Clinicopathological features and prognostic analysis of signet ring cell gastric carcinoma: a population-based study
Introduction
Gastric cancer is the fifth most common cancer in the world and is the third leading cause of cancer mortality (1). Histologically, gastric cancer exhibits obvious heterogeneity at the constructional and cytological levels and usually coexists with several histological components (2). Signet ring cell gastric carcinoma is a histological diagnosis based on microscopic characteristics as described by the World Health Organization (3). Gastric signet ring cell carcinomas (SRCs) are described as being isolated or a micro-community of malignant cells with intracytoplasmic mucins accounting for more than 50% of the tumors (3). In other classifications, it is classified into “diffuse-type” in the Lauren, “anaplastic carcinoma” in Japan, and “infiltrative type” in the Ming classification. It is generally believed that the biological behaviors of SRC differ from that of other histological types. However, the reports of clinicopathologic features and prognostic impacts were still inconsistent. Some studies reported that the long-term survival rate of SRC was better than that of the AC type in early GC (4). Others revealed no significant differences between them (5-7) or whether or not SRC had a worse prognosis (8) in advanced GC. Therefore, to better understanding the prognostic impact of SRC, it is necessary to include a larger volume of patients with consistency in pathological diagnosis. This study aimed to retrospectively estimate the differences in the clinicopathological characteristics and the overall survival of SRC when compared to WMD and PD in patients with gastric cancer.
Methods
Patient selection
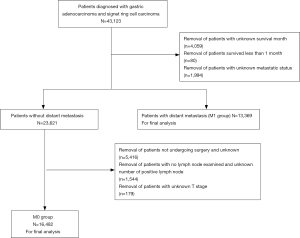
Data was collected from the SEER Regs Custom Data (with additional treatment fields), Nov 2017 Sub (1973–2015 varying) (https://seer.cancer.gov/). This study analyzed records from 2004 to 2015. The pT, pN, and pM of records were determined respectively with SEER data, which included collaborative stage extension (2004+), collaborative stage (CS) regional nodes positive (1988+), and CS metastases (mets) at distant sites (dx) (2004+). The American Joint Committee on Cancer (AJCC) staging manual (8th edition) was used to restage cases from this duration, and patients with less than 15 nodes dissected were classified as pNx. SRC was defined by WHO classification as an adenocarcinoma containing intra-cellular mucin comprising more than 50% of the tumors in the SEER database. Patients between 20 and 70 years, with a pathologic confirmation of gastric signet ring cell carcinoma and gastric adenocarcinoma were included. The International Classification of Diseases code M-8490/3 was used for patients with signet ring cell gastric carcinoma, and codes M-8140/3, M-8145/3, M-8210/3, M-8211/3, M-8255/3, M-8260/3, M-8263/3, M-8310/3, M-8323/3, M-8480/3, M-8481/3 were used to identify adenocarcinoma. Exclusion criteria were as follows: patients with unknown vital status, unknown metastatic status, undifferentiated and unknown histological type, and who died within 1 month. Additionally, in terms of the M0 group, patients not undergoing surgery or unknown surgery status, with no lymph node examined, an unknown number of positive lymph nodes, and unknown T stage were excluded. Due to small population sizes (N=1,117) in well-differentiated AC, patients were divided into three groups: SRC, WMD, and PD for further analysis. The primary endpoint was determining the 5-year overall survival (OS).
Statistical analysis
The demographic and clinical characteristics were compared among groups by independent t-test and χ2 tests. The Kaplan Meier (KM) method was used to generate the survival curves, and then the log-rank test was performed. Long-term survival was assessed using the 5-year overall survival rate. Cox regression hazard model was used for univariable and multivariable analysis. Subgroup analysis of OS in each stage were displayed using forest plot. Prognostic factors consisted of histological type; age at diagnosis; gender; race; T and N stage; distant metastasis; metastasis to bone, brain, liver, and lung; tumor size; tumor site; and presence of radiation therapy. A P<0.05 value was considered as statistically significant for all analyses. All data analyses were performed by SPSS version 23.0 and Stata 12.0.
Results
Patient demographics
A total of 29,851 patients diagnosed with AC or SRC were analyzed, of whom 16,482 were patients absent from distant metastasis and who received gastrectomy (M0 group); 13,369 had distant metastasis (M1 group). A consort diagram is shown in Figure 1. As shown in Table 1, of the M0 group, 3,715 patients were recorded as SRC, and 12,767 patients were recorded as AC. Of these AC patients, 5,312 were well- and moderately differentiated, while 7,455 were PD. The age at diagnosis was younger in the SRC patients than in the WMD or PD patients (SRC: 60 yrs; WMD: 66 yrs; PD: 63 yrs; SRC vs. WMD and PD: t-test P<0.001). SRC had a higher proportion of females (SRC: 47.4%; WMD: 27.2%; PD: 34.0%; χ2 test P<0.001). In the M1 group, 4,059 patients were with SRC, 2948 were WMD, and 6,362 were PD. The demographics were similar to the M0 group, SRC patients were younger (SRC: 57 yrs; WMD: 64 yrs; PD: 61 yrs; SRC vs. WMD and PD: t-test P<0.001) and more were female (SRC: 50.3%; WMD: 35.7%; PD: 32.1%; χ2 test P<0.001) (Table S1).
Table1
| Variables | Signet ring cell carcinoma (A) (N=3,715) | Well and moderately differentiated AC (B) (N=5,312) | Poorly differentiated AC (C) (N=7,455) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | P (A vs. B) | N | % | P (A vs. C) | |||
| Age (yrs) | <0.001 | <0.001 | ||||||||
| Mean | 60 | 66 | 63 | |||||||
| SD | 12.2 | 9.8 | 10.9 | |||||||
| Gender | <0.001 | <0.001 | ||||||||
| Male | 1,954 | 52.6 | 3,866 | 72.8 | 4,919 | 66 | ||||
| Female | 1,761 | 47.4 | 1,446 | 27.2 | 2,536 | 34 | ||||
| Race | 0.018 | 0.749 | ||||||||
| White | 2,460 | 66.5 | 3,579 | 67.6 | 4,892 | 65.8 | ||||
| Black | 484 | 13.1 | 755 | 14.3 | 989 | 13.3 | ||||
| Othera | 754 | 20.4 | 963 | 18.2 | 1,553 | 20.9 | ||||
| AJCC stage | <0.001 | <0.001 | ||||||||
| I | 412 | 21.0 | 869 | 37.4 | 642 | 16.8 | ||||
| II | 432 | 22.0 | 728 | 31.4 | 1,051 | 27.5 | ||||
| III | 1,119 | 57.0 | 725 | 31.2 | 2,133 | 55.8 | ||||
| T stage | <0.001 | <0.001 | ||||||||
| T1 | 816 | 22 | 1,897 | 35.7 | 1,250 | 16.8 | ||||
| T2 | 367 | 9.9 | 806 | 15.2 | 889 | 11.9 | ||||
| T3 | 1,210 | 32.6 | 1,627 | 30.6 | 2,829 | 37.9 | ||||
| T4a | 1,020 | 27.5 | 750 | 14.1 | 1,938 | 26 | ||||
| T4b | 302 | 8.1 | 232 | 4.4 | 549 | 7.4 | ||||
| Node stage | <0.001 | <0.001 | ||||||||
| N0 | 625 | 31.8 | 1,250 | 53.8 | 1,108 | 29.0 | ||||
| N1 | 228 | 11.6 | 389 | 16.8 | 621 | 16.2 | ||||
| N2 | 300 | 15.3 | 329 | 14.2 | 743 | 19.4 | ||||
| N3a | 438 | 22.3 | 274 | 11.8 | 828 | 21.6 | ||||
| N3b | 372 | 19.0 | 80 | 3.4 | 526 | 13.7 | ||||
| Examined LN | <0.001 | 0.130 | ||||||||
| ≥15 | 1,963 | 52.8 | 2,322 | 43.7 | 3,826 | 51.3 | ||||
| <15b | 1,752 | 47.2 | 2,990 | 56.3 | 3,629 | 48.7 | ||||
| Tumor location | <0.001 | <0.001 | ||||||||
| Upper stomach | 695 | 26.4 | 2,282 | 53.1 | 2,520 | 43.6 | ||||
| Middle stomach | 402 | 15.2 | 392 | 9.1 | 696 | 12.1 | ||||
| Lower stomach | 1,185 | 44.9 | 1,420 | 33 | 2,028 | 35.1 | ||||
| Overlapping | 355 | 13.5 | 203 | 4.7 | 531 | 9.2 | ||||
| Tumor size (cm) | <0.001 | 0.230 | ||||||||
| ≥5 | 1,324 | 42.8 | 1,525 | 31.9 | 2,923 | 55.9 | ||||
| <5 | 1,770 | 57.2 | 3,254 | 68.1 | 3,707 | 44.1 | ||||
yrs, years; SD, standard deviation; LN, lymph node; AJCC, American Joint Committee on Cancer. aincludes American Indian/AK Native, Asian/Pacific Islander; bexamined lymph nodes <15 was defined as pNx.
Tumor features
In the M0 group, SRC was more frequently in the lower stomach (gastric antrum and pylorus) while AC was found mostly in the upper third of the stomach (cardia and gastric fundus). A higher percentage of patients with signet ring cell carcinoma was also likely in overlapping type (13.5% vs. 4.7% vs. 9.2%; P<0.001). More signet ring cell carcinoma presented with tumor stages T4a and T4b (35.6% vs. 28.5% vs. 33.4%; P<0.001). A higher rate of patients was diagnosed with node stages N3a and N3b in the SRC type (41.3% vs. 15.2% vs. 35.3%; P<0.001) (Table 1). Signet ring cell carcinoma patients was more common in AJCC stage IV (52.2% vs. 24.9% vs. 46.0%; P<0.001). In the M1 group, SRC had no significant difference from PD in tumor features, except that SRC was with more advanced T (P<0.001). While compared with WMD, SRC also had more advanced T (P<0.001) and node stage (P=0.014) and was more common in the overlapping type (P<0.001) (Table S1).
Table S1
| Variables | Signet ring cell carcinoma (A) (N=4,059) | Well and moderately differentiated AC (B) (N=2,948) | Poorly differentiated AC (C) (N=6,362) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | P (A vs. B) | N | % | P (A vs. C) | |||
| Age (yrs) | <0.001 | <0.001 | ||||||||
| Mean | 57 | 64 | 61 | |||||||
| SD | 13.1 | 10.7 | 12.2 | |||||||
| Gender | <0.001 | <0.001 | ||||||||
| Male | 2,016 | 49.7 | 2,228 | 75.6 | 4,318 | 67.9 | ||||
| Female | 2,043 | 50.3 | 720 | 24.4 | 2,044 | 32.1 | ||||
| Race | <0.001 | 0.621 | ||||||||
| White | 2,941 | 72.7 | 2,137 | 72.6 | 4,596 | 72.4 | ||||
| Black | 490 | 12.1 | 478 | 16.2 | 807 | 12.7 | ||||
| Othera | 616 | 15.2 | 328 | 11.1 | 944 | 14.9 | ||||
| T stage | <0.001 | <0.001 | ||||||||
| T1 | 545 | 21.1 | 623 | 34.3 | 998 | 24.4 | ||||
| T2 | 217 | 8.4 | 87 | 4.8 | 247 | 6.0 | ||||
| T3 | 493 | 19.1 | 408 | 22.5 | 928 | 22.7 | ||||
| T4a | 445 | 17.2 | 215 | 11.8 | 669 | 16.3 | ||||
| T4b | 883 | 34.2 | 484 | 26.6 | 1,255 | 30.6 | ||||
| Node stage | 0.014 | 0.067 | ||||||||
| N0 | 20 | 6.9 | 15 | 10.7 | 37 | 7.9 | ||||
| N1 | 17 | 5.8 | 15 | 10.7 | 45 | 9.6 | ||||
| N2 | 52 | 17.9 | 33 | 23.6 | 70 | 14.9 | ||||
| N3a | 67 | 23.0 | 33 | 23.6 | 135 | 28.7 | ||||
| N3b | 135 | 46.4 | 38 | 27.1 | 184 | 39.1 | ||||
| Surgery | <0.001 | 0.691 | ||||||||
| Yes | 738 | 18.4 | 424 | 14.5 | 1,179 | 18.7 | ||||
| No | 3,280 | 81.6 | 2,503 | 85.5 | 5,133 | 81.3 | ||||
| Examined LN | 0.014 | 0.067 | ||||||||
| ≥15 | 292 | 42.0 | 140 | 35.9 | 471 | 42.9 | ||||
| <15b | 389 | 58.0 | 250 | 64.1 | 618 | 57.1 | ||||
| Tumor location | <0.001 | 0.878 | ||||||||
| Upper stomach | 744 | 28.8 | 1,484 | 61.9 | 2,531 | 52.6 | ||||
| Middle stomach | 531 | 20.6 | 241 | 10.1 | 584 | 12.1 | ||||
| Lower stomach | 771 | 29.9 | 489 | 20.4 | 1,060 | 22.0 | ||||
| Overlapping | 533 | 20.7 | 184 | 7.7 | 636 | 13.2 | ||||
| Tumor size (cm) | 0.188 | 0.095 | ||||||||
| ≥5 | 680 | 55.6 | 705 | 53.0 | 471 | 43.3 | ||||
| <5 | 542 | 44.4 | 624 | 47.0 | 618 | 56.7 | ||||
yrs, years; SD, standard deviation; LN, lymph node. aincludes American Indian/AK Native, Asian/Pacific Islander; bexamined LN <15 was defined as pNx.
Metastatic patterns of SRC, WMD, and PD were further evaluated. The distant metastasis of SRC was most common in the bone (46.1%), and the liver was the most common metastatic site in patients with WMD (64.7%) and PD (55.9%) (Figure 2A), while a brain metastasis was least common all the histological types. There was no significant difference in the number of metastatic sites between SRC and WMD (P=0.173) or PD (P=0.181) (Table 2), but SRC tended to have fewer multiple metastases (SRC vs. WMD vs. PD: 19.1% vs. 22.4% vs. 22.6%; P=0.117 and 0.068, respectively) (Figure 2B).
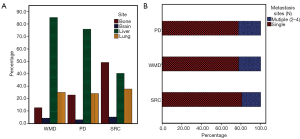
Table 2
| Characteristics | SRC (A) | WMD (B) | PD (C) | |||||
|---|---|---|---|---|---|---|---|---|
| N (%) | P value (A vs. B) | N (%) | P value (B vs. C) | N (%) | P value (A vs. C) | |||
| Metastasis site | <0.001 | <0.001 | <0.001 | |||||
| Bone | 316 (40.1) | 143 (9.9) | 430 (18.2) | |||||
| Brain | 34 (4.3) | 50 (3.5) | 58 (2.5) | |||||
| Liver | 260 (33.0) | 968 (67.0) | 1,421 (60.2) | |||||
| Lung | 178 (22.6) | 284 (19.7) | 452 (19.1) | |||||
| Number of metastasis sites | 0.173 | 0.023 | 0.181 | |||||
| One | 520 (80.9) | 882 (77.6) | 1,452 (77.4) | |||||
| Two | 103 (16.0) | 201 (17.7) | 371 (19.8) | |||||
| Three | 18 (2.8) | 51 (4.5) | 49 (2.6) | |||||
| Four | 2 (0.3) | 2 (0.2) | 5 (0.3) | |||||
SRC, signet ring cell carcinoma; PD, poorly differentiated; WMD, well-to-moderately differentiated.
Survival
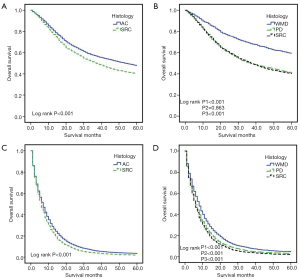
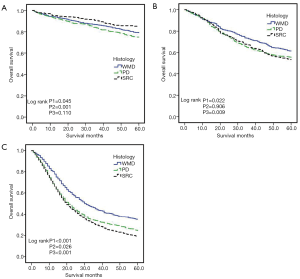
To avoid the impact of inadequately examined LN on the N stage, we only conducted the survival analysis among patients with an examined LN >15 in the M0 group. KM curves, according to histological classification, are shown in Figure 3. In the M0 group, the overall survival of SRC and AC significantly differed (36.7 vs. 40.0 months, respectively; log-rank P<0.001) (Figure 3A). We then divided the non-SRC into WMD and PD groups and compared the OS of SRC with these two groups. SRC demonstrated significantly worse OS than WMD and PD (P1<0.001; P2=0.863; P3<0.001) (Figure 3B). However, in stage I (Figure 4A), SRC was shown to have a better prognosis than WMD and PD. While in stage II, SRC had a similar survival with PD, and both showed worse OS than WMD (Figure 4B). SRC had worse survival than WMD and PD in stage III (Figure 4C). In the M1 group, SRC demonstrated significantly worse survival than AC (Figure 3C). SRC also had a worse prognosis than WMD and PD (Figure 3D).
Predictors of mortality
Univariate analysis results are listed in Table 3. SRC was a prognostic risk factor (univariate Cox HR: 1.78; 95% CI: 1.62–1.96; P<0.001). Prognostic factors including age at diagnosis (≥70), race (white), advanced tumor stage, advanced node stage, tumor location, larger tumor size (≥5 cm) were correlated with increased mortality. Multivariable analysis results from the Cox regression model are shown in Table 3. SRC was an independent unfavorable predictor of survival (multivariable Cox HR: 1.47; 95% CI: 1.37–1.57; P<0.001). Age at diagnosis (multivariable Cox HR: 1.59; 95% CI: 1.48–1.70; P<0.001), white race, advanced tumor stage, advanced node stage, and larger tumor size (≥5 cm) were independently associated with mortality. Then, we further performed the univariable and multivariable analysis at each stage (Figure 5). The results showed that SRC, WMD, and PD had similar overall survival rates in stage I whereas both SRC and PD exhibited a worse OS than WMD in stage II and SRC had worse survival than WMD and PD in stage III. In the M1 group, multivariable analysis showed SRC; age ≥70; advanced node stage; no surgery; lymph node retrieved (<15); metastasis to bone, brain, liver and lung; tumor location; and absence of radiation therapy were independent prognostic factors of stage IV gastric cancer (Table 4); meanwhile, SRC revealed the worst survival. In general, SRC had a worse prognosis with disease progression.
Table 3
| Characteristics | Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Histology | |||||
| WMD | 1 | 1 | |||
| PD | 1.77 (1.62–1.93) | <0.001 | 1.28 (1.21–1.35) | <0.001 | |
| SRC | 1.78 (1.62–1.96) | <0.001 | 1.47 (1.37–1.57) | <0.001 | |
| SRC (vs. PD) | 1.01 (0.93–1.09) | 0.864 | |||
| Age (yrs) | |||||
| <70 | 1 | 1 | |||
| ≥70 | 1.34 (1.25–1.43) | <0.001 | 1.59 (1.48–1.70) | <0.001 | |
| Gender | |||||
| Male | 1 | ||||
| Female | 0.95 (0.89–1.02) | 0.153 | |||
| Race | |||||
| White | 1 | 1 | |||
| Black | 1.03 (0.94–1.14) | 0.531 | |||
| Othera | 0.73 (0.67–0.79) | <0.001 | 0.78 (0.72–0.85) | <0.001 | |
| Tumor stage | |||||
| T1 | 1 | 1 | |||
| T2/T3/T4 | 4.15 (3.67–4.71) | <0.001 | 2.49 (2.19–2.85) | <0.001 | |
| Node stage | |||||
| Negative | 1 | 1 | |||
| Positive | 3.55 (3.26–3.87) | <0.001 | 2.64 (2.41–2.89) | <0.001 | |
| Tumor location | |||||
| Upper stomach | 1 | 1 | |||
| Middle stomach | 0.81 (0.71–0.92) | 0.001 | 0.76 (0.67–0.87) | <0.001 | |
| Lower stomach | 0.87 (0.80–0.95) | 0.002 | 0.83 (0.76–0.91) | <0.001 | |
| Overlapping | 1.30 (1.16–1.47) | <0.001 | 1.02 (0.90– 1.15) | 0.808 | |
| Tumor size (cm) | |||||
| <5 | 1 | 1 | |||
| ≥5 | 1.72 (1.60–1.85) | <0.001 | 1.19 (1.11–1.28) | <0.001 | |
| Radiation | |||||
| No | 1 | ||||
| Yes | 1.02 (0.96–1.09) | 0.555 | |||
SRC, signet ring cell carcinoma; PD, poorly differentiated; WMD, well-to-moderately differentiated; HR, hazard ratio; CI, confidence interval; yrs, years; LN, lymph node. aincludes: American Indian/AK Native, Asian/Pacific Islander.
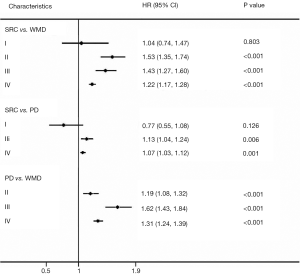
Table 4
| Characteristics | Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Histology | |||||
| WMD | 1 | 1 | |||
| PD | 1.19 (1.17–1.25) | <0.001 | 1.22 (1.17–1.28) | <0.001 | |
| SRC | 1.29 (1.22–1.35) | <0.001 | 1.31 (1.24–1.39) | <0.001 | |
| SRC (vs. PD) | 1.08 (1.04–1.13) | <0.001 | 1.07 (1.03–1,12) | 0.001 | |
| Age (yrs) | |||||
| <70 | 1 | 1 | |||
| ≥70 | 1.19 (1.14–1.23) | <0.001 | 1.22 (1.17–1.27) | <0.001 | |
| Gender | |||||
| Male | 1 | ||||
| Female | 1.01 (0.97–1.04) | 0.791 | |||
| Race | |||||
| White | 1 | ||||
| Black | 1.04 (0.97–1.11) | 0.121 | |||
| Othera | 0.73 (0.69–0.77) | 0.134 | |||
| Tumor stage | |||||
| T1 | 1 | 1 | |||
| T2/T3/T4 | 0.91 (0.86–0.96) | <0.001 | 1.02 (0.97–1.08) | 0.385 | |
| Node stage | |||||
| Negative | 1 | 1 | |||
| Positive | 1.50 (1.31–1.71) | <0.001 | 1.47 (1.25–1.72) | <0.001 | |
| Surgery | |||||
| No | 1 | 1 | |||
| Yes | 0.61 (0.58–0.64) | 0.001 | 0.74 (0.68–0.81) | <0.001 | |
| LN retrieved | |||||
| <15 | 1 | 1 | |||
| ≥15 | 0.83 (0.76–0.91) | 0.001 | 0.85 (077–0.94) | 0.001 | |
| Tumor location | |||||
| Upper stomach | 1 | 1 | |||
| Middle stomach | 1.08 (1.01–1.15) | 0.028 | 0.72 (0.66–0.79) | <0.001 | |
| Lower stomach | 1.01 (0.97–1.06) | 0.758 | 0.78 (0.73–0.83) | 0.202 | |
| Overlapping | 1.17 (1.10–1.21) | <0.001 | 0.94 (0.86–1.03) | <0.001 | |
| Bone metastasis | |||||
| No | 1 | 1 | |||
| Yes | 1.40 (1.30–1.52) | <0.001 | 1.28 (1.16–1.41) | <0.001 | |
| Brain metastasis | |||||
| No | 1 | 1 | |||
| Yes | 1.39 (1.16–1.67) | <0.001 | 1.44 (1.17–1.76) | <0.001 | |
| Liver metastasis | |||||
| No | 1 | 1 | |||
| Yes | 1.08 (1.02–1.14) | <0.001 | 1.10 (1.04–1.18) | 0.003 | |
| Lung metastasis | |||||
| No | 1 | 1 | |||
| Yes | 1.31 (1.22–1.42) | <0.001 | 1.25 (1.12–1.39) | <0.001 | |
| Number of sites | |||||
| One | 1 | 1 | |||
| Two | 1.32 (1.21–1.45) | <0.001 | 1.03 (0.90–1.18) | 0.654 | |
| Three | 1.26 (1.03–1.55) | 0.023 | 0.82 (0.63–1.07) | 0.147 | |
| Four | 2.20 (1.10–4.41) | 0.026 | 0.99 (0.46–2.09) | 0.969 | |
| Tumor size (cm) | |||||
| <5 | 1 | ||||
| ≥5 | 1.05 (0.99–1.11) | 0.135 | |||
| Radiation | |||||
| No | 1 | 1 | |||
| Yes | 0.87 (0.83–0.91) | <0.001 | 0.92 (0.87–0.96) | 0.001 | |
SRC, signet ring cell carcinoma; PD, poorly differentiated; WMD, well-to-moderately differentiated; HR, hazard ratio; CI, confidence interval; yrs, years; LN, lymph node. aincludes: American Indian/AK Native, Asian/Pacific Islander.
Discussion
According to reports, the prevalence of SRC in the stomach ranges from 3.4–39% (9). In this study, 25.1% of the total patients had SRC. In respect of survival of SRC, Jiang et al. (7) reported that SRC was associated with a better survival rate in early gastric cancer while exhibiting a similar prognosis with AC in the advanced stage. Taghavi et al. (10) also suggested that there was no significant difference in the long-term survival between SRC and AC for advanced gastric cancer. However, there are considerable prognostic differences between Asian and American patients (11). The reasons are multifactorial, and SRC might have a different prognostic impact between Asian and American populations. Thus, the prognostic impact of SRC was only evaluated in American populations.
Meanwhile, due to the heterogeneity of gastric cancer, we further divided AC into WMD and PD. The results demonstrated that after adjustment for other prognostic factors, OS had no significant difference among SRC, PD, and WMD in stage I. With the progress of stages, the prognosis of SRC became gradually worse when compared to WMD and PD. Our study suggests that in American populations, SRC resembles hereditary diffuse gastric cancer (HDGC) in the characteristics and prognosis, which is inert in the mucosal layer at early stages and ultimately converts into aggressive phenotype in advanced GC (2). Thus, early aggressive screening and dissection can significantly improve SRC carcinoma patients’ overall survival. Extensive therapy should be considered in advanced and metastatic gastric SRC.
Relatively high percentages of inadequate lymph node dissection were found in our study, including 47.2%, 56.3%, and 48.7% of patients with an examined LN <15 for the SRC, WMD, and PD in the M0 group, respectively. The study indicated that most American gastric cancer patients underwent D1 or D0 lymphadenectomy, which might lead to inadequate LN examination (12). Reid-Lombardo et al. found that the proportion of D1 lymphadenectomy was 56.7% in America (13). A National Cancer Data Base (NCDB) study (14) included 129,666 GC patients, and about 50% of them had more than 15 lymph nodes examined, which was similar to this study.
A higher rate of SRC type was diagnosed in younger patients and in female patients than AC type, which is consistent with previous studies (2,10,15). The mechanism that SRC gastric cancer accounts for a more significant proportion among young and female patients has not yet been explained. One theory is that sex hormones might influence the histological type. The staining pattern of ERβ in young patients is distinctive between signet ring cell carcinomas and other adenocarcinomas, implying that the pathogenesis of SRC differs from that of other cell types (16).
These two tumors also existed at different anatomical locations. Whereas signet ring cell carcinoma presented more in the body, lower stomach, and as an overlapping type, AC was more likely to present proximally. Numerous studies have reported that SRC type is more frequently observed with subserosa (T3) or serosa (T4), and is associated with a higher primary tumor metastasis to lymph node rate (15,17,18), which is consistent with our study. Moreover, the patients of SRC carcinoma with distant metastasis were more numerous than those of AC.
About one-third of patients with gastric cancer were diagnosed with stage IV (19). A previous study indicated that liver metastasis was the most common site of hematogenous metastasis in GC (20), and bone metastasis was rare (21). However, we found that in SRC, bone metastasis accounted for nearly half of the patients with known metastasis sites. Also, the proportion of liver and lung metastasis were also relatively high for SRC patients. Therefore, it is necessary to carry out multi-type imaging techniques and multiple sites examination for SRC. Peritoneal metastasis was also more commonly found among gastric SRC carcinoma during surgery (17,22,23). Thus, studies demonstrated that a lower rate of curative resection was performed in the SRC histological type (22,24-26). The SEER database mainly included hematogenous metastasis, and there were no data about peritoneal metastasis. Thus, we could not compare the differences in peritoneal metastasis between SRC and AC.
Studies have shown that gene expression can also be different between SRC and adenocarcinoma in gastric cancer (27-29). The manifestations of signet ring cell carcinoma distinguished from AC might support gene expression results from a clinical standpoint. Molecular and genomic analysis of gastric cancer was conducted by The Cancer Genome Atlas Research Network (TCGA), and four subtypes were proposed (27). The analysis results may increase our comprehension of occurrence and development of gastric cancer and help to predict long-term survival more accurately in different cell types (27). It is also possible to make identifying molecular biomarkers that are specific to the SRC subtype (28,29) and the relative therapeutic targets. Finally, it helps us predict patients' sensitivity to chemotherapy (28) and select individualized treatment regimens.
Several limitations existed in our study. First, it was a retrospective study, and selection biases were thus inevitable. Second, the SEER database did not contain detailed information about surgical procedures and adjuvant treatment, which might have an impact on the prognosis. Third, there were no data about whether patients received preoperative therapy that might affect pathological stage. Fourth, detailed pathologic review was unclear in SEER database, pathologic diagnosis might be differed in different institutions.
In conclusion, SRC was significantly different from AC in clinicopathologic characteristics. Although the prognosis of SRC was similar to AC in the early stage, it had a poorer prognostic impact with the progression of the disease. Different therapeutic regimens and imaging evaluation should be applied according to histological types of gastric cancer.
Acknowledgments
Funding: This study was funded by grants from
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.09.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This article was approved by an independent ethical committee review board at Liaoning Cancer Hospital & Institute Ethical Committee (ID: 20181226). The individual informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Chon HJ, Hyung WJ, Kim C, et al. Differential prognostic implications of gastric signet ring cell carcinoma: stage adjusted analysis from a single high-volume center in Asia. Ann Surg 2017;265:946-53. [Crossref] [PubMed]
- Bosman FT, Carneiro F, Hruban RH, et al. WHO classification of tumours of the digestive system. International Agency for Research on Cancer. 4th ed. 2010.
- Hyung WJ, Noh SH, Lee JH, et al. Early gastric carcinoma with signet ring cell histology. Cancer 2002;94:78-83. [Crossref] [PubMed]
- Kunisaki C, Shimada H, Nomura M, et al. Therapeutic strategy for signet ring cell carcinoma of the stomach. Br J Surg 2004;91:1319-24. [Crossref] [PubMed]
- Zhang M, Zhu G, Zhang H, et al. Clinicopathologic features of gastric carcinoma with signet ring cell histology. J Gastrointest Surg 2010;14:601-6. [Crossref] [PubMed]
- Jiang CG, Wang ZN, Sun Z, et al. Clinicopathologic characteristics and prognosis of signet ring cell carcinoma of the stomach: results from a Chinese mono-institutional study. J Surg Oncol 2011;103:700-3. [Crossref] [PubMed]
- Piessen G, Messager M, Leteurtre E, et al. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg 2009;250:878-87. [Crossref] [PubMed]
- Theuer CP, Nastanski F, Brewster WR, et al. Signet ring cell histology is associated with unique clinical features but does not affect gastric cancer survival. Am Surg 1999;65:915-21. [PubMed]
- Taghavi S, Jayarajan SN, Davey A, et al. Prognostic significance of signet ring gastric cancer. J Clin Oncol 2012;30:3493-8. [Crossref] [PubMed]
- Jin H, Pinheiro PS, Callahan KE, et al. Examining the gastric cancer survival gap between Asians and whites in the United States. Gastric Cancer 2017;20:573-82. [Crossref] [PubMed]
- Strong VE, Yoon SS. Extended lymphadenectomy in gastric cancer is debatable. World J Surg 2013;37:1773-7. [Crossref] [PubMed]
- Reid-Lombardo KM, Gay G, Patel-Parekh L, et al. Treatment of gastric adenocarcinoma may differ among hospital types in the United States, a report from the National Cancer Data Base. J Gastrointest Surg 2007;11:410-9; discussion 419-20. [Crossref] [PubMed]
- Zhao B, Leichman LP, Horgan S, et al. Evaluation of treatment and outcomes for Hispanic patients with gastric cancer at Commission on Cancer-accredited centers in the United States. J Surg Oncol 2019;119:941-7. [Crossref] [PubMed]
- Kwon KJ, Shim KN, Song EM, et al. Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Gastric Cancer 2014;17:43-53. [Crossref] [PubMed]
- Matsuyama S, Ohkura Y, Eguchi H, et al. Estrogen receptor beta is expressed in human stomach adenocarcinoma. J Cancer Res Clin Oncol 2002;128:319-24. [Crossref] [PubMed]
- Jiang H, Zhang H, Tian L, et al. The difference in clinic-pathological features between signet ring cell carcinoma and gastric mucinous adenocarcinoma. Tumor biology 2013;34:2625-31. [Crossref] [PubMed]
- Kao YC, Fang WL, Wang RF, et al. Clinicopathological differences in signet ring cell adenocarcinoma between early and advanced gastric cancer. Gastric Cancer 2019;22:255-63. [Crossref] [PubMed]
- Jim MA, Pinheiro PS, Carreira H, et al. Stomach cancer survival in the United States by race and stage (2001-2009): findings from the CONCORD-2 study. Cancer 2017;123:4994-5013. [Crossref] [PubMed]
- Li J, Xi H, Cui J, et al. Minimally invasive surgery as a treatment option for gastric cancer with liver metastasis: a comparison with open surgery. Surg Endosc 2018;32:1422-33. [Crossref] [PubMed]
- Nakamura K, Tomioku M, Nabeshima K, et al. Clinicopathologic features and clinical outcomes of gastric cancer patients with bone metastasis. Tokai J Exp Clin Med 2014;39:193-8. [PubMed]
- Kim JP, Kim SC, Yang HK. Prognostic significance of signet ring cell carcinoma of the stomach. Surg Oncol 1994;3:221-7. [Crossref] [PubMed]
- Otsuji E, Yamaguchi T, Sawai K, et al. Characterization of signet ring cell carcinoma of the stomach. J Surg Oncol 1998;67:216-20. [Crossref] [PubMed]
- Yokota T, Kunii Y, Teshima S, et al. Signet ring cell carcinoma of the stomach: a clinicopathological comparison with the other histological types. Tohoku J Exp Med 1998;186:121-30. [Crossref] [PubMed]
- Li C, Kim S, Lai JF, et al. Advanced gastric carcinoma with signet ring cell histology. Oncology 2007;72:64-8. [Crossref] [PubMed]
- Kim DY, Park YK, Joo JK, et al. Clinicopathological characteristics of signet ring cell carcinoma of the stomach. ANZ J Surg 2004;74:1060-4. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Tan IB, Ivanova T, Lim KH, et al. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology 2011;141:476-85. [Crossref] [PubMed]
- Shah MA, Khanin R, Tang L, et al. Molecular classification of gastric cancer: a new paradigm. Clin Cancer Res 2011;17:2693-701. [Crossref] [PubMed]