
Stereotactic ablative radiotherapy for oligometastatic breast cancer in elderly patients
Introduction
Breast cancer (BC) is the most frequently diagnosed neoplasm and the leading cause of cancer-related death in women, accounting for 25% of all female neoplasm and 14% of cancer-related deaths globally (1). According to the National Cancer Institute Surveillance, Epidemiology and End Results Cancer Statistics Review, 43% of patients with newly diagnosed BCs were aged ≥65 years and 20% were aged ≥75 years, and a majority of deaths by BC occurred in these age groups (2). As the incidence of elderly BC is increasing, the absolute number of elderly patients with metastatic disease is also on the rise. Metastatic BC (MBC) remains a virtually incurable disease, with a reported median overall survival (OS) of approximately 2 years. However, improved OS (up to 5 years) has been recently observed for certain subtypes, particularly in HER2-positive disease (3). The most frequent sites of metastasis are bone structures, lungs, regional lymph nodes, brain and liver. The oligometastatic state is a previous step to the generalized metastatic disease (4).
The principle of oligometastases was well popularized in 1995 by Hellman and Weichselbaum who hypothesized that metastatic disease occurs in a step-wise manner, initially with limited metastases followed by progression to widespread disease. Early on, metastases may be limited in number and location based on the interaction of tumor cells with target organs in a “seed and soil” pattern (4). With improvements in imaging, including positron emission tomography/computed tomography (PET/CT) and magnetic resonance imaging (MRI), identification of isolated metastatic deposits is accomplished with higher sensitivity and specificity. A significantly greater proportion of patients may be identified early in the metastatic spectrum and offered potentially curative local treatment (5).
It is in these situations where stereotactic ablative radiotherapy (SABR) can play an important role in the evolution of these patients. SABR and hypofractionated schedules that enable fewer but larger doses per treatment make radiation a more acceptable option for elderly patients for whom long treatment schedules requiring multiple hospital visits may not be advisable (6). Furthermore, these elderly with several comorbidities patients, also considered “fragile patients” do not tolerate aggressive systemic treatments (7). Therefore, SABR arises as one of the pillars of treatment in this particular subset of patients. Recent technological advances in imaging and radiation treatment planning have enabled the precise, safe delivery of high doses per treatment session. Some new data supports the use of SABR and hypofractionated regimens in older patients across multiple tumor sites (7,8).
In elderly patients, it is important to administer personalized therapies using parameters based on a geriatric assessment, which guarantees the patients’ benefit and quality of life (9,10). These parameters must be evaluated through prospective clinical trials, given that this age group is currently underrepresented in these studies (11).
Therefore, the aim of this review is to summarize the use of SABR in elderly oligometastatic patients of primary BC.
SABR: a new approach to oncological treatment
Technological development in recent years has allowed SABR to become more widely used for the treatment of oligometastases and primary tumors (12). This technique enables highly conformal and accurate delivery of high doses of radiation. Moreover, SABR is a form of short course radiotherapy (RT) (one to five fraction regimens). Evidence suggests that higher doses per fraction with SABR are associated with better local control (LC) and OS in patients with lung cancer, liver metastases, bone metastases, among others (13-15).
The advantages of SABR include: high LC, acceptable toxicity profile, non-invasive treatment and ability to safely target multiple metastatic lesions. Another advantage of SABR could be the potential to induce an abscopal effect, especially in those malignancies strongly associated with an immune response (including BC) (16-18).
In order to perform an accurate and effective SABR treatment, many considerations should be addressed beforehand (19):
- Patient should be positioned correctly (during both CT simulation and treatment);
- Patient immobilisation system;
- 4D-CT scan should be used to reduce the organ motion related to the respiratory cycle;
- MRI could be used depending on each individual case;
- Breath hold, gating and tracking techniques could be used for tumours in motion.
Figure 1 shows a SABR treatment planning on the liver of an elderly patient, following the considerations mentioned above.
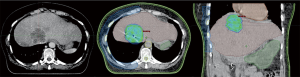
Since the introduction of the concept of “oligometastatic subjects”, the use of SABR in this group of patients has considerably increased, with very acceptable outcomes being reported. The recent randomised phase II trial (SABR-COMET study) showed improved results in oligometastatic disease treated with SABR (15). This trial included 99 patients randomised 1:2 to palliative standard of care versus SABR to all metastatic lesions (maximum of 5 lesions). OS was superior in the SABR group (41 vs. 28 months, P=0.09) and progression-free survival (PFS) also was better in the SABR group with 12 vs. 6 months (P=0.001).
In relation to SABR dosage, there is evidence that a biologically effective dose (BED) of at least 100 Gy constitutes a strong predictive factor of long-term LC after treatment (20,21).
To the best of our knowledge, there is limited clinical data available regarding the impact of SABR in elderly patients, or in oligometastatic BC. Age is not a limiting factor that justifies denying this treatment to any patient, even elderly ones, due to the fact that SABR is a non-invasive and well-tolerated therapy (22).
Are elderly cancer patients eligible for SABR?
In the general population, eligibility for SABR treatment follows these criteria: (I) controlled primary tumor; (II) up to three lesions in the same organ; (III) up to five lesions in total; (IV) life expectancy longer than 6 months; (V) no chemotherapy treatment 4 weeks prior, during or 2 weeks after RT (15).
At the moment, there are no specific SABR criteria that can apply to the elderly population as a whole. Given that this is a heterogeneous subgroup of patients, treatment-related decisions can be complex due to performance status, comorbidities, geriatric syndromes and personal choices, as some patients tend to prefer supportive care rather than aggressive therapies (23,24). For these reasons, patients should be informed about their therapeutic options and prognosis. Family members should also play a role in assisting patients in the decision-making process (25).
In this scenario, it seems reasonable to first establish which patients will definitely not benefit from SABR treatment. The unfit subgroup of elderly patients described in Table 1, have shown no clear benefit from radiation treatment. In contrast, the rest of the population, can potentially benefit from radiotherapy. This data could be useful for identifying potential treatment candidates from this heterogeneous group of patients.
Table 1
| Extracranial sites | Patients category | ||
|---|---|---|---|
| Fit | Borderline | Unfit | |
| Liver (26) | |||
| Performance status (ECOG) | 0-1 | 2-3 | 4 |
| Extrahepatic disease | Absent | Controlled | Progressing |
| Number of lesions | ≤3 | 4 | ≥5 |
| Size of lesions | ≤3 | >3 ≤6 | >6 |
| OARs distance (mm) | >8 | 8–5 | <5 |
| Liver function | Child A | Child B | Child C |
| Free liver volume (cc) | >1,000 | <1,000 ≥700 | >700 |
| Groningen Frailty Index (score) | <4 | 4 | >4 |
| Vulnerable Elders Survey-13 (score) | <4 | 4 | >4 |
| Lung (27) | |||
| Performance status (ECOG) | 0-1 | 2-3 | 4 |
| Size of lesions (cm) | <5 | 5–7 | >7 |
| At least 2 cm from the hilium, major bronchus, heart, great vessel, and esophagus (cm) | >2 | 2 | <2 |
| Controllable primary disease | Yes | Yes | No |
| Groningen Frailty Index (score) | <4 | 4 | >4 |
| Vulnerable Elders Survey-13 (score) | <4 | 4 | >4 |
| Bone (28) | |||
| Performance status | ECOG 0-1 | ECOG 2-3 | ECOG 4 |
| Number of vertebral bodies per region | 1 | 2 | 3 |
| Number of vertebral regions affected | >3 | 2–3 | <2 |
| Tumor distance to the spinal cord | No | NA | Yes |
| Myeloma or lymphoma histology | No | NA | Yes |
| Previous irradiation | No | NA | Yes |
| Contraindications for MRI | No | NA | Yes |
| Cervical spine involved | No | NA | Yes |
| Groningen Frailty Index (score) | <4 | 4 | >4 |
| Vulnerable Elders Survey-13 (score) | <4 | 4 | >4 |
| Intracraneal sites (29) | |||
| Performance status (ECOG) | 0-1 | 2-3 | 4 |
| Life expectancy (months) | >3 | 3 | <3 |
| Lesion size (cm) | <3 | 3 | >3 |
| Number of lesions | <4 | 4 | >4 |
| Controllable primary disease | Yes | NA | No |
| Meningeal or ependymal tumor spread | No | NA | Yes |
| Groningen Frailty Index (score) | <4 | 4 | >4 |
| Vulnerable Elders Survey-13 (score) | <4 | 4 | >4 |
ECOG, Eastern Cooperative Oncology Group; OAR, organs at risk; MRI, magnetic resonance imaging; NA, not available.
In 2016, researchers participating in the study by Dagan et al. (30) strongly agreed to consider SABR as an appropriate technique in all oligometastatic patients with adequate performance status, without age restrictions. Rosenbluth (31) introduced the idea of not using chronological age but physiological age when deciding on different RT schemes. For these reasons, geriatric assessment tools can be helpful to predict tolerance to RT and possible side effects. These scales usually measure performance status, comorbidities, cognitive function, mobility, psychological state, nutritional profile, economic status and social support (32-34). For instance, patients with dementia may not be able to express pain. A correct immobilization for delivering SABR might not be achievable in patients with Parkinson’s disease. Patients with mobility limitations may not be positioned adequately for RT. Other comorbidities might make it difficult for patients to tolerate breath-holding techniques or abdominal compression (34). Moreover, large fractionation schedules can have an impact on the quality of life of patients with mobility limitations or socioeconomic problems (23).
Use of SABR in elderly oligometastatic BC patients
Numerous reports on MBC show a variable, but usually limited median survival, ranging from 8 months to as long as 48 months (35-37). The current standard of treatment for MBC is based on a systemic approach, even though the odds of achieving a sustained complete response are extremely low (38-40).
The role of radiotherapy in the management of oligometastatic neoplasms has experienced a paradigm shift with the development of high-dose, high-precision techniques, and radical treatment has become a reality for patients previously intended for palliative care.
Several authors (26,27,41,42) have reported that SABR is a feasible, safe and effective technique as treatment for elderly metastatic patients, achieving high rates of prolonged LC in different treatment sites. However, existing clinical evidence backing ablative therapies mostly consists of observational studies with great heterogeneity among their designs. Even then, an ablative approach in this group of patients seems to be clinically relevant.
How effective is SABR in achieving LC and PFS in oligometastatic BC patients?
As observed in Table 2, clinical results regarding LC seem to be very promising, attaining between 75–100% rate at 1-year and between 73–97% at the 2-year mark (28,29,43-57). Articles reporting elderly patients’ results, specifically show similar LC rates to those reached in more heterogeneous subject groups. This fact raises the question of whether this technique should be offered as a standard treatment in patients with good performance status, instead of other more palliatively-oriented alternatives.
Table 2
| Author | Study type | n | MTs, n | Elderly patient, n | Primary neoplasm | MT location(s), n | RT dose | Local control rate | Median OS (months) | PFS (months) | Toxicity ≥ G3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yang et al. (41) | Retrospective | 136 | 186 | NR | Breast | Brain | 21 Gy/1–3 fx | 1-y: 90%; 2-y: 73% | 17,6 | 14.8 | No |
| Xu et al. (42) | Retrospective | 103 | 283 | NR | Breast | Brain | 20 Gy/1 fx | NR | TN: 10; Others: 18 | NR | No |
| Dyer et al. (43) | Retrospective | 51 | 51 | 11 | Breast | Brain | NR | NR | 16.2 | NR | NR |
| Gagnon et al. (44) | Retrospective | 18 | NR | 4 | Breast | Spinal cord | 21–28 Gy/3–4 fx | NR | 21 | NR | No |
| Muacevic et al. (45) | Retrospective | 151 | 620 | 114 | Breast | Brain: 620 | 15–41 Gy/1–5 fr | 1-y 94% | 10 | NR | No |
| Onal et al. (46) | Retrospective | 22 | 29 | 5 | Breast | Liver: 29 | 18 Gy ×3 fr | 1-y: 100%; 2-y: 88% | Not reached | 7.4 | 4.54% |
| Vern-Gross et al. (47) | Retrospective | 154 | NR | 4 | Breast | Brain | 9–24 Gy single fr | HER2−: 1-y 76.5%; 3-y 59.5%; HER2+ 1-y 79.4%; 3-y 55.9% | 8.4 | NR | NR |
| Sharma et al. (48) | Retrospective | 206 | 327 | 76 | CCR, lung, melanoma, sarcoma, breast (7), other | Lung | 51–60×3 fr; 30×1 fr; 50–60×5 fr; 48 ×6 fr; 56 ×7 fr; 49×7 fr | 2-y: 85%; 3-y: 83%; 5-y: 81% | 33 | 13 | 2.40% |
| Kased et al. (49) | Retrospective | 176 | 348 | NR | Breast | Brain | 19 Gy ×1 fr | 1-y: 90%; 2-y: 83% | 16 | 8.6 | NR |
| Cho et al. (50) | Retrospective | 131 | NR | NR | Breast | Brain | 14–24 Gy single fx | NR | 15.7 | NR | NR |
| Scorsetti et al. (51) | Prospective | 33 | 47 | NR | Breast | Liver: 33; lung 14 | 18,75 Gy ×3 fr; −25 Gy ×3 fr | 1-y: 98%; 2-y: 90% | 48 | 11 | No |
| Trovo et al. (52) | Prospective | 54 | 92 | NR | Breast | Bones:60; nodes:23; lung: 4; liver: 5 | 30–45 Gy/3 fx; 60 Gy/25 fx | 2-y: 97% | NR. Actuarial 2-y survival: 95% | 1-y: 75%; 2-y: 53% | No |
| Gerszten et al. (53) | Prospective | 50 | 68 | NR | Breast | Spinal cord | 15–22, 5 Gy/1 fx | 100% | NR | NR | No |
MT, metastasis.
Only 7 out of the 17 articles included reported results involving PFS (667 patients), describe periods fluctuating between 7.4 and 24 months, with considerable variability among them (43,47,49,50,52,53,55). This could be attributed to the heterogeneity of study designs and patient selection. The shortest PFS periods reported represent patients with liver and brain metastases, an occurrence that had no apparent association with the subjects’ prognostic factors such as size and number of metastases, hormonal status or timing of treatment (28,43-48,50-53,55,57).
How does MT location impact LC?
In general, articles included in this review, report LC results with very good outcomes. When analyzed by metastatic location, there seems to be an increased control rate in patients affected exclusively by bone metastases, even reaching a 10-year LC rate of 100% as published by Lancia et al. (57). This suggest that SABR could play a fundamental role in this group of patients, given that a traditional course of radical-intent RT treatment might become a challenging task, even risking the chance of not being able to complete this therapy.
Could SABR improve OS in elderly patients?
Most of the patient groups encompassed in the articles in this review have not specifically included elderly patients. When a general analysis is performed on these series, only 13 out of the 17 total studies have reported survival outcomes. As such, the data provided is quite heterogeneous, ranging from 8 to 48 months, with one study (28) even reaching an actuarial survival up to 120 months. Thus, it is not possible to extrapolate these results to an old population. Even then, it stands out that, in Muacevic’s et al. study (46), 114 elderly patients were included and achieved a median OS of 10 months. A similar case can be seen on the studies published by Sharma et al. and Dyer et al., which also specified to have involved the inclusion of aging individuals, reaching a median OS of 33 and 16.2 months, respectively. These seem as very promising results in a group of subjects that, in addition to their advanced age, usually present a myriad of other comorbidities (even though this topic was not reported in any of these references) and where their life expectancy is not generally defined by the presence of an oncologic disease.
Is it a safe treatment in elderly patients?
The possible side effects derived from radiotherapy must always be taken into consideration when deciding to deliver a treatment. This is especially important in elder patients, as toxicity derived from RT can have a bigger impact on their quality of life as compared to the general population (58). Moreover, it is known that standard palliative radiation is often well-tolerated (59), which sets a high bar for SABR treatments.
Data derived from the studies analyzed in this review shows that SABR has a good adverse effect profile, with grade ≥3 toxicities ranging from none (Scorsetti et al.) to 4.5% (Onal et al.) (26,47). It must be noted, however, that the toxicity present in the subgroup of elder patients is not specified in any of the studies. Moreover, a great number of these do not report any data on adverse effects.
In the recent SABR-COMET study, oligometastatic patients presented similar rates of grade ≥3 toxicity in both the standard RT and the SABR arms (15). In contrast, patients treated with SABR presented higher rates of grade 2 adverse effects (29% vs. 9%), and three treatment-related deaths occurred in this arm (vs. none in the control arm). These results, however, were not stratified by age.
In the particular case of brain metastases, it must be taken into account that traditional palliative whole brain radiotherapy (WBRT) has largely been associated with a decline in cognitive status and quality of life (60). This can be critical in the elder population. In this scenario, SRS represents an opportunity to avoid these toxicities. SRS seems to be a safe treatment with minimal acute side effects. And, even though radionecrosis can be present as a finding in control MRI in up to 34% of cases, only 10–17% are actually symptomatic (61,62). Again, none of the evidence that we analyzed specified any differences regarding the subgroup of older patients.
Regarding bone lesions, the main side effect derived from SABR is an increased risk of fracture (63). This risk is especially relevant for elder patients, as it is well known that bone fractures in this population has a considerable impact in quality of life and OS (64). Moreover, the higher prevalence of osteoporosis in this subgroup of patients could pose a greater risk of complications from SABR treatment. A study by Kam et al. that included patients up to 79 years old reported a 10% rate of vertebral fractures (65). However, the age of these patients was not specified. Moreover, we did not find any studies that specifically evaluated the risk of fracture in elder patients.
Which tools can we use for a better selection of elderly patients?
Although aging produces physiological changes in organ function and pharmacokinetics, chronological age does not necessarily translate into biological age. In elderly patients, symptoms caused by the tumor must be added to the previously present comorbidities that affect their functional, psychological and daily performance state. For these reasons, geriatric evaluation tools that better define the risks associated with treatments are essential in the decision-making process.
Comprehensive geriatric assessment (CGA)
This tool has been shown to be useful for detecting reversible factors that can influence treatment outcomes (malnutrition, inadequate social support, comorbidities). Moreover, it can estimate mortality risks based on performance status, comorbidities and presence of geriatric syndromes (66,67).
However, given that the CGA takes a considerable amount of time to complete, simpler screening tests are often used in order to select patients who can benefit from a more comprehensive assessment. These include:
- Vulnerable Elderly Survey (VES): evaluates age, function and activity. A score ≥3 indicates a higher risk of functional impairment and, therefore, conducting the CGA is recommended (68);
- The Groningen Frailty Index: assesses diminished functions in four domains: physical, cognitive, social and psychological. A score of ≥4 predicts a higher risk of frailty (69);
- Test Timed Up and Go: patients requiring more than 10 seconds to complete the exercise, needing to use their arms to stand up or following an erroneous path should undergo CGA (70,71);
- 7-item Physical performance: requires 10 minutes to complete. If the total score is less than 20, CGA is advisable. This test has shown to be more sensitive than the Karnofsky Performance Status (KPS) to recognize patients at risk of functional impairment (71).
Functional status and quality of life (QoL)
In the assessment of performance status (PS), there are many aspects of physical limitations that are not reflected, especially those related to instrumental activities that may interfere with the adhesion to diagnostic or therapeutic processes. An accurate assessment of functional status and QoL is necessary.
Sprave et al. (56) have recently published one of the few articles comparing QoL in this subgroup of patients. This is a prespecified secondary analysis of a randomized trial which assessed the QoL, fatigue and emotional distress after SABR versus conventional 3DCRT as part of the palliative management of painful spinal metastases. Its results show that SABR does not imply worse QoL than 3DCRT, but the limitations of the study manifest the need for further research that corroborates these findings (72).
Despite an increasing trend to assess QoL (66), no consensus has been reached on its definition and how to measure it. Although a number of tools have been validated for evaluation, none has specifically been developed based on the needs of elderly patients.
Furthermore, only relative consensus has been reached on important aspects of QoL, such as functional capacity, role functioning, social interaction and community, wellbeing, somatic sensation and life satisfaction (67).
There are few published articles on QoL in elderly cancer patients. Moreover, this population constitutes a poorly represented group in clinical trials. Future studies should try to improve this representation in order to expand the knowledge on QoL and find specific tools to evaluate it.
Conclusions
A SABR approach in oligometastatic BC poses a promising therapeutic option, with excellent clinical results, such as long-term LC, low toxicity and an increase in OS in particular cases. Even though there is limited evidence available, SABR in elderly patients represents an auspicious option that avoids more invasive therapeutic strategies that involve hospitalization (and their intrinsic risks) and allows for a short-course, tolerable, safe and effective treatment. Further studies are required to improve patient selection, establish the most effective fractionation schemes for each localization and evaluate the impact of this kind of treatment on short-, medium- and long-term quality of life.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Vincent Vinh-Hung and Nam P Nguyen) for the series “Radiotherapy for Breast Cancer in Advanced Age” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.08.29). The series “Radiotherapy for Breast Cancer in Advanced Age” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2013. National Cancer Institute. Available online: http://seer.cancer.gov/csr/1975_2013. Accessed May 15th, 2017.
- Malmgren JA, Mayer M, Atwood MK, et al. Differential presentation and survival of de novo and recurrent metastatic breast cancer over time: 1990–2010. Breast Cancer Res Treat 2018;167:579-90. [Crossref] [PubMed]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Oh D, Ahn YC, Seo JM, et al. Potentially curative stereotactic body radiation therapy (SBRT) for single or oligometastasis to the lung. Acta Oncol 2012;51:596-602. [Crossref] [PubMed]
- Rubio C, Morera R, Hernando O, et al. Extracranial stereotactic body radiotherapy. Review of main SBRT features and indications in primary tumors. Rep Pract Oncol Radiother 2013;18:387-96. [Crossref] [PubMed]
- Brunner TB, Nestle U, Grosu AL, et al. SBRT in pancreatic cancer: what is the therapeutic window? Radiother Oncol 2015;114:109-16. [Crossref] [PubMed]
- Kim SK, Wu CC, Horowitz DP. Stereotactic body radiotherapy for the pancreas: a critical review for the medical oncologist. J Gastrointest Oncol 2016;7:479-86. [Crossref] [PubMed]
- Hurria A, Cirrincione CT, Muss HB, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol 2011;29:1290-6. [Crossref] [PubMed]
- Williams GR, Deal AM, Jolly TA, et al. Feasibility of geriatric assessment in community oncology clinics. J Geriatr Oncol 2014;5:245-51. [Crossref] [PubMed]
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA 2004;291:2720-6. [Crossref] [PubMed]
- Carey Sampson M, Katz A, Constine LS. Stereotactic body radiation therapy for extracranial oligometastases: does the sword have a double edge? Semin Radiat Oncol 2006;16:67-76. [Crossref] [PubMed]
- Timmerman RD, Kavanagh BD, Cho LC, et al. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol 2007;25:947-52. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancer (SABR-COMET): a randomised, phase 2, open label trial. Lancet 2019;393:2051-8. [Crossref] [PubMed]
- Chicas-Sett R, Morales-Orue I, Rodriguez-Abreu D, et al. Combining radiotherapy and ipilimumab induces clinically relevant radiation-induced abscopal effects in metastatic melanoma patients: A systematic review. Clin Transl Radiat Oncol 2017;9:5-11. [Crossref] [PubMed]
- Chicas-Sett R, Morales-Orue I, Castilla-Martinez J, et al. Stereotactic ablative Radiotherapy combined with immune checkpoint inhibitors reboots the immune response assisted by immunotherapy in metastatic lung cancer: A systematic review. Int J Mol Sci 2019;20:2173. [Crossref] [PubMed]
- Morales-Orue I, Chicas-Sett R, Lara PC. Nanoparticles as a promising method to enhance the abscopal effect in the era of new targeted therapies. Rep Pract Oncol Radiother 2019;24:86-91. [Crossref] [PubMed]
- Scorsetti M, Arcangeli S, Tozzi A, et al. Is stereotactic body radiation therapy an attractive option for unresectable liver metastasis? A preliminary report from a phase 2 trial. Int J Radiat Oncol Biol Phys 2013;86:336-42. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011;81:1352-8. [Crossref] [PubMed]
- Figlia V, Mazzola R, Cuccia F, et al. Hypo-fractionated stereotactic radiation therapy for lung malignancies by means of helical tomotherapy: report of feasibility by a single-center experience. Radiol Med 2018;123:406-14. [Crossref] [PubMed]
- Scorsetti M, Clerici E, Comito T. Stereotactic body radiation therapy for liver metastases. J Gastrointest Oncol 2014;5:190-7. [PubMed]
- Chang S, Goldstein NE, Dharmarajan KV. Managing an Older Adult with Cancer: Considerations for Radiation Oncologists. Biomed Res Int 2017;2017:1695101. [Crossref] [PubMed]
- Rosati LM, Herman J. Role of Stereotactic Body Radiotherapy in the Treatment of Elderly and Poor Performance Status Patients With Pancreatic Cancer. J Oncol Pract 2017;13:157-66. [Crossref] [PubMed]
- Martinez KA, Kurian AW, Hawley ST, et al. How can we best respect patient autonomy in breast cancer treatment decisions? Breast Cancer Manag 2015;4:53-64. [Crossref] [PubMed]
- Scorsetti M, Clerici E, Navarria P, et al. The role of stereotactic body radiation therapy (SBRT) in the treatment of oligometastatic disease in the elderly. Br J Radiol 2015;88:20150111. [Crossref] [PubMed]
- Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol 2009;27:1579-84. [Crossref] [PubMed]
- Milano MT, Katz A, Zhang H, et al. Oligometastatic breast cancer treated with hypofractionated stereotactic radiotherapy: Some patients survive longer than a decade. Radiother Oncol 2019;131:45-51. [Crossref] [PubMed]
- Gagnon GJ, Henderson FC, Gehan EA, et al. Cyberknife radiosurgery for breast cancer spine metastases: a matched-pair analysis. Cancer 2007;110:1796-802. [Crossref] [PubMed]
- Dagan R, Lo S, Redmond K, et al. A multi-national report on stereotactic body radiotherapy for oligometastases: Patient selection and follow-up Acta Oncologica 2016;55:633-7. [Crossref] [PubMed]
- Rosenbluth B. The Use of Radiation Therapy in the Geriatric Population. Clin Geriatr Med 2012;28:105-14. [Crossref] [PubMed]
- Hazzard WR, Halter JB. Hazzard’s Geriatric Medicine and Gerontology. 6th edition. New York, NY: McGraw-Hill Medical, 2009.
- Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014;32:2595-603. [Crossref] [PubMed]
- Kunkler IH, Audisio R, Belkacemi Y, et al. Review of current best practice and priorities for research in radiation oncology for elderly patients with cancer: The international society of geriatric oncology (SIOG) task force. Ann Oncol 2014;25:2134-46. [Crossref] [PubMed]
- Hortobagyi GN. Can we cure limited metastatic breast cancer? J Clin Oncol 2002;20:620. [Crossref] [PubMed]
- Pagani O, Senkus E, Wood W, et al. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Inst 2010;102:456e63.
- Tan SH, Wolff AC. Treatment of metastatic breast cancer. In: Harris JR, Lippman ME, Morrow M, et al. editors. Diseases of the breast. 4th edition. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins, 2010:877e919.
- Tomiak E, Piccart M, Mignolet F, et al. Characterisation of complete responders to combination chemotherapy for advanced breast cancer: a retrospective EORTC Breast Group study. Eur J Cancer 1996;32A:1876e87.
- Greenberg PA, Hortobagyi GN, Smith TL, et al. Longterm follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J Clin Oncol 1996;14:2197e205.
- Yamamoto N, Katsumata N, Watanabe T, et al. Clinical characteristics of patients with metastatic breast cancer with complete remission following systemic treatment. Jpn J Clin Oncol 1998;28:368e73.
- Takeda A, Sanuki N, Eriguchi T, et al. Stereotactic ablative body radiation therapy for octogenarians with non small cell lung cancer. Int J Radiat Oncol Biol Phys 2013;86:257-63. [Crossref] [PubMed]
- Sandhu AP, Lau SK, Rahn D, et al. Stereotactic body radiation therapy in octogenarians with stage I lung cancer. Clin Lung Cancer 2014;15:131-5. [Crossref] [PubMed]
- Yang TJ, Oh JH, Folkert MR, et al. Outcomes and prognostic factors in women with 1 to 3 breast cancer brain metastases treated with definitive stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2014;90:518-25. [Crossref] [PubMed]
- Xu Z, Schlesinger D, Toulmin S, et al. Impact of triple-negative phenotype on prognosis of patients with breast cancer brain metastases. Int J Radiat Oncol Biol Phys 2012;84:612-8. [Crossref] [PubMed]
- Dyer MA, Kelly P, Chen YH, et al. Importance of Extracranial Disease Status and Tumor Subtype for Patients Undergoing Radiosurgery for Breast Cancer Brain Metastases. Int J Radiat Oncol Biol Phys 2012;83:e479-86. [Crossref] [PubMed]
- Muacevic A, Kreth FW, Tonn JC, et al. Stereotactic radiosurgery for multiple brain metastases from breast carcinoma. Cancer 2004;100:1705e11.
- Onal C, Cem Guler O, Akkus Yildrim B. Treatment outcomes of breast cancer liver metastasis treated with stereotactic body radiotherapy. Breast 2018;42:150-6. [Crossref] [PubMed]
- Vern-Gross TZ, Lawrence J, Case D, et al. Breast cancer subtype affects patterns of failure of brain metastases after treatment with stereotactic radiosurgery. J Neurooncol 2012;110:381-8. [Crossref] [PubMed]
- Sharma A, Duijm M, Oomen-de Hoop E, et al. Survival and prognostic factors of pulmonary oligometastases treated with stereotactic body radiotherapy. Acta Oncol 2019;58:74-80. [Crossref] [PubMed]
- Kased N, Binder D, McDermott M, et al. Gamma Knife radiosurgery for brain metastases from primary breast cancer. Int J Radiat Oncol Biol Phys 2009;75:1132-40. [Crossref] [PubMed]
- Cho E, Rubinstein L, Stevenson P, et al. The use of stereotactic radiosurgery for brain metastases from breast cancer: Who benefits most? Breast Cancer Res Treat 2015;149:743-9. [Crossref] [PubMed]
- Scorsetti M, Franceschini D, De Rose F, et al. Stereotactic body radiation therapy: A promising chance for oligometastatic breast cancer. Breast 2016;26:11-7. [Crossref] [PubMed]
- Trovo M, Furlan C, Polesel J, et al. Radical radiation therapy for oligometastatic breast cancer: Results of a prospective phase II trial. Radiother Oncol 2018;126:177-80. [Crossref] [PubMed]
- Gerszten PC, Burton S, Welch W, et al. Single-Fraction Radiosurgery for the Treatment of Spinal Breast Metastases. Cancer 2005;104:2244-54. [Crossref] [PubMed]
- Milano MT, Zhang H, Metcalfe S, et al. Oligometastatic breast cancer treated with curative-intent stereotactic body radiation therapy. Breast Cancer Res Treat 2009;115:601-8. [Crossref] [PubMed]
- Sprave T, Verma V, Förster R, et al. Quality of Life Following Stereotactic Body Radiotherapy Versus Three-Dimensional Conformal Radiotherapy for Vertebral Metastases: Secondary Analysis of an Exploratory Phase II Randomized Trial. Anticancer Res 2018;38:4961-8. [Crossref] [PubMed]
- Lancia A, Ingrosso G, Carosi A, et al. Oligometastatic cancer in elderly patients: the “blitzkrieg” radiotherapy approach. Aging Clin Exp Res 2019;31:109-14. [Crossref] [PubMed]
- O'Donovan A, Leech M, Gillham C. Assessment and management of radiotherapy induced toxicity in older patients. J Geriatr Oncol 2017;8:421-7. [Crossref] [PubMed]
- Rich SE, Chow R, Raman S, et al. Update of the systematic review of palliative radiation therapy fractionation for bone metastases. Radiother Oncol 2018;126:547-57. [Crossref] [PubMed]
- Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality of life results. J Clin Oncol 2013;31:65e72.
- Le Rhun E, Dhermain F, Vogin G, et al. Radionecrosis after stereotactic radiotherapy for brain metastases. Expert Rev Neurother 2016;16:903-14. [Crossref] [PubMed]
- Kohutek ZA, Yamada Y, Chan TA, et al. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol 2015;125:149-56. [Crossref] [PubMed]
- Zeng KL, Tseng CL, Soliman H, et al. Stereotactic Body Radiotherapy (SBRT) for Oligometastatic Spine Metastases: An Overview Front Oncol 2019;9:337. [Crossref] [PubMed]
- Amin S, Achenbach SJ, Atkinson EJ, et al. III Trends in fracture incidence: a population-based study over 20 years. J Bone Miner Res 2014;29:581-9. [Crossref] [PubMed]
- Kam TY, Hong Chan O, Man Hung A, et al. Utilization of stereotactic ablative radiotherapy in oligometastatic & oligoprogressive skeletal metastases: Results and pattern of failure. Asia-Pac J Clin Oncol 2019;15:14-9. [Crossref] [PubMed]
- Repetto L, Ausili-Cefaro G, Gallo C, et al. Quality of life in elderly cancer patients. Ann Oncol 2001;12:S49-52. [Crossref] [PubMed]
- Bowling A, Grundy E. Activities of daily living: changes in functional ability in three samples of elderly and very elderly people. Age Ageing 1997;26:107-14. [Crossref] [PubMed]
- Dempster M, Donnelly M. How well do elderly people complete individualised quality of life measures: an exploratory study. Qual Life Res 2000;9:369-75. [Crossref] [PubMed]
- Ingram SS, Seo PH, Sloane R, et al. The association between oral health and general health and quality of life in older male cancer patients. J Am Geriatr Soc 2005;53:1504-9. [Crossref] [PubMed]
- Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the impostors. Spine 1990;15:1-4. [Crossref] [PubMed]
- Chow E, Nguyen J, Zhang L, et al. International field testing of the reliability and validity of the EORTC QLQ-BM22 module to assess health-related quality of life in patients with bone metastases. Cancer 2012;118:1457-65. [Crossref] [PubMed]
- Weis J, Arraras JI, Conroy T, et al. Development of an EORTC quality of life phase III module measuring cancerrelated fatigue (EORTC QLQ-FA13). Psychooncology 2013;22:1002-7. [Crossref] [PubMed]


