A daily quality assurance routine for ultrasounds in vitro experiments
Introduction
Ultrasounds (US) are extensively used in medicine both for diagnostic imaging and for noninvasive therapies. Their high-intensity and focused version are recently emerging as a promising weapon for solid tumors treatment (1). Therapeutic strategies are based on the capability of acoustic waves to interact with biological soft tissues through thermal and non-thermal physical mechanisms, to produce a wide range of bioeffects. As thermal bioeffects are referred changes in living cells and tissues resulting from temperature increase due to the conversion of acoustic energy into heat. In relation with the total acoustic power delivered and the duration of the exposure, different temperatures could be reached. In the low-power and low-duration acoustic applications, formerly that one used in the diagnostic medical sonography applications, energy absorption is limited and temperature rise is well below 2-3 °C (2). This rise cannot be neglected for pre-natal ecography where the temperature of the fetus should not safely rise more than 0.5 °C above its normal temperature (3). Even though there is no experimentation that can prove a causal link between birth defects and in utero ultrasound exposure, the relation between brain malformations and US exposure longer than 30 minutes in mice is been demonstrated (4). Increasing energies beyond the range of diagnostic ultrasound, the range of the hyperthermia (to 40-43 °C) is found, in which the ultrasound are used to heal or to enhance others therapies (5). Over, with temperatures higher than 43 °C, it possible to reach the thermal ablation of tissues, with an efficacy that depends on the duration of the exposure and on the cellular types (6,7). Nowadays the clinical application of the High Intensity Focused Ultrasound (HIFU) systems is limited to few procedures, every one based on the employment of the thermal effects. The most established of these is the treatment of uterine fibroids (8), for which, the Magnetic Resonance guided Focused Ultrasound Surgery (MRgFUS) ExAblate 2000 (InSightec Ltd, Tirat Carmel, Israel) received in 2004 the FDA approval. In the meanwhile, HIFU systems guided by both magnetic resonance and US, underwent clinical trials for fields in which have proven them reliability: palliative treatment of bone metastasis (9), breast (10) and prostate (11) cancer but also in treatment of the essential tremor (12). Some of these systems are daily employed in Europe thanks to the CE mark approval and time seems to be ripe for liver treatment too (13). Much more work is required to push on the clinical employment of non-thermal US for therapy applications. Large part of non-thermal mechanisms could be referred to mechanical effects, such as radiation force, radiation torque and acoustic streaming which act as physical forces applied on tissues (2). One of the most relevant non-thermal effects is the cavitation, described as the formation, growth, oscillation and collapse of gaseous microbubbles within tissues (14). The negative pressure peak makes water and volatile substances pass from the liquid to the gas state at a very small scale [depending on US amplitude and frequency (15)]. Bubbles of gas begin oscillate around a neutral position, expanding and rarefacting their volume in a harmonic way. In the low intensity regime, the phenomenon is almost stable, generating some micro-streaming that induces small sized pores into the cell’s plasma membrane (16). Increasing energy can turn cavitation into unstable, making bubbles collapse with the generation of a powerful micro-jet (17) that can damage permanently the lipid membrane. At the highest energies, cavitation can lead to a complete tissue disruption, useful feature for an application like histotripsy (18). It is impossible to totally avoid cavitation during an ablation treatment; for this reason this phenomenon is often used as an advantage in solid tumor treatment, but it’s the most widely investigated non-thermal bioeffects for its non-disruptive potential. Ultrasound mediated Targeted Drug Delivery (UmTDD) (19) and SonoDynamic Therapy (SDT) (20) are two of the novel promising therapeutic applications of Focused UltraSound (FUS) employing mechanical effects. Extensive physical and biological studies have been carried out and there are encouraging evidences to suggest that FUS exposure is a powerful stimulus to induce stress response and apoptosis with minimal lysis in several cancer cell lines (21-24); hence, in non-surgical cancer treatment, US apoptotic anticancer therapy is beginning to emerge as a preferred approach to achieving tumor cell killing. Furthermore, US irradiation of cancer cell lines in conjunction with hyperthermia (25), photodynamic therapy (26), radiotherapy (27,28) and chemotherapy (29) produces a synergistic effect to cell death in vitro, described as the overall decrease in number of viable cells and inhibition of cancer cell proliferation. In order to investigate the potential of US in these applications, in vitro researches are essential, to identify and better understand molecular mechanisms induced within cells and tissues. In a few in vitro experiments, clinical systems are also used to guide and monitor the beam, some examples are the magnetic resonance imaging (19,30) and the US (23). In other experiments authors reserve a part of the study to the acoustic characterization, employing professional instruments such as US power meter (14,21,22,27,31) or hydrophones (24,25,32-35) to determine the averaged (in space, time and both of them) intensity (W/cm2) or the pressure field (MPa). In these works, cells are grown onto supports of various kind and nature, like flasks (36), tubes (25,37), and Petri dishes (38,39), and exposed to the protocol in study into water basins (40) in which the transducer is housed. This is an easy way to ensure a strong acoustic coupling of the whole apparatus, but at the same time carry with it a lot of uncertainty: to avoid stationary wave arise, beams are often addressed to cells with a non-perpendicular path, but that can cause unpredicted reflections and cells exposition to an high spatial variability (17). For focused clinical systems, the use of cultures plates is preferred (41,42). Plates are housed in a fixed geometry setup, coupled with the transducers bath. Flasks, plates, tubes or Petri dishes, if not specifically designed (34,43), are made of plastic materials (polyethylene, polystyrene), which are not impermeable to ultrasound and could potentially absorb a large part of the energy delivered (25,37). In order to obtain repeatable and precise results an accurate ultrasound characterization and control is needed, like it is done in the clinical field, where a daily quality assurance (DQA) is a routine for the HIFU applications (44,45). Our belief is that, if the role of a physical variable of interest is investigated, the capability of the apparatus to control it should be checked before every session. In the following we will show the tests we have carried out in order to obtain useful data from our in vitro researches, considering that our initial interest is on the power delivered and the focus position in the space.
Materials and methods
Focused ultrasound system
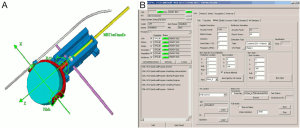
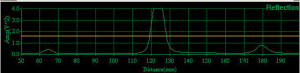
The source of US, is the InSightec ExAblate 2100 (InSightec Ltd, Tirat Carmel, Israel) MRgFUS equipment available in our institute (LaTO srl, Cefalù (PA), Italy) (46). It incorporates a 208 elements phased array transducer which can operate with a frequency that varies from 0.9 to 1.3 MHz and an energy from 100 to 6,000 J. This transducer has five degrees of freedom in the space: it can be elevated (Z), moved in the horizontal plane (XY) and rotated around the two axes that form this plane (roll and pitch angles). The transducer can be moved through his axis thanks to CGA-CSA. 4.75.24.[00] software (Figure 1). It also is possible to modify power, frequency and duration of the signal. A continuous impulse with 1 MHz frequency, 20 s duration and a variable intensity (<10 W/cm2) have been preferred for the purpose of assess the ranges commonly used in “low intensity” in vitro experiments (47). Below, we have characterized the radiation force. The software used (Figure 1), gives the possibility to monitor the reflection (Figure 2) in the hypersonic field giving a single pulse of desired power before the requested insonation. This feature reduces the probability of damaging the transducer, and gives the possibility to monitor the distance of the objects receiving the acoustic energy. Spot position in the space is one of the relevant variables that has been investigated too.
Radiation force and weighing system
US are the result of a mechanical perturbation of a medium, like air or water, which is elastic and continuous. This perturbation generates the travel of a pressure wave that runs from the emitter to the receiver. Real bodies are not perfect emitter and their vibration produces not just a single traveling plane wave, but a field to which, every single object within is subjected. This pressure is said “radiating” (PRad N/cm2) and the integration of its value all across a surface is the Radiation Force FRad. It is difficult to obtain a complete understanding of this pressure field, PRad(S), but it is possible to apply a simplification when the total acoustic power output
This expression is reliable for high absorbing (α≈1), low reflecting bodies (2). A correct estimation of the radiation force means a correct evaluation of transducer power and then of the intensity brought to the target spot. An easy and cheap way to measure radiation force indirectly is made by the acquisition of the apparent weight reduction of a high absorbing target. This reduction is the result of the difference between the real weight force of the target and the radiation force of the hypersonic beam, supposed to act in opposition into the vertical direction. For this purpose we employed a high precision (0.01 g) digital scale for generic purpose. The scale was placed over the top of a water phantom, with its weighing plate firmly linked to the guides of the acoustic target, as shown in Figure 3. An external command was added to avoid the perturbations of the system caused by the movement induced by cyclic tare procedures. Then an acrylic-glass support for the MR pelvic coil was put around the weighing system to shield it (Figure 3) from the environmental perturbations. Those evidence were averaged and transcribed into a spreadsheet with a resolution of 1 record for second.
3D modeling
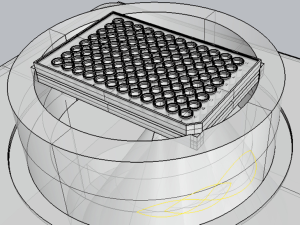
To overcome the deficit of an US guide and predict beams path, we have developed the 3D model shown in Figure 4. The model reproduces the essential parts of the ExAblate 2100 in the in vitro configuration. The coupling and positioning system for culture plates, supplied by InSightec, are also represented. It has a water phantom with two plastic rings specially carved to house the standards culture plates and keep them in a fixed position in the space. These plates, used in in vitro studies (26,41,48), have standard dimension.
Gel phantom targets
Following the procedure described in (49), a transparent polyacrylamide hydrogel, supplemented with bovine serum albumin (BSA) has been realized. This phantom has the feature to be optically transparent at room temperature, becoming opaque once overheated, thanks to protein denaturation of BSA. We filled with this gel some of the 96 wells of a special plate with a membrane at the bottom (Gas Permeable Plates. Coy Laboratory Products, Grass Lake, MI, United States), in order to verify the geometrical accuracy of our automatic system.
Custom software
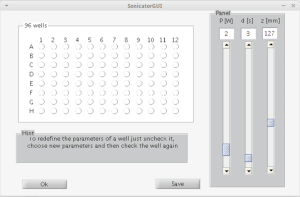
Geometrical calibration is an iterative process. The CGA-CSA has the capability to execute multiple insonations with different features reading the information from an *.ini file (stress test panel, Figure 1B); but the coordinates have to be known. In order to automatically execute these operations, a MatLab® (MathWorks) custom code was been wrote (Figure 5). This reduces the preparation phase and gives to the user the possibility to plan the parameters of each well before the calibration phase. This consists in the process to establish a relationship between the inner reference system of the FUS apparatus and the system of the code. Output of the MatLab® (MathWorks) code is an *.ini file that can be read by the machine as described above.
Results
Radiation force tests
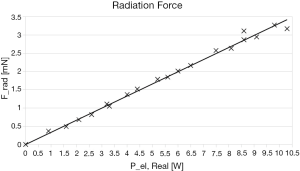
A total of 213 tests to evaluate the radiation force have been performed. The data are listed in Table 1. During each insonation, CGA-CSA software displays the real electrical power Pel,Real emitted by the transducer, this data are reported in Table 1. Trials are made with a mean duration d of 20 seconds and an average of 11 repetitions for each power. Frad is obtained multiplying the apparent weight reduction w, read from the scale, for the gravitational acceleration g. For every value of radiation force, the type A uncertainty U% has been calculated with a confidence interval of the 95 percentile. There’s no value with an uncertainty over 5%. The maximum type A uncertainty recorded is 4.42% for the lower power of 1 W which produces an apparent weight reduction w of 0.037 g: too close to the sensitivity of the scale. Those medium values were fitted with a straight line forced to the origin of the axes trough an ordinary least squares regression:
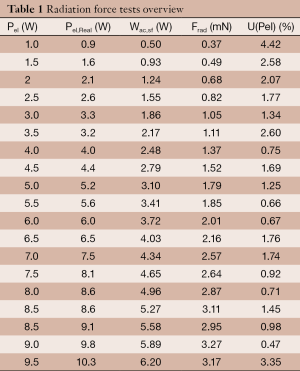
Full table
The data and the fit are shown in the Figure 6, the coefficient of determination, R2, is 0.9981.
Geometrical tests
An area of the plate, that is shadowed by the ring of the coupling system, was been identified thanks to the 3D model (Figure 4). This kind of interference is reasonable that would not have been recognized without any kind of imaging. To verify the correspondence between wells coordinates in the 3D model and in the real system, a test has been done looking the insonation effect on the water surface (acoustic streaming) in some well of the plate (see Figure 7). The 96-wells plate position was been not always the same, so a calibration of coordinates is needed to guarantee the correct position of each insonations. Usually a 5 mm correction of coordinates was been enough. The software stores the last values used and suggests these to the user for the next calibration. Calibration in the z direction is done comparing reflection graph in the acquisition part of the CGA-CSA software, with the predicted z into the 3D model. In Figure 2, the reflection from the water table at the head of the well (z≈125 mm) is visible. The software reliability was tested with the phantoms described above. All the prescribed wells were insonated with an adequate spatial precision, as shown by the evident proteins denaturation in Figure 8.


Discussion
Agreeing with the Buldakov et al.’s statement (14), it is very difficult to derive solid theories from the in vitro US experimentations accomplished, and this is due to a lack of systematic works that study the influence of changing each and all of the parameters involved. Going beyond this, a series of controls to ensure the repeatability and the accuracy of our in vitro experiments was been established. The spots position in the space was assessed employing custom made software based on a 3D model of the system. Its reliability was first verified visually, and then established with the protein denaturation of some specifically made gel phantoms placed inside a 96 well plate. The acoustic power delivered was assessed with a series of test, obtaining a linear relation between the electric power requested by the transducer and the radiation force perceived by the target (Eq. [2]). The experience gained allowed us to write two separate sessions of controls that fit our needs in an easy and cheap manner, making our results almost operator independent; everyone who materially executes the tests has to:
Power check
- Make a low power (1 W) insonation, without moving the transducer, to obtain the z coordinate of the free surface zfs (mm) into the water phantom;
- Prepare the ExAblate 2100 with the InSightec in vitro system, without any culture plate. Fill it of degassed water to the height of the housing of the plate in the lower ring;
- Put on the weighing system described above (see Figure 3);
- Prepare the insonation with z=zfs –10 (mm) and do tree different tests to verify the correspondence between what is get and Table 2.
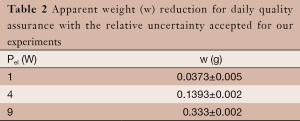 Table 2 Apparent weight (w) reduction for daily quality assurance with the relative uncertainty accepted for our experiments
Table 2 Apparent weight (w) reduction for daily quality assurance with the relative uncertainty accepted for our experiments
Full table
Spot position check
- Prepare the ExAblate 2100 system with the in vitro support (Figure 6) and the drilled plate, ensuring that water reaches the bottom of the wells;
- Start Sonicator, and retrieve the default H6 well’s coordinates from it;
- Perform a low power (≤5 W) insonation with these coordinates;
- Visually verify x and y position, if z is correct, a little cloud of vapor (not hot!) should come from the surface. If the location is not correct try subtracting/adding 1 mm on x and y coordinates and search the reflection plot for free surface distance. Iterate this process till the bottom of the H6 well is targeted with precision;
- Put the right H6 coordinates in Sonicator and repeat steps from (II) to (V) for the A7 well;
- Ask Sonicator to plan the treatment of 3 different wells and visually verify their correct execution;
These are just examples of how an in vitro DQA can be articulated. Our intent is to promote such a kind of behavior, and suggest everyone who is involved into these researches the adoption of his own procedure.
Acknowledgments
The Authors wish to thank the InSightec team for the support into the workstation usage.
Funding: This work was supported by the European Community with the PON01_01059 that belongs to the FESR and FdR founds for the Italian National Operative Program “research and competitiveness” (PON “R&C”) 2007-2013, Axis I, Action I.3.2.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “High intensity focused ultrasounds”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.09.02). The series “High intensity focused ultrasounds” was commissioned by the editorial office without any funding or sponsorship. GIF served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Translational Cancer Research. GR served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schlesinger D, Benedict S, Diederich C, et al. MR-guided focused ultrasound surgery, present and future. Med Phys 2013;40:080901 [PubMed]
- National Council on Radiation Protection and Measurements. eds. Biological effects of ultrasound: mechanisms and clinical implications. Bethesda, MD: The Council, c1983.
- National Council on Radiation Protection and Measurements. Diagnostic Ultrasound Safety: A summary of the technical report “Exposure Criteria for Medical Diagnostic Ultrasound: II. Criteria Based on all Known Mechanisms”. Available online: HTTP://www.ncrponline.org/Publications/Reports/Misc_PDFs/Ultrasound%20Summary--NCRP.pdf
- Ang ES Jr, Gluncic V, Duque A, et al. Prenatal exposure to ultrasound waves impacts neuronal migration in mice. Proc Natl Acad Sci U S A 2006;103:12903-10. [PubMed]
- Hildebrandt B, Wust P, Ahlers O, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol 2002;43:33-56. [PubMed]
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 1984;10:787-800. [PubMed]
- Dewhirst MW, Viglianti BL, Lora-Michiels M, et al. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia 2003;19:267-94. [PubMed]
- Taran FA, Tempany CM, Regan L, et al. Magnetic resonance-guided focused ultrasound (MRgFUS) compared with abdominal hysterectomy for treatment of uterine leiomyomas. Ultrasound Obstet Gynecol 2009;34:572-8. [PubMed]
- Ripamonti C, Fulfaro F. Malignant bone pain: pathophysiology and treatments. Curr Rev Pain 2000;4:187-96. [PubMed]
- Li S, Wu PH. Magnetic resonance image-guided versus ultrasound-guided high-intensity focused ultrasound in the treatment of breast cancer. Chin J Cancer 2013;32:441-52. [PubMed]
- Dickinson L, Ahmed HU, Kirkham AP, et al. A multi-centre prospective development study evaluating focal therapy using high intensity focused ultrasound for localised prostate cancer: The INDEX study. Contemp Clin Trials 2013;36:68-80. [PubMed]
- Elias WJ, Huss D, Voss T, et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med 2013;369:640-8. [PubMed]
- Li CX, Xu GL, Jiang ZY, et al. Analysis of clinical effect of high-intensity focused ultrasound on liver cancer. World J Gastroenterol 2004;10:2201-4. [PubMed]
- Buldakov MA, Hassan MA, Zhao QL, et al. Influence of changing pulse repetition frequency on chemical and biological effects induced by low-intensity ultrasound in vitro. Ultrason Sonochem 2009;16:392-7. [PubMed]
- Brotchie A, Grieser F, Ashokkumar M. Effect of power and frequency on bubble-size distributions in acoustic cavitation. Phys Rev Lett 2009;102:084302 [PubMed]
- Marmottant P, Hilgenfeldt S. Controlled vesicle deformation and lysis by single oscillating bubbles. Nature 2003;423:153-6. [PubMed]
- Ohl CD, Arora M, Ikink R, et al. Sonoporation from jetting cavitation bubbles. Biophys J 2006;91:4285-95. [PubMed]
- Maxwell AD, Wang TY, Cain CA, et al. Cavitation clouds created by shock scattering from bubbles during histotripsy. J Acoust Soc Am 2011;130:1888-98. [PubMed]
- Gourevich D, Hertzberg Y, Volovick A, et al. Ultrasound-mediated targeted drug delivery generated by multifocal beam patterns: an in vitro study. Ultrasound Med Biol 2013;39:507-14. [PubMed]
- Kolárová H, Bajgar R, Tománková K, et al. In vitro study of reactive oxygen species production during photodynamic therapy in ultrasound-pretreated cancer cells. Physiol Res 2007;56:S27-S32. [PubMed]
- Feril LB Jr, Kondo T, Cui ZG, et al. Apoptosis induced by the sonomechanical effects of low intensity pulsed ultrasound in a human leukemia cell line. Cancer Lett 2005;221:145-52. [PubMed]
- Jiang Z, Wu W, Qian ML. Cellular damage and apoptosis along with changes in NF-kappa B expression were induced with contrast agent enhanced ultrasound in gastric cancer cells and hepatoma cells. Cancer Cell Int 2012;12:8. [PubMed]
- Hirokawa N, Koito K, Okada F, et al. High-intensity focused ultrasound induced apoptosis with caspase 3, 8, and 9/6 activation in rat hepatoma. J Med Ultrason 2009;36:177-85.
- Miller DL, Dou C. Induction of apoptosis in sonoporation and ultrasonic gene transfer. Ultrasound Med Biol 2009;35:144-54. [PubMed]
- Liu Y, Kon T, Li C, et al. High intensity focused ultrasound-induced gene activation in sublethally injured tumor cells in vitro. J Acoust Soc Am 2005;118:3328-36. [PubMed]
- Harada Y, Ogawa K, Irie Y, et al. Ultrasound activation of TiO2 in melanoma tumors. J Control Release 2011;149:190-5. [PubMed]
- Buldakov MA, Feril LB Jr, Tachibana K, et al. Low-intensity pulsed ultrasound enhances cell killing induced by X-irradiation. Ultrason Sonochem 2014;21:40-2. [PubMed]
- Borasi G, Melzer A, Russo G, et al. Cancer therapy combining high-intensity focused ultrasound and megavoltage radiation. Int J Radiat Oncol Biol Phys 2014;89:926-7. [PubMed]
- Eisenbrey JR, Huang P, Hsu J, et al. Ultrasound triggered cell death in vitro with doxorubicin loaded poly lactic-acid contrast agents. Ultrasonics 2009;49:628-33. [PubMed]
- Gourevich D, Gerold B, Arditti F, et al. Ultrasound activated nano-encapsulated targeted drug delivery and tumour cell poration. Adv Exp Med Biol 2012;733:135-44. [PubMed]
- Tang W, Liu Q, Wang X, et al. Involvement of caspase 8 in apoptosis induced by ultrasound-activated hematoporphyrin in sarcoma 180 cells in vitro. J Ultrasound Med 2008;27:645-56. [PubMed]
- Abdollahi A, Domhan S, Jenne JW, et al. Apoptosis signals in lymphoblasts induced by focused ultrasound. FASEB J 2004;18:1413-4. [PubMed]
- Colucci V, Strichartz G, Jolesz F, et al. Focused ultrasound effects on nerve action potential in vitro. Ultrasound Med Biol 2009;35:1737-47. [PubMed]
- Graham SM, Carlisle R, Choi JJ, et al. Inertial cavitation to non-invasively trigger and monitor intratumoral release of drug from intravenously delivered liposomes. J Control Release 2014;178:101-7. [PubMed]
- Feng Y, Tian Z, Wan M. Bioeffects of low-intensity ultrasound in vitro: apoptosis, protein profile alteration, and potential molecular mechanism. J Ultrasound Med 2010;29:963-74. [PubMed]
- Phillips LC, Klibanov AL, Wamhoff BR, et al. Targeted gene transfection from microbubbles into vascular smooth muscle cells using focused, ultrasound-mediated delivery. Ultrasound Med Biol 2010;36:1470-80. [PubMed]
- Honda H, Kondo T, Zhao QL, et al. Role of intracellular calcium ions and reactive oxygen species in apoptosis induced by ultrasound. Ultrasound Med Biol 2004;30:683-92. [PubMed]
- Furusawa Y, Fujiwara Y, Campbell P, et al. DNA double-strand breaks induced by cavitational mechanical effects of ultrasound in cancer cell lines. PLoS One 2012;7:e29012 [PubMed]
- Hassan MA, Furusawa Y, Minemura M, et al. Ultrasound-induced new cellular mechanism involved in drug resistance. PLoS One 2012;7:e48291 [PubMed]
- Yumita N, Iwase Y, Nishi K, et al. Involvement of reactive oxygen species in sonodynamically induced apoptosis using a novel porphyrin derivative. Theranostics 2012;2:880-8. [PubMed]
- Gourevich D, Dogadkin O, Volovick A, et al. Ultrasound-mediated targeted drug delivery with a novel cyclodextrin-based drug carrier by mechanical and thermal mechanisms. J Control Release 2013;170:316-24. [PubMed]
- Zhang Y, Deng J, Feng J, et al. Enhancement of antitumor vaccine in ablated hepatocellular carcinoma by high-intensity focused ultrasound. World J Gastroenterol 2010;16:3584-91. [PubMed]
- Borrelli MJ, O’Brien WD Jr, Hamilton E, et al. Influences of microbubble diameter and ultrasonic parameters on in vitro sonothrombolysis efficacy. J Vasc Interv Radiol 2012;23:1677-84. [PubMed]
- Gorny KR, Hangiandreou NJ, Hesley GK, et al. MR guided focused ultrasound: technical acceptance measures for a clinical system. Phys Med Biol 2006;51:3155-73. [PubMed]
- InSightec Ltd. Operator’s Manual For the ExAblate® System for Treatments of Uterine Fibroids and Adenomyosis Original Instructions. February 2012.
- Militello C, Vitabile S, Russo G, et al. A Semi-automatic Multi-seed Region-Growing Approach for Uterine Fibroids Segmentation in MRgFUS Treatment. Complex, Intelligent, and Software Intensive Systems (CISIS), 2013 Seventh International Conference on 2013;176-82.
- Yu T, Wang Z, Mason TJ. A review of research into the uses of low level ultrasound in cancer therapy. Ultrason Sonochem 2004;11:95-103. [PubMed]
- Matsuo M, Yamaguchi K, Feril LB Jr, et al. Synergistic inhibition of malignant melanoma proliferation by melphalan combined with ultrasound and microbubbles. Ultrason Sonochem 2011;18:1218-24. [PubMed]
- Lafon C, Zderic V, Noble ML, et al. Gel phantom for use in high-intensity focused ultrasound dosimetry. Ultrasound Med Biol 2005;31:1383-9. [PubMed]