
Prazosin inhibits the growth and mobility of osteosarcoma cells
Introduction
Osteosarcoma is a primary malignant bone tumor that frequently occurs in adolescents and children, commonly occurring in the femur, tibia and humerus (1-3). Its incidence rate is 3–4/million worldwide, being slightly higher in males than in females (4,5). Due to the high aggressive and rapid metastasis of osteosarcoma, general treatment is severely limited to patients that exhibit metastasis, leading to a poor prognosis of less than 20% (6,7). However, given the advancement of osteosarcoma treatment technologies in recent years (surgical resection, chemotherapy, and radiotherapy) the survival of patients with osteosarcoma had generally improved, but has more recently stagnated (8). Therefore, it is critical that we find a new anticancer agent that inhibits the metastasis and invasion of osteosarcoma.
Recent research has worked towards this end, searching for new anti-cancer drugs and re-evaluating known drugs that have been used for other diseases, to find their potential anti-tumor effects (9). Prazosin [1-(4-amino-6,7-dimethoxy-quinazolin-2-yl)-4-(2-furoyl) piperazine], an α1-adrenoceptor antagonist, had generally been used as a sympatholytic drug for the treatment of hypertension and posttraumatic stress disorder (PTSD) (9-11). Recently however, Lin et al. demonstrated that Prazosin also displays an anti-proliferative effect in prostate cancer cells; it accomplished this by inducing apoptosis via resulting in DNA damage stress (12). Similarly, Fuchs et al. confirm that Prazosin treatment inhibits the growth of medullary thyroid carcinoma (MTC) cells, also inducing apoptosis (9). Further studies have also revealed that Prazosin reduces cell proliferation and increases docetaxel-induced toxicity in prostate cancer cells, by modulating autophagy and apoptosis (13).
However, these studies have generally focused on the anti-proliferative effects of Prazosin without specifically studying whether Prazosin affects tumor metastasis in osteosarcoma. Therefore, in the present study, we have investigated Prazosin’s underlying mechanisms and its effects on the biological behaviors of osteosarcoma cells. In brief, we have observed that Prazosin displays a significant depression of the proliferation, migration, and invasion properties of osteosarcoma cell lines MG63 and 143B, and induced apoptosis, suggesting that it may serve as a novel anti-cancer agent.
Methods
Cell culture
Osteosarcoma cell lines MG63 and 143B were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). They were cultured in Dulbecco’s Modified Eagle Medium (DMEM) medium (HyClone, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, USA) at 37 °C with 5% CO2.
CCK8 assay
The MG63 and 143B cells were treated with different concentrations of Prazosin (0, 2.5, 5, 7.5, 10, 15, 20, 30, 40, and 50 µM; MedChemExpress, USA), with Prazosin’s effect on cell viability being analyzed by CCK8 assay. After treatment for 24 h, cells were cultured with CCK8 reagent (10 µL/well; Beijing Solarbio Science & Technology, Beijing, China) at 37 °C for 90 min with absorbance being measured at 450 nm.
The effect of Prazosin on MG63 and 143B cells were further analyzed. Approximately 1×103 cells were seeded into each well of a 96-well plate and cultured for 24 h. Cells were then treated with either Prazosin or with dimethyl sulfoxide (DMSO) as negative control (NC). Following this treatment for 0, 24, 48, and 72 h, cells were cultured with CCK8 reagent at 37 °C for 90 min with absorbance being measured at 450 nm.
Clonogenic assay
About 500 of the cells treated with either Prazosin or DMSO for 24 h were seeded into mediums to be cultured at 37 °C with 5% CO2 for 2 weeks. Afterwards, the colonies were fixed with 1 mL of 4% paraformaldehyde for 30 min, followed by staining with crystal violet for 30 min. Photographs were then taken and the number of colonies was counted.
Transwell assay
Transwell chambers were performed to assess the migration and invasion abilities of MG63 and 143B cells. Before assaying, the Transwell chambers were coated with Matrigel. Following treatment with Prazosin or DMSO for 24 h, the cells in serum-free culture medium were transferred to the upper chamber, with complete medium being added to the lower chamber. After incubation for 24 h, the invaded cells were fixed with 4% paraformaldehyde, and stained with 0.1% crystal violet for 20 min. Photograph was then taken and the number of invaded cells was counted under the microscope. The migration assay was similarly conducted except for the absence of Matrigel.
Flow cytometry assay
In order to assess apoptosis of the cells, the treated MG63 and 143B cells were cultured in serum-free medium for 24 h in order to induce starvation. They were then stained with the Annexin V-FITC-PI apoptosis detection kit (4A Biotech, China) as according to the instructions. The apoptosis rate was analyzed using a flow cytometer (BD FACSCanto II, BD Biosciences, USA), and calculated using BD FACSDiva software.
Western blot assay
Cells were lysed using ice-cold RIPA Lysis Buffer (CWBIO, China) after 24 h of Prazosin or DMSO treatment to extract proteins. Twenty µg of protein from each sample was electrophoresed on 10% 24 sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, and then transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, USA). The membrane was blocked with 5% non-fat milk for 1 h prior to incubation with primary antibodies at 4 °C overnight. Following incubation with secondary antibody for 1 h, the signal was then developed using an enhanced chemiluminescence detection kit. Bcl-2, Bax, active caspase 3, P70, cyclin D1 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibodies were purchased from Proteintech Group (USA), and Akt, p-Akt, mTOR and p-mTOR antibodies were obtained from Cell Signaling Technology (USA). The horseradish peroxidase-conjugated secondary antibodies were obtained from Proteintech Group.
Statistical analysis
All experiments were repeated in triplicate with the values being presented as mean ± standard deviation (SD). SPSS 18.0 statistical software was utilized to carry out statistical analysis. Student’s t-test or one-way analysis of variance (ANOVA) was performed for statistical analysis between groups, and P<0.05 was considered statistically significant.
Results
Prazosin inhibits the growth of MG63 and 143B cells
In order to investigate whether Prazosin effects the cell growth of osteosarcoma, MG63 and 143B cells were treated with different concentrations of Prazosin (0, 2.5, 5, 7.5, 10, 15, 20, 30, 40, and 50 µM). We found that Prazosin showed no effect on the cell viability of MG63 cells at concentrations lower than 10 µM (Figure 1A), whereas it showed significant inhibitory effect at greater concentrations. Similar inhibitory effects were observed in 143B cells (Figure 1B). The half maximal inhibitory concentration (IC50) concentration was 25.29 µM in MG63 cells and 35.28 µM in 143B cells, and 20 µM of Prazosin was used in MG63 cells and 25 µM was used in 143B cells in all the rest experiments as its appropriate effects. In order to substantiate the inhibition of Prazosin in the proliferation of MG63 and 143B cells, CCK8 assay was performed. As shown in Figure 1C, Prazosin significantly blocked the proliferation of MG63 cells when compared with NC cells (P<0.05), and Prazosin also inhibited the proliferation of 143B cells in a time-dependent manner (Figure 1D). Colony-forming assay was performed to further confirm the effect of Prazosin on clonogenic abilities of MG63 and 143B cells. Our data revealed a significant decrease in the number and size of colonies in Prazosin treated cells compared with NC group (P<0.05, Figure 1E). Collectively, these data suggest that Prazosin inhibits the viability and proliferation of osteosarcoma cells in vitro.
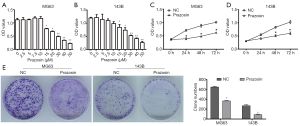
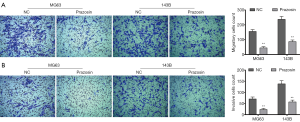
Prazosin decreases the migration and invasion abilities of MG63 and 143B cells
It is well known that the invasion and metastasis properties of cancer cells are the main causes for poor prognosis in osteosarcoma. Therefore, we examined Prazosin’s effect on these properties in MG63 and 143B cells using a Transwell assay in vitro. As shown in Figure 2A, Prazosin led to a significant decrease in the migration abilities of MG63 and 143B cells compared with NC group (P<0.01). Meanwhile, the number of invasive cells was also significantly decreased by Prazosin treatment (P<0.01, Figure 2B). Overall, Prazosin displays a significant anti-cancer role in the migration and invasion abilities of osteosarcoma cells.
Prazosin induces apoptosis in MG63 and 143B cells
Given that Prazosin was found to inhibit cell growth, cell apoptosis was assessed using flow cytometry to determine the effect of Prazosin on cell survival of osteosarcoma. Flow cytometric analysis showed that Prazosin significantly increased the rate of apoptosis induced by starvation in MG63 cells compared with NC group (P<0.05, Figure 3A). Additionally, the rate of apoptosis in 143B cells was also promoted by Prazosin (Figure 3A). To investigate which apoptotic pathway was induced by Prazosin, western blotting was used to detect the expression level of Bcl-2, Bax and active caspase 3. As expected, the expression level of anti-apoptotic protein Bcl-2 was down-regulated by Prazosin compared with NC group (P<0.01), while expression level of pro-apoptotic proteins Bax and active caspase 3 was simultaneously up-regulated (P<0.01, Figure 3B). The above suggests that Prazosin induces the mitochondrial apoptotic pathway in MG63 and 143B cells.

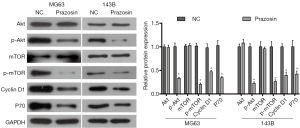
Prazosin inhibits activity of the Akt/mTOR pathway in MG63 and 143B cells
As everyone knows, the Akt/mTOR pathway plays a pivotal role in regulating cell growth and survival, being particularly involved in progression of osteosarcoma. In the present study, we examined the expression levels of crucial proteins in the Akt/mTOR pathway to confirm whether the pathway is involved in the anticancer effects of Prazosin. Our data showed that the phosphorylation levels of Akt (p-Akt) and mTOR (p-mTOR) were significantly attenuated by Prazosin in both MG63 and 143B cells. Furthermore, it showed that the expression levels of downstream proteins p70S6k (P70) and cyclin D1 were also decreased (P<0.05, Figure 4). This data suggests that the Akt/mTOR pathway might be involved in the anti-tumor effects of Prazosin.
Discussion
Metastasis is an important factor affecting the prognosis of patients with osteosarcoma, especially the occurrence of lung metastasis (14). Therefore, blocking tumor metastasis and invasion is a necessary strategy for osteosarcoma therapy. It has been well-documented that Prazosin enhances the apoptosis rate in a number of tumor cells, displaying anti-growth activity in prostate cancer (12,13,15), glioblastoma (16), and MTC cells (9). In the present study, we have tested and identified Prazosin as a novel anti-cancer agent in osteosarcoma cells. We have shown that Prazosin possesses a significant inhibitory effect on cell viability of osteosarcoma MG63 and 143B cells in a dose- and time-dependent manner in vitro. Furthermore, we’ve shown that Prazosin inhibits both cell migration and invasion, and also induces apoptosis, further displaying an anti-tumor role in the progression and motility of osteosarcoma cells.
Dysregulated apoptosis is one of the common hallmarks of tumor cells, reducing apoptosis is a general mechanism of survival of osteosarcoma cells. In this study, our data confirmed a significant induction in apoptosis in Prazosin treated cells compared with the NC group. It has been shown that apoptosis involves numerous proteolytic events mainly mediated by the family of cysteine proteases, including the activated caspase 3, a pivotal executioner to trigger apoptosis (17). It has already shown that Prazosin induces apoptosis in prostate cancer PC-3 cells by triggering mitochondria mediated caspase cascades through targeting DNA (12). Our data confirm that Prazosin induces apoptosis by up-regulating the level of active caspase 3 in MG63 and 143B cells, suggesting that activating the caspase cascades is involved in the promotion of apoptosis induced by Prazosin. The Bcl-2 family also plays an essential role in initiation of apoptosis by triggering the mitochondrial pathway (18,19). In general, a decreasing ratio of Bcl-2, a pivotal anti-apoptotic protein located in the mitochondria, to Bax, a crucial pro-apoptotic protein mainly located in the cytoplasm, promotes apoptosis (20,21). In our study, a significant decrease in the expression level of Bcl-2 and concurrent increase in Bax were observed in MG63 and 143B cells exposed to Prazosin, indicating that apoptosis promoted by Prazosin is specifically triggered in the mitochondrial pathway. Autophagy, also known as type II programmed cell death, is another way of causing cell death. Prazosin also has been reported to be able to induce autophagy in prostate cancer (13,15) and H9C2 cells (22). As described above, Prazosin might be used as an effective anti-tumor agent for cancer therapy, and whether Prazosin induces autophagy in osteosarcoma cells will be the focus of our further study.
Abnormalities in the PI3K/Akt/mTOR pathway are frequently observed in various cancers and are correlated with tumor cell growth and survival. Aberration of PI3K/Akt pathway is frequently observed in cancers including osteosarcoma (23). More specifically, growing evidence reveals that some anti-tumor agents, such as Ferulic acid (8), Lupeol (24) and Andrographolide (25), blocks the PI3K/Akt/mTOR pathway, exerting significant inhibitory effects on cell growth and survival of osteosarcoma and even enhancing the anti-cancer effects of cisplatin (26). Furthermore, Yang et al. report that the Akt/mTOR pathway is involved in Prazosin-induced autophagy in rat embryonic ventricular myoblast H9C2 cells (22). The Akt pathway is also observed to be inhibited by Prazosin in glioblastoma-initiating cells (16). Consistent with these previous studies, we have found that the phosphorylation levels of Akt and mTOR were down-regulated by Prazosin. Similarly, we found that expression levels of P70 and cyclin D1 were also decreased. P70, a downstream target protein of mTOR, modulates protein synthesis and is involved in cell proliferation and cell cycle. It has been demonstrated that P70 is over-activated in osteosarcoma tissues, being associated with the progression of osteosarcoma (27). Cyclin D1, a key regulator in the cell cycle, promotes cell proliferation. Cyclin D1 overexpression is frequently observed in tumors and associated with tumorigenesis (28). Given the above, the Akt/mTOR pathway may be involved in the anti-tumor effects of Prazosin on the proliferation, invasion, and survival of osteosarcoma cells.
Conclusions
In the current study, we highlight that Prazosin inhibits the cell growth and motility of MG63 and 143B cells. We have also shown that it promotes apoptosis in the mitochondrial pathway. In sum, as Prazosin inhibits cell survival and metastasis, our data suggests that it may serve as a potential anti-cancer agent in osteosarcoma therapy.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.09.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of The First Central Hospital of Baoding (No. 2019061). Individual informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Geller DS, Gorlick R. Osteosarcoma: a review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol 2010;8:705-18. [PubMed]
- Kansara M, Teng MW, Smyth MJ, et al. Translational biology of osteosarcoma. Nat Rev Cancer 2014;14:722-35. [Crossref] [PubMed]
- Zhang H, Guo X, Feng X, et al. MiRNA-543 promotes osteosarcoma cell proliferation and glycolysis by partially suppressing PRMT9 and stabilizing HIF-1α protein. Oncotarget 2017;8:2342-55. [PubMed]
- Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology, and end results program. Cancer 2009;115:1531-43. [Crossref] [PubMed]
- Anderson ME. Update on survival in osteosarcoma. Orthop Clin North Am 2016;47:283-92. [Crossref] [PubMed]
- Allison DC, Carney SC, Ahlmann ER, et al. A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma 2012;2012:704872. [Crossref] [PubMed]
- Jin H, Jin X, Zhang H, et al. Circular RNA hsa-circ-0016347 promotes proliferation, invasion and metastasis of osteosarcoma cells. Oncotarget 2017;8:25571-81. [PubMed]
- Wang T, Gong X, Jiang R, et al. Ferulic acid inhibits proliferation and promotes apoptosis via blockage of PI3K/Akt pathway in osteosarcoma cell. Am J Transl Res 2016;8:968-80. [PubMed]
- Fuchs R, Schwach G, Stracke A, et al. The anti-hypertensive drug Prazosin induces apoptosis in the medullary thyroid carcinoma cell line TT. Anticancer Res 2015;35:31-8. [PubMed]
- Nakamura T, Kamishikiryo J, Morita T. Prazosin-stimulated release of hepatic triacylglyceride lipase from primary cultured rat hepatocytes is involved in the regulation of cAMP-dependent protein kinase through activation of the Ca(2+)/calmodulin-dependent protein kinase-II. Pharmacol Rep 2016;68:649-53. [Crossref] [PubMed]
- Petrakis IL, Desai N, Gueorguieva R, et al. Prazosin for veterans with posttraumatic stress disorder and comorbid alcohol dependence: a clinical trial. Alcohol Clin Exp Res 2016;40:178-86. [Crossref] [PubMed]
- Lin SC, Chueh SC, Hsiao CJ, et al. Prazosin displays anticancer activity against human prostate cancers: targeting DNA and cell cycle. Neoplasia 2007;9:830-9. [Crossref] [PubMed]
- Spencer BH, McDermott CM, Chess-Williams R, et al. Prazosin but not tamsulosin sensitises PC-3 and LNCaP prostate cancer cells to docetaxel. Pharmacology 2018;102:10-8. [Crossref] [PubMed]
- Faisham WI, Mat Saad AZ, Alsaigh LN, et al. Prognostic factors and survival rate of osteosarcoma: a single-institution study. Asia Pac J Clin Oncol 2017;13:e104-10. [Crossref] [PubMed]
- Forbes A, Anoopkumar-Dukie S, Chess-Williams R, et al. Relative cytotoxic potencies and cell death mechanisms of α1-adrenoceptor antagonists in prostate cancer cell lines. Prostate 2016;76:757-66. [Crossref] [PubMed]
- Assad Kahn S, Costa SL, Gholamin S, et al. The anti-hypertensive drug Prazosin inhibits glioblastoma growth via the PKCδ-dependent inhibition of the AKT pathway. EMBO Mol Med 2016;8:511-26. [Crossref] [PubMed]
- Gui D, Guo Y, Wang F, et al. Astragaloside IV, a novel antioxidant, prevents glucose-induced podocyte apoptosis in vitro and in vivo. PLoS One 2012;7:e39824. [Crossref] [PubMed]
- Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell 2011;21:92-101. [Crossref] [PubMed]
- Wu R, Tang S, Wang M, et al. MicroRNA-497 induces apoptosis and suppresses proliferation via the Bcl-2/Bax-caspase9-caspase3 pathway and cyclin D2 protein in HUVECs. PLoS One 2016;11:e0167052. [Crossref] [PubMed]
- Soares NCP, Teodoro AJ, Oliveira FL, et al. Lycopene induce apoptosis in human prostate cells and alters the expression of Bax and Bcl-2 genes. LWT-Food Science and Technology 2014;59:1290-7. [Crossref]
- Liu G, Wang T, Wang T, et al. Effects of apoptosis-related proteins caspase-3, Bax and Bcl-2 on cerebral ischemia rats. Biomed Rep 2013;1:861-7. [Crossref] [PubMed]
- Yang YF, Wu CC, Chen WP, et al. Prazosin induces p53-mediated autophagic cell death in H9C2 cells. Naunyn Schmiedebergs Arch Pharmacol 2011;384:209-16. [Crossref] [PubMed]
- Chen J, Yao D, Yuan H, et al. Dipsacus asperoides polysaccharide induces apoptosis in osteosarcoma cells by modulating the PI3K/Akt pathway. Carbohydr Polym 2013;95:780-4. [Crossref] [PubMed]
- Liu Y, Bi T, Dai W, et al. Lupeol induces apoptosis and cell cycle arrest of human osteosarcoma cells through PI3K/AKT/mTOR pathway. Technol Cancer Res Treat 2016;15:NP16-24. [Crossref] [PubMed]
- Liu Y, Zhang Y, Zou J, et al. Andrographolide induces autophagic cell death and inhibits invasion and metastasis of human osteosarcoma cells in an autophagy-dependent manner. Cell Physiol Biochem 2017;44:1396-410. [Crossref] [PubMed]
- Liu B, Yan S, Qu L, et al. Celecoxib enhances anticancer effect of cisplatin and induces anoikis in osteosarcoma via PI3K/Akt pathway. Cancer Cell Int 2017;17:1. [Crossref] [PubMed]
- Zhou Q, Deng Z, Zhu Y, et al. mTOR/p70S6K signal transduction pathway contributes to osteosarcoma progression and patients' prognosis. Med Oncol 2010;27:1239-45. [Crossref] [PubMed]
- Qie S, Diehl JA. Cyclin D1, cancer progression, and opportunities in cancer treatment. J Mol Med (Berl) 2016;94:1313-26. [Crossref] [PubMed]


