802 | 1.1118855 | 2.4472978 |
| Crabtree | 1.8368011 | 1.0681913 | 3.1584585 |
| Hamaji | 1.579457 | 1.0551697 | 2.3642497 |
| Mokhles | 1.782833 | 1.1317002 | 2.8086004 |
| Verstegen | 2.0083621 | 1.4169319 | 2.8466566 |
| Combined | 1.7689382 | 1.2226687 | 2.5592725 |
| Age/sex/tumour size/stage/CCI/FEV1/tumour site | |||
| Crabtree | 1.6228749 | 0.84796333 | 3.1059396 |
| Hamaji | 1.4343506 | 0.97291517 | 2.1146364 |
| Mokhles | 1.6216862 | 0.9721716 | 2.7051458 |
| Verstegen | 1.9035183 | 1.3119954 | 2.7617338 |
| Combined | 1.6495802 | 1.1118855 | 2.4472977 |
| Age/sex/tumour size/stage/CCI/FEV1/tumour site/pathology | |||
| Hamaji | 1.1559277 | 0.62260908 | 2.1460795 |
| Mokhles | 1.5628695 | 0.57187903 | 4.2711153 |
| Verstegen | 2.27895 | 1.3025346 | 3.9873126 |
| Combined | 1.6228749 | 0.84796335 | 3.1059396 |
| Age/sex/tumour size/stage/CCI/FEV1/tumour site/pathology/WHO performance score | |||
| Verstegen | 1.732 | 0.62119561 | 4.8291135 |
| Mokhles | 0.917 | 0.42207512 | 1.9922732 |
| Combined | 1.1559276 | 0.62260911 | 2.1460795 |
| SBRT vs. lobectomy | |||
| Age/sex/tumour size | |||
| Verstegen | 2.5995011 | 1.6162257 | 4.1809793 |
| Mokhles | 2.1960237 | 1.0460625 | 4.6101642 |
| Hamaji | 1.9456136 | 0.8923775 | 4.2419405 |
| Eba | 1.8368011 | 1.0681913 | 3.1584585 |
| Cornwell | 1.8996218 | 0.93906474 | 3.8427203 |
| Combined | 2.0443205 | 1.1500961 | 3.6338233 |
| Age/sex/tumour size/stage/CCI/FEV1 | |||
| Verstegen | 2.4252729 | 1.4882857 | 3.9521639 |
| Mokhles | 1.8709387 | 0.90483546 | 3.8685613 |
| Hamaji | 1.5760972 | 0.78540981 | 3.1627851 |
| Cornwell | 1.6228749 | 0.84796333 | 3.1059396 |
| Combined | 1.836801 | 1.0681913 | 3.1584586 |
| Age/sex/tumour size/stage/CCI/FEV1/tumour site/pathology | |||
| Verstegen | 2.27895 | 1.3025346 | 3.9873126 |
| Mokhles | 1.5628695 | 0.57187903 | 4.2711153 |
| Hamaji | 1.1559277 | 0.62260908 | 2.1460795 |
| Combined | 1.6228749 | 0.84796335 | 3.1059396 |
| Age/sex/tumoursize/stage/CCI/FEV1/tumour site/pathology/WHO performance score | |||
| Verstegen | 1.732 | 0.62119561 | 4.8291135 |
| Mokhles | 0.917 | 0.42207512 | 1.9922732 |
| Combined | 1.1559276 | 0.62260911 | 2.1460795 |
hr, hazard ratio; ul, upper CI limit; ll, lower CI limit; CCI, Charlson Comorbidity Index; FEV1, forced expiratory volume in one second.
Table S4
| Matched and comparable | Study number | Heterogeneity | Meta-analysis results | ||||
|---|---|---|---|---|---|---|---|
| I2 | P | HR 95% CI | P | Z | |||
| Age | 11 | 28.90% | 0.17 | 1.685 (1.502–1.892) | 0.000 | 8.87 | |
| Sex | 12 | 23.30% | 0.215 | 1.814 (1.672–1.968) | 0.000 | 14.33 | |
| Tumour size | 12 | 23.30% | 0.215 | 1.814 (1.672–1.968) | 0.000 | 14.33 | |
| Tumour site | 7 | 38.30% | 0.137 | 1.793 (1.542–2.084) | 0.000 | 7.59 | |
| Pathology | 7 | 4.20% | 0.394 | 1.665 (1.494–1.856) | 0.000 | 9.21 | |
| FEV1 | 6 | 12.50% | 0.335 | 1.630 (1.255–2.117) | 0.000 | 3.66 | |
| CCI | 8 | 13.80% | 0.322 | 1.775 (1.681–1.875) | 0.000 | 20.56 | |
| WHO performance score | 3 | 0 | 0.56 | 1.302 (0.901–1.882) | 0.16 | 1.41 | |
| Stage | 10 | 25.60% | 0.207 | 1.831 (1.653–2.027) | 0.000 | 11.64 | |
OS, overall survival; SBRT, stereotactic body radiotherapy; CCI, Charlson Comorbidity Index; FEV1, forced expiratory volume in one second.
Table S5
| Study omitted | hr | ll | ul |
|---|---|---|---|
| WHO performance score | |||
| Matsuo | 1.1559277 | 0.62260908 | 2.1460795 |
| Mokhles | 1.2482964 | 0.84137028 | 1.8520312 |
| Verstegen | 1.4418236 | 0.94897151 | 2.1906402 |
| Combined | 1.3021361 | 0.90105694 | 1.8817441 |
| CCI | |||
| Verstegen | 1.7811882 | 1.6861216 | 1.8816148 |
| Puri | 1.6464717 | 1.475185 | 1.837647 |
| Mokhles | 1.7331141 | 1.5635493 | 1.9210681 |
| Matsuo | 1.7591522 | 1.6143737 | 1.9169145 |
| Hamaji | 1.7368838 | 1.5980154 | 1.8878198 |
| Crabtree | 1.7366304 | 1.5642656 | 1.9279879 |
| Cornwell | 1.7380165 | 1.5949125 | 1.8939604 |
| Yerokun | 1.782964 | 1.587473 | 2.0025289 |
| Combined | 1.744878 | 1.6019877 | 1.9005136 |
| FEV1 | |||
| Verstegen | 1.754642 | 1.3294036 | 2.3159022 |
| Mokhles | 1.6232841 | 1.2388787 | 2.1269646 |
| Matsuo | 1.7603524 | 1.2806404 | 2.4197586 |
| Crabtree | 1.6226661 | 1.1938679 | 2.2054746 |
| Cornwell | 1.5605381 | 1.1904382 | 2.0456996 |
| Hamaji | 1.5022434 | 1.1308502 | 1.9956095 |
| Combined | 1.6301331 | 1.2552573 | 2.1169636 |
| Pathology | |||
| Verstegen | 1.6849387 | 1.5099949 | 1.880151 |
| Robinson | 1.6445134 | 1.3653029 | 1.9808236 |
| Mokhles | 1.6844364 | 1.4025178 | 2.0230231 |
| Matsuo | 1.7211684 | 1.4475336 | 2.0465298 |
| Hamaji | 1.645655 | 1.4742202 | 1.8370256 |
| Cornwell | 1.6536372 | 1.4825866 | 1.8444222 |
| Yerokun | 1.730846 | 1.3010579 | 2.3026092 |
| Combined | 1.6711456 | 1.4659635 | 1.905046 |
| Tumour site | |||
| Ye | 1.7877356 | 1.5173078 | 2.1063612 |
| Rosen | 1.6519463 | 1.4746035 | 1.8506172 |
| Verstegen | 1.8348652 | 1.6249032 | 2.0719573 |
| Mokhles | 1.7890148 | 1.5162021 | 2.1109152 |
| Hamaji | 1.760498 | 1.5058082 | 2.0582657 |
| Crabtree | 1.8011726 | 1.5160294 | 2.1399472 |
| Yerokun | 1.9698706 | 1.7690516 | 2.1934862 |
| Combined | 1.7928256 | 1.5420132 | 2.0844334 |
| Tumour size/sex | |||
| Yerokun | 1.8682752 | 1.7224461 | 2.0264506 |
| Eba | 1.8133087 | 1.6874046 | 1.948607 |
| Verstegen | 1.827387 | 1.7110267 | 1.9516605 |
| Cornwell | 1.8080132 | 1.6648475 | 1.9634902 |
| Crabtree | 1.8175399 | 1.663331 | 1.9860457 |
| Hamaji | 1.8048924 | 1.6622403 | 1.9597869 |
| Matsuo | 1.8289365 | 1.6869751 | 1.982844 |
| Mokhles | 1.8129864 | 1.6601011 | 1.9799514 |
| Puri | 1.7996904 | 1.5719037 | 2.0604858 |
| Robinson | 1.8072671 | 1.6516297 | 1.9775708 |
| Rosen | 1.7636899 | 1.6402458 | 1.8964244 |
| Ye | 1.8124709 | 1.6599579 | 1.9789964 |
| Combined | 1.8141657 | 1.6722329 | 1.9681452 |
| Age | |||
| Yerokun | 1.6628933 | 1.3799418 | 2.0038629 |
| Eba | 1.6912272 | 1.5269233 | 1.8732109 |
| Verstegen | 1.7187033 | 1.5561492 | 1.8982375 |
| Cornwell | 1.6729795 | 1.4894521 | 1.8791207 |
| Crabtree | 1.6797321 | 1.4780302 | 1.9089595 |
| Hamaji | 1.666374 | 1.4838452 | 1.8713557 |
| Matsuo | 1.7038975 | 1.5108503 | 1.9216111 |
| Mokhles | 1.6779509 | 1.4815413 | 1.9003987 |
| Puri | 1.5947312 | 1.3609457 | 1.8686769 |
| Puri | 1.7457726 | 1.5927151 | 1.9135388 |
| Ye | 1.6781578 | 1.4827392 | 1.8993316 |
| Combined | 1.6854631 | 1.5018507 | 1.8915234 |
| Stage | |||
| Verstegen | 1.8585182 | 1.7230624 | 2.0046227 |
| Cornwell | 1.8199849 | 1.6401489 | 2.0195391 |
| Crabtree | 1.8321621 | 1.6365567 | 2.0511467 |
| Hamaji | 1.8151941 | 1.6352527 | 2.0149362 |
| Mokhles | 1.8243045 | 1.6318126 | 2.0395031 |
| Puri | 1.7718397 | 1.4668602 | 2.1402283 |
| Puri | 1.8623133 | 1.7650927 | 1.9648887 |
| Robinson | 1.8146738 | 1.6156299 | 2.0382395 |
| Rosen | 1.7402323 | 1.5199064 | 1.992497 |
| Ye | 1.8234518 | 1.6317147 | 2.037719 |
| Combined | 1.830589 | 1.653334 | 2.0268475 |
hr, hazard ratio; ul, upper CI limit; ll, lower CI limit; OS, overall survival; SBRT, stereotactic body radiotherapy; CCI, Charlson Comorbidity Index; FEV1, forced expiratory volume in one second.
Search strategies of all databases besides PubMed
Table S1
| Study | Matching characteristics |
|---|---|
| Ye et al. 2018 | Age, male/female, tumour size, clinical staging, pathologic cell type*, tumour location, smoking status, SUVmax, COPD |
| Varlotto et al. 2013 | NA |
| Verstegen et al. 2013 | Age, male/female, tumour size, clinical staging, CCI, FEV1, pathologic cell type, tumour location, WHO performance score |
| Rosen et al. 2016 | Age*, male/female, tumour size, clinical staging, pathologic cell type*, tumour location, grade*, facility type, race, spanish hispanic origin, primary payer, median income, high school degree, urban/rural, facility location |
| Robinson et al. 2013 | Age*, male/female, tumour size, clinical staging, CCI*, FEV1*, pathologic cell type, DLCO*, ACE score*, FVC*, race, SUVmax |
| Puri et al. 2012 | Age, male/female*, clinical staging, FEV1*, DLCO*, ACE score |
| Puri et al. 2015 | Age, male/female, tumour size, clinical staging, CCI, facility type, race, urban location, income >$35,000/year, chemotherapy*, median survival* |
| Mokhles et al. 2015 | Age, male/female, tumour size, clinical staging, CCI, FEV1, pathologic cell type, tumour location, hypertension, WHO performance score |
| Matsuo et al. 2014 | Age, male/female, tumour size, CCI, FEV1, pathologic cell type*, performance status (0:1) |
| Kastelijn et al. 2015 | NA |
| Hamaji et al. 2015 | Age, male/female, tumour size, clinical staging, CCI, FEV1, pathologic cell type, tumour location, smoking status, comorbidities, serum CEA, serum SCC antigen, mortality within 30 days of treatment, follow-up period |
| Eba et al. 2016 | Age, male/female, tumour size, C/T ratio |
| Crabtree et al. 2014 | Age, male/female, tumour size, clinical staging, CCI, FEV1, tumour location, hypertension, smoking status, race, weight (lb), DLCO* |
| Cornwell et al. 2018 | Age, male/female, tumour size, clinical staging, CCI, FEV1, pathologic cell type, hypertension, smoking status, mediastinal staging via EBUS* |
| Yerokun et al. 2017 | Age, male/female, tumour size, CCI, pathologic cell type, tumour location, facility type, insurance status, distance to hospital |
*, the characteristics have significant differences between SBRT and surgery (P<0.05). NA, not applicable; FEV1, forced expiratory volume in one second; CEA, carcinoembryonic antigen; DLCO, diffusing capacity of lung for carbon monoxide; ACE, adult comorbidity evaluation; FVC, forced vital capacity; SUVmax, maximum standardized uptake value; CCI, Charlson Comorbidity Index; SCC, squamous cell carcinoma.
Table S2
| Study omitted | hr | ul | ll |
|---|---|---|---|
| OS | |||
| Yerokun | 1.8400707 | 1.7465854 | 1.9385599 |
| Eba | 1.8073877 | 1.7228516 | 1.8960718 |
| Kastelijn | 1.8094635 | 1.7246432 | 1.8984553 |
| Verstegen | 1.8136526 | 1.7286876 | 1.9027938 |
| Cornwell | 1.8069048 | 1.7223189 | 1.8956448 |
| Crabtree | 1.8104978 | 1.7254543 | 1.8997327 |
| Hamaji | 1.8056992 | 1.7210512 | 1.8945105 |
| Matsuo | 1.8142195 | 1.7289299 | 1.9037163 |
| Mokhles | 1.8091191 | 1.724434 | 1.897963 |
| Puri | 1.7940363 | 1.6668215 | 1.9309602 |
| Puri | 1.8213148 | 1.7353703 | 1.9115158 |
| Robinson | 1.8073045 | 1.722218 | 1.8965948 |
| Rosen | 1.7663994 | 1.6751817 | 1.862584 |
| Varlotto | 1.8086122 | 1.7235931 | 1.8978251 |
| Ye | 1.808966 | 1.7243375 | 1.8977481 |
| Combined | 1.8089472 | 1.7243604 | 1.8976834 |
| CSS | |||
| Cornwell | 1.3648237 | 0.44740593 | 4.1634312 |
| Hamaji | 0.91862035 | 0.49507394 | 1.7045199 |
| Puri | 2.1175666 | 0.78394288 | 5.7199168 |
| Robinson | 1.75502 | 0.43099326 | 7.1465039 |
| Combined | 1.4895626 | 0.58800853 | 3.7734091 |
| RFS and DFS | |||
| Hamaji | 2.0039792 | 1.3983246 | 2.8719602 |
| Crabtree | 2.408608 | 1.6349978 | 3.5482571 |
| Kastelijn | 2.5226545 | 1.7660397 | 3.6034217 |
| Cornwell | 2.1214278 | 1.5168952 | 2.9668865 |
| Combined | 2.2454038 | 1.6462356 | 3.0626468 |
| RFS | |||
| Verstegen | 0.99619269 | 0.5796833 | 1.7119689 |
| Mokhles | 0.71148819 | 0.23445702 | 2.159097 |
| Ye | 0.55184406 | 0.26303679 | 1.1577539 |
| Varlotto | 0.73461908 | 0.23294972 | 2.3166597 |
| Combined | 0.73248023 | 0.33585692 | 1.5974877 |
| LRC | |||
| Robinson | 2.8066239 | 0.55296022 | 14.245398 |
| Hamaji | 1.4087356 | 0.96393794 | 2.05878 |
| Crabtree | 3.5956235 | 0.5200488 | 24.860184 |
| Combined | 2.2168428 | 0.68552249 | 7.1688269 |
| RCR | |||
| Crabtree | 1.28017 | 0.28629088 | 5.72437 |
| Hamaji | 1.0520202 | 0.55643898 | 1.9889807 |
| Robinson | 1.5083785 | 0.81575012 | 2.7890964 |
| Combined | 1.2287541 | 0.65902543 | 2.2910143 |
| L-RCR | |||
| Kastelijn | 0.90567803 | 0.28358454 | 2.8924453 |
| Verstegen | 1.754505 | 0.88387251 | 3.4827282 |
| Mokhles | 0.93215638 | 0.26229578 | 3.3127315 |
| Ye | 1.0030768 | 0.28693643 | 3.5065713 |
| Combined | 1.1059231 | 0.44077615 | 2.7748005 |
| DCR | |||
| Kastelijn | 1.3352301 | 0.68837976 | 2.5899065 |
| Verstegen | 1.5862026 | 0.90612304 | 2.7767076 |
| Crabtree | 1.4209255 | 0.66007811 | 3.0587735 |
| Hamaji | 1.0237905 | 0.73714757 | 1.4218956 |
| Mokhles | 1.3104142 | 0.67754769 | 2.5344126 |
| Robinson | 1.3211111 | 0.64130294 | 2.7215447 |
| Combined | 1.3150553 | 0.7484743 | 2.3105276 |
| Lobectomy vs. SBRT OS | |||
| Verstegen | 2.0371864 | 1.8275077 | 2.2709227 |
| Rosen | 2.0443203 | 1.1500962 | 3.6338234 |
| Mokhles | 2.0352147 | 1.3764541 | 3.0092535 |
| Hamaji | 1.8959923 | 1.2384275 | 2.9027023 |
| Eba | 1.9469334 | 1.4940101 | 2.5371647 |
| Cornwell | 1.9151517 | 1.3214743 | 2.7755408 |
| Combined | 1.9953141 | 1.452325 | 2.7413137 |
hr, hazard ratio; ul, upper CI limit; ll, lower CI limit; OS, overall survival; CSS, cause-specific survival; FFP, freedom from progression; RFS, recurrence-free survival; DFS, disease-free survival; LCR, local control rate; RCR, regional control rate; L-RCR, loco-regional control rate; DCR, distant control rate; SABR, stereotactic ablation radiotherapy.
Table S3
| Study omitted | hr | ll | ul |
|---|---|---|---|
| SBRT vs. surgery | |||
| Age/sex/tumour size/stage/CCI/FEV1 | |||
| Cornwell | 1.6495802 | 1.1118855 | 2.4472978 |
| Crabtree | 1.8368011 | 1.0681913 | 3.1584585 |
| Hamaji | 1.579457 | 1.0551697 | 2.3642497 |
| Mokhles | 1.782833 | 1.1317002 | 2.8086004 |
| Verstegen | 2.0083621 | 1.4169319 | 2.8466566 |
| Combined | 1.7689382 | 1.2226687 | 2.5592725 |
| Age/sex/tumour size/stage/CCI/FEV1/tumour site | |||
| Crabtree | 1.6228749 | 0.84796333 | 3.1059396 |
| Hamaji | 1.4343506 | 0.97291517 | 2.1146364 |
| Mokhles | 1.6216862 | 0.9721716 | 2.7051458 |
| Verstegen | 1.9035183 | 1.3119954 | 2.7617338 |
| Combined | 1.6495802 | 1.1118855 | 2.4472977 |
| Age/sex/tumour size/stage/CCI/FEV1/tumour site/pathology | |||
| Hamaji | 1.1559277 | 0.62260908 | 2.1460795 |
| Mokhles | 1.5628695 | 0.57187903 | 4.2711153 |
| Verstegen | 2.27895 | 1.3025346 | 3.9873126 |
| Combined | 1.6228749 | 0.84796335 | 3.1059396 |
| Age/sex/tumour size/stage/CCI/FEV1/tumour site/pathology/WHO performance score | |||
| Verstegen | 1.732 | 0.62119561 | 4.8291135 |
| Mokhles | 0.917 | 0.42207512 | 1.9922732 |
| Combined | 1.1559276 | 0.62260911 | 2.1460795 |
| SBRT vs. lobectomy | |||
| Age/sex/tumour size | |||
| Verstegen | 2.5995011 | 1.6162257 | 4.1809793 |
| Mokhles | 2.1960237 | 1.0460625 | 4.6101642 |
| Hamaji | 1.9456136 | 0.8923775 | 4.2419405 |
| Eba | 1.8368011 | 1.0681913 | 3.1584585 |
| Cornwell | 1.8996218 | 0.93906474 | 3.8427203 |
| Combined | 2.0443205 | 1.1500961 | 3.6338233 |
| Age/sex/tumour size/stage/CCI/FEV1 | |||
| Verstegen | 2.4252729 | 1.4882857 | 3.9521639 |
| Mokhles | 1.8709387 | 0.90483546 | 3.8685613 |
| Hamaji | 1.5760972 | 0.78540981 | 3.1627851 |
| Cornwell | 1.6228749 | 0.84796333 | 3.1059396 |
| Combined | 1.836801 | 1.0681913 | 3.1584586 |
| Age/sex/tumour size/stage/CCI/FEV1/tumour site/pathology | |||
| Verstegen | 2.27895 | 1.3025346 | 3.9873126 |
| Mokhles | 1.5628695 | 0.57187903 | 4.2711153 |
| Hamaji | 1.1559277 | 0.62260908 | 2.1460795 |
| Combined | 1.6228749 | 0.84796335 | 3.1059396 |
| Age/sex/tumoursize/stage/CCI/FEV1/tumour site/pathology/WHO performance score | |||
| Verstegen | 1.732 | 0.62119561 | 4.8291135 |
| Mokhles | 0.917 | 0.42207512 | 1.9922732 |
| Combined | 1.1559276 | 0.62260911 | 2.1460795 |
hr, hazard ratio; ul, upper CI limit; ll, lower CI limit; CCI, Charlson Comorbidity Index; FEV1, forced expiratory volume in one second.
Table S4
| Matched and comparable | Study number | Heterogeneity | Meta-analysis results | ||||
|---|---|---|---|---|---|---|---|
| I2 | P | HR 95% CI | P | Z | |||
| Age | 11 | 28.90% | 0.17 | 1.685 (1.502–1.892) | 0.000 | 8.87 | |
| Sex | 12 | 23.30% | 0.215 | 1.814 (1.672–1.968) | 0.000 | 14.33 | |
| Tumour size | 12 | 23.30% | 0.215 | 1.814 (1.672–1.968) | 0.000 | 14.33 | |
| Tumour site | 7 | 38.30% | 0.137 | 1.793 (1.542–2.084) | 0.000 | 7.59 | |
| Pathology | 7 | 4.20% | 0.394 | 1.665 (1.494–1.856) | 0.000 | 9.21 | |
| FEV1 | 6 | 12.50% | 0.335 | 1.630 (1.255–2.117) | 0.000 | 3.66 | |
| CCI | 8 | 13.80% | 0.322 | 1.775 (1.681–1.875) | 0.000 | 20.56 | |
| WHO performance score | 3 | 0 | 0.56 | 1.302 (0.901–1.882) | 0.16 | 1.41 | |
| Stage | 10 | 25.60% | 0.207 | 1.831 (1.653–2.027) | 0.000 | 11.64 | |
OS, overall survival; SBRT, stereotactic body radiotherapy; CCI, Charlson Comorbidity Index; FEV1, forced expiratory volume in one second.
Table S5
| Study omitted | hr | ll | ul |
|---|---|---|---|
| WHO performance score | |||
| Matsuo | 1.1559277 | 0.62260908 | 2.1460795 |
| Mokhles | 1.2482964 | 0.84137028 | 1.8520312 |
| Verstegen | 1.4418236 | 0.94897151 | 2.1906402 |
| Combined | 1.3021361 | 0.90105694 | 1.8817441 |
| CCI | |||
| Verstegen | 1.7811882 | 1.6861216 | 1.8816148 |
| Puri | 1.6464717 | 1.475185 | 1.837647 |
| Mokhles | 1.7331141 | 1.5635493 | 1.9210681 |
| Matsuo | 1.7591522 | 1.6143737 | 1.9169145 |
| Hamaji | 1.7368838 | 1.5980154 | 1.8878198 |
| Crabtree | 1.7366304 | 1.5642656 | 1.9279879 |
| Cornwell | 1.7380165 | 1.5949125 | 1.8939604 |
| Yerokun | 1.782964 | 1.587473 | 2.0025289 |
| Combined | 1.744878 | 1.6019877 | 1.9005136 |
| FEV1 | |||
| Verstegen | 1.754642 | 1.3294036 | 2.3159022 |
| Mokhles | 1.6232841 | 1.2388787 | 2.1269646 |
| Matsuo | 1.7603524 | 1.2806404 | 2.4197586 |
| Crabtree | 1.6226661 | 1.1938679 | 2.2054746 |
| Cornwell | 1.5605381 | 1.1904382 | 2.0456996 |
| Hamaji | 1.5022434 | 1.1308502 | 1.9956095 |
| Combined | 1.6301331 | 1.2552573 | 2.1169636 |
| Pathology | |||
| Verstegen | 1.6849387 | 1.5099949 | 1.880151 |
| Robinson | 1.6445134 | 1.3653029 | 1.9808236 |
| Mokhles | 1.6844364 | 1.4025178 | 2.0230231 |
| Matsuo | 1.7211684 | 1.4475336 | 2.0465298 |
| Hamaji | 1.645655 | 1.4742202 | 1.8370256 |
| Cornwell | 1.6536372 | 1.4825866 | 1.8444222 |
| Yerokun | 1.730846 | 1.3010579 | 2.3026092 |
| Combined | 1.6711456 | 1.4659635 | 1.905046 |
| Tumour site | |||
| Ye | 1.7877356 | 1.5173078 | 2.1063612 |
| Rosen | 1.6519463 | 1.4746035 | 1.8506172 |
| Verstegen | 1.8348652 | 1.6249032 | 2.0719573 |
| Mokhles | 1.7890148 | 1.5162021 | 2.1109152 |
| Hamaji | 1.760498 | 1.5058082 | 2.0582657 |
| Crabtree | 1.8011726 | 1.5160294 | 2.1399472 |
| Yerokun | 1.9698706 | 1.7690516 | 2.1934862 |
| Combined | 1.7928256 | 1.5420132 | 2.0844334 |
| Tumour size/sex | |||
| Yerokun | 1.8682752 | 1.7224461 | 2.0264506 |
| Eba | 1.8133087 | 1.6874046 | 1.948607 |
| Verstegen | 1.827387 | 1.7110267 | 1.9516605 |
| Cornwell | 1.8080132 | 1.6648475 | 1.9634902 |
| Crabtree | 1.8175399 | 1.663331 | 1.9860457 |
| Hamaji | 1.8048924 | 1.6622403 | 1.9597869 |
| Matsuo | 1.8289365 | 1.6869751 | 1.982844 |
| Mokhles | 1.8129864 | 1.6601011 | 1.9799514 |
| Puri | 1.7996904 | 1.5719037 | 2.0604858 |
| Robinson | 1.8072671 | 1.6516297 | 1.9775708 |
| Rosen | 1.7636899 | 1.6402458 | 1.8964244 |
| Ye | 1.8124709 | 1.6599579 | 1.9789964 |
| Combined | 1.8141657 | 1.6722329 | 1.9681452 |
| Age | |||
| Yerokun | 1.6628933 | 1.3799418 | 2.0038629 |
| Eba | 1.6912272 | 1.5269233 | 1.8732109 |
| Verstegen | 1.7187033 | 1.5561492 | 1.8982375 |
| Cornwell | 1.6729795 | 1.4894521 | 1.8791207 |
| Crabtree | 1.6797321 | 1.4780302 | 1.9089595 |
| Hamaji | 1.666374 | 1.4838452 | 1.8713557 |
| Matsuo | 1.7038975 | 1.5108503 | 1.9216111 |
| Mokhles | 1.6779509 | 1.4815413 | 1.9003987 |
| Puri | 1.5947312 | 1.3609457 | 1.8686769 |
| Puri | 1.7457726 | 1.5927151 | 1.9135388 |
| Ye | 1.6781578 | 1.4827392 | 1.8993316 |
| Combined | 1.6854631 | 1.5018507 | 1.8915234 |
| Stage | |||
| Verstegen | 1.8585182 | 1.7230624 | 2.0046227 |
| Cornwell | 1.8199849 | 1.6401489 | 2.0195391 |
| Crabtree | 1.8321621 | 1.6365567 | 2.0511467 |
| Hamaji | 1.8151941 | 1.6352527 | 2.0149362 |
| Mokhles | 1.8243045 | 1.6318126 | 2.0395031 |
| Puri | 1.7718397 | 1.4668602 | 2.1402283 |
| Puri | 1.8623133 | 1.7650927 | 1.9648887 |
| Robinson | 1.8146738 | 1.6156299 | 2.0382395 |
| Rosen | 1.7402323 | 1.5199064 | 1.992497 |
| Ye | 1.8234518 | 1.6317147 | 2.037719 |
| Combined | 1.830589 | 1.653334 | 2.0268475 |
hr, hazard ratio; ul, upper CI limit; ll, lower CI limit; OS, overall survival; SBRT, stereotactic body radiotherapy; CCI, Charlson Comorbidity Index; FEV1, forced expiratory volume in one second.
Acknowledgments
Funding:
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.41). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen WQ, Sun KX, Zheng RS, et al. Report of Cancer Incidence and Mortality in Different Areas of China, 2014. Zhonghua Zhong Liu Za Zhi 2018;40:894-9. [PubMed]
- Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol 2016;893:1-19. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2014, based on November 2016 SEER data submission, posted to the SEER web site, April 2017. Bethesda, MD: National Cancer Institute; 2017.
- Carney DN. Lung cancer--time to move on from chemotherapy. N Engl J Med 2002;346:126-8. [Crossref] [PubMed]
- Chute JP, Chen T, Feigal E, et al. Twenty years of phase III trials for patients with extensive-stage small-cell lung cancer: perceptible progress. J Clin Oncol 1999;17:1794-801. [Crossref] [PubMed]
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-e313S.
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Samson P, Keogan K, Crabtree T, et al. Interpreting survival data from clinical trials of surgery versus stereotactic body radiation therapy in operable Stage I non-small cell lung cancer patients. Lung Cancer 2017;103:6-10. [Crossref] [PubMed]
- Verstegen NE, Oosterhuis J W, Palma DA, et al. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol 2013;24:1543-8. [Crossref] [PubMed]
- Robinson CG, Dewees TA, Naqa IME, et al. Patterns of Failure after Stereotactic Body Radiation Therapy or Lobar Resection for Clinical Stage I Non–Small-Cell Lung Cancer. J Thorac Oncol 2013;8:192-201. [PubMed]
- Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the Quality of non-randomised studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [Crossref] [PubMed]
- Ye L, Xu F, Shi S, et al. A SUVmax-based propensity matched analysis of stereotactic body radiotherapy versus surgery in stage I non-small cell lung cancer: unveiling the role of 18F-FDG PET/CT in clinical decision-making. Clin Transl Oncol 2018;20:1026-34. [Crossref] [PubMed]
- Varlotto J, Fakiris A, Flickinger J, et al. Matched-pair and propensity score comparisons of outcomes of patients with clinical stage I non-small cell lung cancer treated with resection or stereotactic radiosurgery. Cancer 2013;119:2683-91. [Crossref] [PubMed]
- Rosen JE, Salazar MC, Wang Z, et al. Lobectomy versus stereotactic body radiotherapy in healthy patients with stage I lung cancer. J Thorac Cardiovasc Surg 2016;152:44-54.e9. [Crossref] [PubMed]
- Puri V, Crabtree TD, Kymes S, et al. A comparison of surgical intervention and stereotactic body radiation therapy for stage I lung cancer in high-risk patients: a decision analysis. J Thorac Cardiovasc Surg 2012;143:428-36. [Crossref] [PubMed]
- Puri V, Crabtree TD, Bell JM, et al. Treatment Outcomes in Stage I Lung Cancer: A Comparison of Surgery and Stereotactic Body Radiation Therapy. J Thorac Oncol 2015;10:1776-84. [Crossref] [PubMed]
- Mokhles S, Verstegen N, Maat A P, et al. Comparison of clinical outcome of stage I non-small cell lung cancer treated surgically or with stereotactic radiotherapy: results from propensity score analysis. Lung Cancer 2015;87:283-9. [Crossref] [PubMed]
- Matsuo Y, Chen F, Hamaji M, et al. Comparison of long-term survival outcomes between stereotactic body radiotherapy and sublobar resection for stage I non-small-cell lung cancer in patients at high risk for lobectomy: A propensity score matching analysis. Eur J Cancer 2014;50:2932-8. [Crossref] [PubMed]
- Kastelijn EA, El Sharouni SY, Hofman FN, et al. Clinical Outcomes in Early-stage NSCLC Treated with Stereotactic Body Radiotherapy Versus Surgical Resection. Anticancer Res 2015;35:5607-14. [PubMed]
- Hamaji M, Chen F, Matsuo Y, et al. Video-assisted thoracoscopic lobectomy versusstereotactic radiotherapy for stage I lung cancer. Ann Thorac Surg 2015;99:1122-9. [Crossref] [PubMed]
- Eba J, Nakamura K, Mizusawa J, et al. Stereotactic body radiotherapy versus lobectomy for operable clinical stage IA lung adenocarcinoma: comparison of survival, outcomes in two clinical trials with propensity score analysis (JCOG1313-A). Jpn J Clin Oncol 2016;46:748-53. [Crossref] [PubMed]
- Crabtree T, Puri V, Robinson C, et al. Analysis of First Recurrence and Survival in Patients with Stage I Non-Small Cell Lung Cancer Treated with Surgical Resection or Stereotactic Radiation Therapy. J Thorac Cardiovasc Surg 2014;147:1183-91. [Crossref] [PubMed]
- Cornwell LD, Echeverria AE, Samuelian J, et al. Video-assisted thoracoscopic lobectomy is associated with greater recurrence-free survival than stereotactic body radiotherapy for clinical stage I lung cancer. J Thorac Cardiovasc Surg 2018;155:395-402. [Crossref] [PubMed]
- Yerokun BA, Yang CJ, Gulack BC, et al. A national analysis of wedge resection versus stereotactic body radiation therapy for stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;154:675-86.e4. [Crossref] [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Detterbeck FC, Mazzone PJ, Naidich DP, et al. Screening for lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e78S-e92S.
- Uematsu M, Shioda A, Suda A, et al. Computed tomography-guided frameless stereotactic radiotherapy for stage I non-small cell lung cancer: a 5-year experience. Int J Radiat Oncol Biol Phys 2001;51:666-70. [Crossref] [PubMed]
- Alliance for Clinical Trials in Oncology. Surgery With Or Without Internal Radiation Therapy Compared With Stereotactic Body Radiation Therapy in Treating Patients With High-Risk Stage I Non–Small Cell Lung Cancer. In: ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US); 2000. NLM Identifier: NCT01336894. Available online: https://clinicaltrials.gov/ct2/show/NCT01336894. Accessed September 30, 2016.
- M.D. Anderson Cancer Center. Randomized Study to Compare CyberKnife to Surgical Resection in Stage I Non–small Cell Lung Cancer (STARS). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US); 2000. NLM Identifier: NCT00840749. Available online: https://clinicaltrials.gov/ct2/show/NCT00840749. Accessed September 30, 2016.
- Trial of Either Surgery or Stereotactic Radiotherapy for Early Stage (IA) Lung Cancer (ROSEL). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US); 2000. NLMIdentifier: NCT00687986. Available online: https://clinicaltrials.gov/ct2/show/NCT00687986. Accessed September 30, 2016.
- Schaue D, Ratikan JA, Iwamoto KS, et al. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys 2012;83:1306-10. [Crossref] [PubMed]
- Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589-95. [Crossref] [PubMed]
- Timmerman R, Bastasch M, Saha D, et al. Stereotactic body radiation therapy: normal tissue and tumor control effects with large dose per fraction. Front Radiat Ther Oncol 2011;43:382-94. [Crossref] [PubMed]
- O’Leary DP, Wang JH, Cotter TG, et al. Less stress, more success? Oncological implications of surgery-induced oxidative stress. Gut 2013;62:461-70. [Crossref] [PubMed]
- Snee MP, McParland L, Collinson F, et al. The SABRTooth feasibility trial protocol: a study to determine the feasibility and acceptability of conducting a phase III randomised controlled trial comparing stereotactic ablative radiotherapy (SABR) with surgery in patients with peripheral stage I non–small cell lung cancer (NSCLC) considered to be at higher risk of complications from surgical resection. Pilot Feasibility Stud 2016;2:5. [Crossref] [PubMed]