
Opportunities from modulating ultrasound, from tissue ablation to tissue regeneration
Introduction
The first studies on the biological effects of ultrasound (US) are dated at the beginning of 1900s, when the interaction between living tissues and high intensity and frequency sound waves were analysed (1). Then, in 1940 and 1950 William Fry and collaborators produced deep lesions in brains of cats and monkeys to study the use of focused US for therapeutic tissue ablation, but the lack of a precise imaging system limited their use till two decades ago (2-4). Nowadays, high intensity focused ultrasound (HIFU) systems, in combination with magnetic resonance imaging or diagnostic ultrasound, have given rise to novel therapeutic approaches like MR guided focused ultrasound surgery (MRgFUS) and US guided ultrasound surgery (USgFUS). These systems have become a new medical non-invasive therapeutic approach, thanks to their potentiality to obtain combinations of biological effects by modulating thermal, mechanical and chemical effects produced by the propagation of high and low energy waves through living tissues. The current HIFU clinical approved applications are finalized to the ablation of uterine fibroids, prostatic cancer and the palliative pain treatments of bone metastases. Moreover, different clinical experimentations are on-going worldwide in the field of solid tumours ablation, as well as treatments of neurodegenerative diseases, pain and vascular problems, to maximise the use of this technology (5). The last evolution of the addressing HIFU deep in the tissue is the release of “sonication” from phased array transducers trough small ellipsoid-shaped spots. Multiple spots or “sonication” series, released under MR imaging, are needed to destroy a solid mass in the target volume (6). Although, its initial application has been the targeted non-invasive destruction of solid tumours, since US waves can penetrate the skin and tissue layer reaching a target inside the body, HIFU is also effective in tremor treatments and in arresting haemorrhage from either organs or vessels (7). Besides, novel therapeutic horizons become visible for the near future battle against cancer and neurologic diseases; since drug delivery, blood barrier opening, hyperthermia and cell sensitization to radiation treatments, modulating the waves beam intensity, render the use of US an adjuvant therapy in combination to traditional surgery, radiation or chemotherapy treatments (8). This review article would describe all the clinical and research potentialities deriving from modulating ultrasound beams in order to obtain the desired biological effect.
Physical and biological effects
Although the definition of “ultrasonic dose” is still debated, the main physical consequences caused by the ultrasonic waves’ propagation across tissues are the thermal and the mechanical effects.
Thermal effects are the result of the tissue specific ability to absorb acoustic energy and can be easily monitored in clinical use by MR-thermometry (9). According to the final desired purpose, this effect can be utilized to induce hyperthermia, sensitizing tissues and enlarging membranes and junctions of normal tissue structures, or to provoke cells killing through the phenomenon called “coagulative necrosis”, within ablative regimen. These two biologic effects can be reached alone or in combination, as the focused ultrasound can be directed deep in a target within the tissue, which is destroyed using a temperature above 56 °C for few seconds, while gradually lower temperatures are observed in the areas of tissues reached by lower energy intensities of the ultrasound beam (10). Specifically, when the temperature is lower than 100 °C, tissues are subjected to the so called thermal fixation, in which cells do not undergo lysis and the tissue architecture remains relatively intact, but the cells are non-longer viable (11). Moreover, a sharp direct distinction is histologically visible between necrotic treated and not treated areas, while another less immediate biologic effect, following the ablative necrosis, is the release of a large repertoire of intracellular antigens that stimulate the immunological response (12). In addition to uterine fibroids and solid tumours, ablative temperatures reached by MRgFUS, can be used to develop new treatments for arteriovenous malformations and highly vascularized targets, obtaining thermal coagulation of blood vessels and safe and effective non-invasive bone metastases pain palliation, targeting of the periosteum area and thus resulting in bone denervation and pain relief. Furthermore, a pilot study translated this effect to facet pain alleviation, using low levels of energy to achieve a localized heating effect without damaging adjacent tissues, thanks to the high acoustic absorption and low thermal conductivity of the bone cortex (13).
On the other hand, working with sub-ablative temperatures give clinicians numerous possibilities of bio-effects and applications, the majority of which are under experimentation worldwide. For example, the use of hyperthermia in combination with radiotherapy or chemotherapy is a new object of research aiming to generate radio-sensitising or chemo-sensitising adjuvant treatments of target tissue (14).
Besides, among the mechanical effects produced by HIFU, a significant one is termed cavitation, which can be distinguished in non-inertial and inertial. The first one occurs when a gas-filled bubble, formed in tissue in response to US with high peak negative pressure, interacts with an ultrasound wave producing a stable oscillation of the gas filled bodies in response to positive and negative pressures of the US field.
The inertial cavitation occurs when oscillating bubbles undergo a violent collapse in response to US of specific frequency, pulse length, repetition frequency and pressure amplitude parameters. This collapse produces a rapid temperature and pressure increase that provoke cell lysis and the formation of reactive oxygen species (ROS). As consequence of this phenomenon, sub-ablative temperatures can be applied in presence of microbubbles, allowing the alteration of cell membrane permeability, which can facilitate drug or gene delivery, as well as the selective disruption of the blood-brain barrier (BBB) or blood clot. In particular, blood clot disruption is a new therapeutic opportunity, since maintenance of temperatures below the threshold required for ablation and acoustic cavitation, and can causes changes in endothelial membranes and the fibrinolysis cascade activation (15-17). From this perspective, the transcranial ultrasound application is a promising method of thrombolysis for the acute ischemic stroke treatment (18).
Another interesting mechanical phenomenon occurs when a wave is absorbed or reflected by a fluid. The radiation force due to the fluid moving under pressure produces an acoustic streaming, that causes a velocity gradient which in turn induces shear stress (19,20).
In addition, as the possibilities of modulating ultrasound offer a large and varied panel of combined bio-effect, histotripsy, worth to be mentioned. This new ultrasound ablation method depends on the initiation and maintenance till to cavitation of a bubble cloud to fractionate soft tissue, using short and high-intensity pulses of ultrasound (21,22).
Finally, opposed to the drastic killing effects observable under ablative regimen, regenerative properties have been in vitro and in vivo described in treatments using low-intensity pulsed ultrasound (LIPUS). These regeneration effects have been especially observed for bone and cartilage tissues, involving activation of osteoblasts, osteoclasts, chondrocytes, mesenchymal cells, with the exception of tissues already calcified (23).
Approved clinical employment
The great potentialities in the field of ultrasound’ applications have driven, in recent years, a strong technological improvement in transducer design, energy delivery modes and real time imaging. Particularly, the modern MRgFUS systems represent a good technological combination of advanced acoustic transducers with the anatomic, functional, and thermal guidance of MRI, allowing accurate targeting, real-time temperature monitoring, and closed-loop control of energy deposition deep in the body (24,25) (Table 1).

Full table
Three types of devices have been developed: extracorporeal, intracavitary and interstitial. The first one is used to target tissues readily accessible from an acoustic window through the skin, such as uterine fibroids or breast. The second one is used for trans-rectal and trans-urethral treatments of prostate cancer or for intra-esophageal purpose; whereas the third one is dedicated to the treatment of the biliary duct and other difficult to access targets.
Moreover, the MRI temperature and imaging monitoring system offers far more accurate target details than US, in terms of tumour margin detection, surrounding anatomical details and temperature changes, which need to be monitored during therapy (26,27).
These features have brought the first FDA clearance, in 2004, for the MRgFUS clinical employment in the uterine fibroids treatment. Nowadays, the uterine fibroids and prostate cancer HIFU ablation plus the bone metastases pain palliative treatment is the only application with clinical acceptance. All other ablative treatments on breast, liver, kidney and other cancer targets represent on-going clinical trials.
The 10 years employment of MRgFUS for fibroids ablation in more than ten thousand patients, offers significant retrospective data to affirm that this minimally-invasive technique, results in very few patients reporting serious post-treatment complications and a comparable rate of success in terms of symptoms’ reduction for medium sized fibroids and sensible improving of life quality and fertility preservation, compared to traditional surgical or radiological procedures (28-30). Moreover, although leiomyomas should preferably not exceed 10 cm in size, in 2012 Kim and co-workers introduced an interesting new technique for fibroid ablation, featuring one-layer ablation strategy for lesions larger than 10 cm (31).
Similarly, the prostate cancer ablation is, by now, a dated and consolidated technique that permits to reach the target with ultrasound beam, sparing organs at risk, such as the neurovascular bundle. In 2011, a long term study involving 803 patients, made evident the success of this technique in terms of 8 years overall (89%), cancer-specific (99%) survival rates and metastasis-free survival rate (97%) (32-34). Overall, this technique offers an excellent tumour control and complication rates (urinary or sexual dysfunction) comparable to traditional radiotherapy or radical prostatectomy interventions (35).
Finally, the bone metastasis pain palliation treatment, the third consolidated ablation technique, have sensibly and rapidly improved the life quality of bone metastatic patients, which can remain alive longer, providing them fast and effective pain relief, without ionizing radiation, surgical intervention or serious side effects (36). Studies have reported rapid improvements in both visual acuity scale (VAS) scale and pain-measuring scales, just few days after the treatments, while in all studies no adverse events were recorded and all patients were able to reduce their medications at 3 months from MRgFUS (37,38).
A case report at Cefalù Hospital, Italy, described a 62-year-old patient with primary renal carcinoma, treated with MRgFUS (ExAblate System 2100, InSightec Ltd., Haifa, Israel) for the ablation of metastases sited on pelvic bone under the right iliac wing (39). The treatment was safe and effective in terms of pain reduction and a significant increase in life quality. Moreover, in this case, since the bone was completely eroded by the tumour, in addition to the mere palliative treatment, it was possible to exert high-energy sonications (5,500 joules) within the lesion. In correspondence of the treated area, weeks later it was observed the formation of new bone tissue, in the iliac wing and acetabular roof (39).
Experimental ablative protocols
Many clinical trials are on-going worldwide on the use of the MRgFUS technique for the treatments of solid tumours, for instance breast, liver, pancreas and other abdominal targets, as well as brain cancer and neurological disease treatments.
Nonetheless the efforts made by researchers, the effectiveness and safety of this technique have not yet reached an adequate level of quality to overcome the standard therapeutic protocols (40). The main problem to solve is to achieve a 100% of tumour ablation without positive margins, since a small percentage of cases still show peripheral residual tumour presence, most probably due to a sensitivity limit of the MRI imaging system. Therefore, current experimental MRgFUS ablative protocols include the surgical removal of tissue after ablation.
The group of Gianfelice was the first to report the results of a “treat-and-resect” protocol type applied to two groups of 12 and 17 invasive breast cancer patients, confirming a mean of 88,3% of ablated tumours and the need for larger (>5 mm) safety margins around the MRI visible tumour (41,42). The group of Furusawa recently reported better results on 30 breast cancer patients, using the same type of “treat-and-resect” protocol. In this case, the mean percentage of tumour necrosis was 97% and 50% of patients had 100% necrosis of the ablated tumours (43). Again, Gianfelice and co-workers have described, in 2003, a method to assess the ability of dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) parameters [increase in signal intensity (ISI); maximum difference function (MDF); and positive enhancement integral (PEI)] to monitor residual tumours following MRgFUS treatment of breast cancer. This method achieved a sensitivity of 77% and a specificity of 100% (41,42).
Besides the difficulty of getting 100% of ablation with negative margins, other critical issues on the use of this methodology for the treatment of abdominal targets, such as liver, kidney and pancreas are under investigation (44). Particularly, in these cases the problems are related to the acoustic windows, restricted by the presence of rib cage and bowel, which can distort the ultrasound beam. Intestinal gas presence can cause reflection and unwanted heating, preventing ultrasound energy from reaching the target. Furthermore, the organ motion is another problem to solve, on which different groups of engineers are working worldwide. One solution could consist in focusing other target points, such as blood vessels, close to the organ to treat, as proposed by Ross and colleagues in 2008. In this case the sub-pixel tracking accuracy was measured to be 5.7 ms (SD: ±1.6 ms), sufficient for a real-time use (45).
However, experimental protocols for abdominal cancer ablation have been on-going for the last ten years, with the purpose of tumour control and pain palliation. A recent work of the group of Anzidei conducted on seven selected patients with unresectable primary pancreatic adenocarcinoma showed a successful procedure in 6 out of 7 patients, since in a single patient, the lesion accessibility was limited at treatment time and the procedure was suspended. Follow-up imaging revealed negligible (n=1) or no (n=5) tumour regrowth within the ablation area (46). In 2005, a study reported the results of treatments on 30 patients with hepatic or renal tumours, according to four trial protocols. HIFU exposure resulted in discrete zones of ablation in 25 out of 27 evaluable patients (93%). Ablation of liver tumours was achieved more consistently than that of kidney tumours (100% vs. 67%, radiologically assessed). The adverse event profile was favourable when compared to more invasive techniques (47).
Clinical trials on other oncologic disease are in progress, like treatment for osteoid osteoma, suggesting that MRgFUS treatment can be performed safely with a high rate of success and without apparent treatment-related morbidity (48).
Last but not least, MRgFUS is employed in the neurological field, with application to brain tumour surgery and brain disorders treatments of neuropathic pain, essential tremor and Parkinson disease (49,50). This technology has introduced revolutionary treatment for neuro interventions, due to its ability of precision lesioning, supported by imaging technologies able to visualize the anatomy within the skull, without the need of a craniotomy and a real-time temperature control that allows to safety achieve targets close to nerves, cortical and subcortical regions, and other critical structures.
For decades, therapeutic ultrasound through an intact skull was considered impossible due to disruption of the focused acoustic beam and skull heating. In the 1950’s William Fry applied HIFU to the human brain with the intent to create discrete lesions to treat hyperkinetic disorders such as Parkinson’s disease. Today, both Insightec Ltd and SuperSonic Imagine (Aix en Provence, France) developed an MRgFUS system for brain treatment. The Insightec system, termed Exablate 4000, is in a phase I clinical trial for the treatment of primary and metastatic brain malignancies. The hemispheric phased arrays around the head allow the focus restoring by adjusting each phased-array element according to the thickness of the underlying bone (51,52). Clinical studies are on-going on over 130 patients with neuropathic pain, essential tremor, Parkinson’s disease and obsessive-compulsive disorder, showing very promising results and immediate improvements after treatments (53-55).
In addition, pre-clinical studies, investigating the possibility to combine neuro-functional ablative and non-ablative methods, represent a precious tool to achieve the near-future expansion of MRgFUS treatment in new fields, such as BBB disruption and drug delivery.
Biological effects of ultrasound, a weapon for future ultrasound therapeutic uses
At present, a conspicuous number of potentially interesting US applications, which do not rely solely to the direct tissue destruction, are currently on-going in vitro and preclinical research subjects. These topics are interesting, not only from a purely scientific point of view, but reserve promising potential applications for the introduction of revolutionaries and personalized therapeutic interventions in the next decades.
However, despite the biological research is very intense on several fields, in this proteogenomic era, there is still the need for a deep comprehension of molecular mechanisms sustaining the biological effects of ultrasound. To this aim, the in vitro research is a fundamental step to reach the more advanced pre-clinical studies.
In order to comprehensively discuss the wide types of obtainable biologic effects obtainable in the near future, we will classify them according to the ultrasound power ranges, from the low intensity ultrasound to the ablative powers (Figure 1).
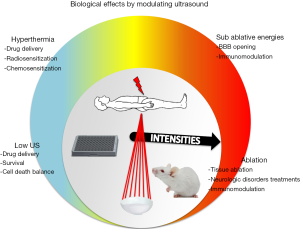
The treatments with LIPUS use energy intensities of the order of mW to 3 W/cm2, pulsed at repetition frequencies ranging from 0.5 to 100 Hz, with a pulse width of milliseconds and frequencies around 1 MHz (56). Their benefits are widely recognized in the field of bone and cartilage regeneration, stimulating fracture healing. The mechanisms by which ultrasound can trigger these effects remain poorly understood, nonetheless the events of bone regeneration are well known and physiologically distinguished in an early inflammatory phase, a reparative phase and a late remodelling phase, sustained by the involvement of multiple factors, including cytokines, hormones and growth factors, released in the extracellular matrix (ECM) and interacting with different cell types (mesenchymal stem cells, endothelial cells, bone and cartilage cells) recruited to the site of tissue damage.
Several pathways have been recognized to be activated by LIPUS, such as TGFβ signalling and MAPK signalling. However, the diversity of current experimental set-ups renders heterogeneous the results of in vitro studies, therefore the overall evaluation of biological effects induced by LIPUS needs the design of dedicated experimental set-ups, in which the different mechanical phenomena can be controlled (57). Furthermore, among the observed effects on tumours, low intensity ultrasound act as modulators of host-tumour response, whereas a study showed that LIPUS can also induce DNA damages, as demonstrated by the presence of gammaH2AX-positive foci in leukaemia cells (58,59). In addition, important evidences suggest that the application of low and sub-lethal intensity ultrasound is able to disturb the balance between survival and apoptosis/cell death. The ability to induce cell death in the absence of necrosis represents a novel approach of apoptotic cancer therapy. Several in vitro studies are evaluating this issue to induce stress response and apoptosis with minimal lysis in several cancer cell lines or to use this approach in conjunction with hyperthermia, photodynamic therapy, radiotherapy and chemotherapy to produce a synergistic effect (15,60,61). In a work conducted by the group of Feril in 2002, the effects of ultrasound on hyperthermia-induced apoptosis was studied on human lymphoma U937 cells, exposed to 44.0 degrees for 10 min. and continuous 1 MHz ultrasound at intensities of 0.5 or 1.0 W/cm2, considered non-thermal energies and sub-threshold for inertial cavitation. They observed that 0.5 W/cm2 in combination with hyperthermia synergistically induced apoptosis, whereas at 1.0 W/cm2 with hyperthermia an augmented instant cell lysis without significant change in apoptosis ratio was showed (62). Furthermore, in a work conducted by the same group in 2008, the networks activated by U937 cells subjected to LIPUS treatment (0.3 W/cm2 for 1 min) has been analysed by global-scale microarrays. Six hours later, apoptosis without cell lysis was observed. The networks of down-regulated genes regarded cell growth and proliferation, gene expression or cell development, while the ones of up-regulated genes resulted associated to cell movement, cell morphology and cell death (63). In addition to that produced by the simple ultrasound treatments, the cell death can be amplified by the contemporary administration of cytotoxic molecules, drugs or genetic material, entering in transiently permeabilized cells by different drug delivery methods, world-wide under study (64).
Although it has to be taken in mind that each biological response to a kind of physic ultrasound setting is, always, cell-type dependent, the mechanisms underlying the transient membrane permeability can be summarised like the following: (I) low intensity ultrasound leading to stable cavitation of microbubbles; (II) high intensity ultrasound leading to inertial cavitation with microbubble collapse; and (III) ultrasound application in the absence of microbubbles. More precisely, using low intensity ultrasound, the endocytic uptake of several drugs is stimulated, while short but intense ultrasound pulses can be applied to induce pore formation and direct cytoplasmic drugs uptake. Hyperthermia effect, with temperature increase between 39 and 41 °C, can also be commonly used alone or synergistically to mechanical effects, for the purposes of drug delivery and can be combined with the use of temperature sensitive liposomes (LTSLs) (65).
Ultrasound intensities can be adapted to create pore sizes correlating with drug size. Larger drugs, such as nanoparticles and gene complexes, will require higher ultrasound intensities in order to allow direct cytoplasmic entry or the use of engineered delivery systems, while small molecules are able to diffuse passively through small pores created at lower ultrasound intensity.
Additionally, the expressions of transgenes can be placed under the control of temperature sensitive promoters, such as those of heat-shock genes.
In a work of Zhong and colleagues the biological membrane opening is shown using a direct approach, by means of electron microscopy. In this way it was possible to observe the formation of pores of diameter between 0.1-0.5 μm following a sonoporation experiment in presence of microbubbles. In particular, the pores are detectable 3 seconds after exposure to ultrasound (parameters used: 1 MHz frequency, 10% duty cycle, 1 kHz repetition rate pulsed, 0.5 MPa peak of negative pressure, in presence of 1% microbubbles) (66). After one minute, the microscopic observation shows that pores were repaired and the membrane was covered with “patches” as protuberances dispersed along the membrane surface. This mechanism appears to be transient, since 1 h after treatment the membrane surface is already more smooth, a sign that cells attempt to return to their original shape.
Instead, the using of grater frequencies can cause cavitation and produce tissue damage if not properly controlled. To overcome this problem, many studies use protocols with pulsed sonication frequency of 1-3 MHz and intensity of 0.5-2.5 W/cm2 (67). However, these conditions are not always very efficient and the improvement of drug delivery protocols needs to be adapted case by case, considering the cells and tissue type under treatment and the type of molecules to delivery. Today, thanks to advances in ultrasound control techniques, many studies are carried out to evaluate the combined effects of ultrasound and chemotherapy and a lot of effort is currently made in the optimization of drug delivery particles that can be addressed in a targeted or non-targeted way. Polymeric nanoparticles, microbubbles and several types of liposomes can be engineered to contain genetic material, drugs or other cytotoxic molecules, that can be locally released under ultrasound control, taking advantage from inducing cavitation or local temperature increase when the LTLs or similar systems are used. Also, they can be specifically delivered combining them with antibodies recognizing specific cell antigens, so that only the targeted cells will be treatment, sparing the surrounding tissue and decreasing the risk of side effects (68-73).
These experimental approaches represent a challenge for numerous cancers and diseases, which today have low chance of success. For example, the efficacies of chemotherapeutic agents are severely restricted in the brain, due to the BBB, that prevents large molecules from penetrating into the parenchyma from the brain vasculature. If focused ultrasound is applied in the presence of microbubbles, the BBB can be temporarily disrupted, allowing the penetration of large molecules to reach image-selected regions of the brain. Kinoshita M. and collaborators demonstrate the ability to drive large-molecule drugs like Herceptin (trastuzumab) and doxorubicin through the BBB without damages to brain tissue, which may allow the use of these drugs to treat primary and metastatic brain tumours (74). The group of Yang reported a BBB permeability increase in rats inoculated with F98 glioma cells within the brain, treated with pulsed-HIFU. The effect of sonication resulted in an accumulation of Evans Blue dye (used as a marker that bounds to albumin, a complex which reaches a molecular weight of about 68.0000 Da), approximately 2-fold higher in the region of the tumour treated with HIFU compared to the contralateral part of brain (75).
The group of Jordão in 2010 has carried out experiments of HIFU mediated drug delivery on murine models of Alzheimer's disease, using an anti-amyloid β antibody, injected in combination with a MRI contrast agent. The authors describe the antibody binding to the plates within minutes from the treatment and reported that the bonds remained visible for at least 4 days, reducing the pathology in terms of number, size and mean of plaque surface area. These early results suggest that focused ultrasound is able of BBB-opening in a reversible, localizable, and non-invasive manner (76).
As regard to other kinds of therapeutic combination with ultrasound, in 1979, already Sapareto and colleagues have explored the field of hyperthermia and radiation combination therapy, in order to sensitize cells to radiation effectiveness. These in vitro studies demonstrated both an optimum temperature range and a practical split thermal treatment method, which provide maximum interaction of heat and radiation in terms of cell death (77).
The effect of radio-sensitization induced by hyperthermia can be attributed to the fact that heat is a pleiotropic damaging agent, altering protein structures and the DNA damage repair (78). Indeed, heat does not induce DNA double-strand breaks but rather appears to inhibit or just delay the DNA damage repair. For example, hyperthermia enhances the IR-induced ATM kinase activity producing the ATM-dependent phosphorylation of H2AX, altering the chromatin structure. Moreover, heat causes protein unfolding, which can lead to protein precipitation and sequestering of the proteins involved in DNA damage repair. Thus, hyperthermia influences several molecular parameters involved in sensitizing tumour cells to radiation and can enhance the potential of targeted radiotherapy (79).
At the end of this long enumeration of ultrasound applications of such disparate biologic effects, it worths mentioning the ability of the ablative process to activate and enhance an anti-tumour immunologic response.
In fact, death by necrosis, rather than apoptosis, damage cells in a more destructive way, allowing the release of intracellular molecules, orienting the immunological response and miming the effect of a natural anti-cancer vaccine, through the modulation of lymphocyte subpopulations (Th1, Th2, Natural Killer, B), sets of cytokines, chemokines and pro-anti-inflammatory molecules towards a target to eliminate. This effect has interesting implications, since cancer cells develop the ability of immuno-surveillance “escaping” during neoplastic progression (80). In this way, after the ablative treatment and the release of tumour-specific intracellular signals, the immunological response against tumour targets is enhanced reducing the risk of metastasis and recurrence. The group of Wu observed an increase in the CD4+ cell population and the CD4+/CD8+ ratio after HIFU treatment in a group of patients with solid tumours (six patients with osteosarcoma, five with hepatocellular carcinoma and five with renal cell carcinoma) (81). Moreover, in patients showing altered lymphocyte percentages before treatment, a re-establishment of normal conditions was observable already a few days after treatment. The group of Xu conducted a similar study on 48 patients with breast cancer undergoing mastectomy, randomly divided into a control group (25 patients) and in a group subjected to thermal ablation with HIFU (23 patients) 1-2 weeks prior to surgical resection. The study has shown that in the resected tumours treated with HIFU a significant increase of DCs, macrophages and B cells infiltrates was observable along the margins of treated regions (82).
Conclusions
This review has enumerated a long list of biologic effects induced by ultrasound, each one having enormous potential future therapeutic implications. However, many efforts are still needed to translate these opportunities into clinical practices. Indeed, while ablation techniques need to be improved in sensibility and specificity of tumour and neurologic targets ablation, drug delivery and other more elegant cell-killing methods need to be better investigated through in vitro and preclinical studies, as variable results are reported among similar type of experiments. In this regard, biologists and physics need to better refine the concept of “acoustic dose” adapted to each kind of biologic system, as the same ultrasound parameters can result in completely different responses, both for the lack of technical reproducibility in acoustic irradiation and the cell and tissue-type dependence of biologic response. Particularly, the aim of shifting the delicate survival/cell death balance, for the purposes of cell killing, needs the support of deep highly throughput technologies, which could facilitate the comprehension of molecular signalling induced by ultrasound and the identification of biological markers of interest in targeted therapies (83). Our group of physics and biologists is working in this direction, for the realization of reproducible performances of in vitro acoustic irradiation and the molecular characterization of tumour responses to combined ultrasound and cell killing sensitizing type of treatments.
Acknowledgments
Funding: This work was supported by PON01_01059 “Development of a new technological platform based on focused ultrasound for non-invasive treatment of tumours and infection”.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “High intensity focused ultrasounds”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.09.04). The series “High intensity focused ultrasounds” was commissioned by the editorial office without any funding or sponsorship. GIF served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Translational Cancer Research. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Harvey EN, Harvey EB, Loomis AL. Further observation on the effect of high frequency sound waves on living matter. Bio Bull 1928;55:459-69.
- Lynn JG, Zwemer RL, Chick AJ, et al. A new method for the generation and use of focused ultrasound in experimental biology. J Gen Physiol 1942;26:179-93. [PubMed]
- Fry WJ, Mosberg WH Jr, Barnard JW, et al. Production of focal destructive lesions in the central nervous system with ultrasound. J Neurosurg 1954;11:471-8. [PubMed]
- Fry WJ, Barnard JW, Fry EJ, et al. Ultrasonic lesions in the mammalian central nervous system. Science 1955;122:517-8. [PubMed]
- Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer 2005;5:321-7. [PubMed]
- Alongi F, Russo G, Spinelli A, et al. Can magnetic resonance image-guided focused ultrasound surgery replace local oncology treatments? A review. Tumori 2011;97:259-64. [PubMed]
- Grossman L, Brock-Abraham C, Carbone N, et al. The 50 best inventions. Time 2011-11-21.
- Borasi G, Russo G, Alongi F, et al. High-intensity focused ultrasound plus concomitant radiotherapy: a new weapon in oncology? J Ther Ultrasound 2013;1:6. [PubMed]
- Wu F, Wang ZB, Cao YD, et al. Heat fixation of cancer cells ablated with high-intensity-focused ultrasound in patients with breast cancer. Am J Surg 2006;192:179-84. [PubMed]
- Zhong P, Xing F, Huang X, et al. HIFU as a neoadjuvant therapy in cancer treatment. AIP Conf Proc 2011;1359:289-94.
- Jang HJ, Lee JY, Lee DH, et al. Current and Future Clinical Applications of High-Intensity Focused Ultrasound (HIFU) for Pancreatic Cancer. Gut Liver 2010;4:S57-61. [PubMed]
- Wu F, Zhou L, Chen WR. Host antitumour immune responses to HIFU ablation. Int J Hyperthermia 2007;23:165-71. [PubMed]
- Weeks EM, Platt MW, Gedroyc W. MRI-guided focused ultrasound (MRgFUS) to treat facet joint osteoarthritis low back pain--case series of an innovative new technique. Eur Radiol 2012;22:2822-35. [PubMed]
- Borasi G, Melzer A, Russo G, et al. Cancer therapy combining high-intensity focused ultrasound and megavoltage radiation. Int J Radiat Oncol Biol Phys 2014;89:926-7. [PubMed]
- Cui H, Yang X. Laser enhanced high-intensity focused ultrasound thrombolysis: an in vitro study. J Acoust Soc Am 2013;133:EL123-8. [PubMed]
- Borrelli MJ, O’Brien WD Jr, Hamilton E, et al. Influences of microbubble diameter and ultrasonic parameters on in vitro sonothrombolysis efficacy. J Vasc Interv Radiol 2012;23:1677-84. [PubMed]
- Vaezy S, Zderic V. Hemorrhage control using high intensity focused ultrasound. Int J Hyperthermia 2007;23:203-11. [PubMed]
- Lapchak PA, Kikuchi K, Butte P, et al. Development of transcranial sonothrombolysis as an alternative stroke therapy: incremental scientific advances toward overcoming substantial barriers. Expert Rev Med Devices 2013;10:201-13. [PubMed]
- Hariharan P, Myers MR, Robinson RA, et al. Characterization of high intensity focused ultrasound transducers using acoustic streaming. J Acoust Soc Am 2008;123:1706-19. [PubMed]
- Myers MR, Hariharan P, Banerjee RK. Direct methods for characterizing high-intensity focused ultrasound transducers using acoustic streaming. J Acoust Soc Am 2008;124:1790-802. [PubMed]
- Vlaisavljevich E, Maxwell A, Warnez M, et al. Histotripsy-induced cavitation cloud initiation thresholds in tissues of different mechanical properties. IEEE Trans Ultrason Ferroelectr Freq Control 2014;61:341-52. [PubMed]
- Lin KW, Kim Y, Maxwell AD, et al. Histotripsy beyond the intrinsic cavitation threshold using very short ultrasound pulses: microtripsy. IEEE Trans Ultrason Ferroelectr Freq Control 2014;61:251-65. [PubMed]
- Claes L, Willie B. The enhancement of bone regeneration by ultrasound. Prog Biophys Mol Biol 2007;93:384-98. [PubMed]
- Jolesz FA, McDannold N. Current status and future potential of MRI-guided focused ultrasound surgery. J Magn Reson Imaging 2008;27:391-9. [PubMed]
- Moonen CT, Quesson B, Salomir R, et al. Thermal therapies in interventional MR imaging. Focused ultrasound. Neuroimaging Clin N Am 2001;11:737-47. [PubMed]
- Cline HE, Hynynen K, Watkins RD, et al. Focused US system for MR imaging-guided tumor ablation. Radiology 1995;194:731-7. [PubMed]
- Salomir R, Delemazure AS, Palussière J, et al. Image-based control of the magnetic resonance imaging-guided focused ultrasound thermotherapy. Top Magn Reson Imaging 2006;17:139-51. [PubMed]
- Hesley GK, Felmlee JP, Gebhart JB, et al. Noninvasive treatment of uterine fibroids: early Mayo Clinic experience with magnetic resonance imaging-guided focused ultrasound. Mayo Clin Proc 2006;81:936-42. [PubMed]
- Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol 2009;34:584-9. [PubMed]
- Gizzo S, Saccardi C, Patrelli TS, et al. Magnetic resonance-guided focused ultrasound myomectomy: safety, efficacy, subsequent fertility and quality-of-life improvements, a systematic review. Reprod Sci 2014;21:465-76. [PubMed]
- Kim YS, Kim JH, Rhim H, et al. Volumetric MR-guided high-intensity focused ultrasound ablation with a one-layer strategy to treat large uterine fibroids: initial clinical outcomes. Radiology 2012;263:600-9. [PubMed]
- Crouzet S, Rouviere O, Martin X, et al. High-intensity focused ultrasound as focal therapy of prostate cancer. Curr Opin Urol 2014;24:225-30. [PubMed]
- Crouzet S, Rebillard X, Chevallier D, et al. Multicentric oncologic outcomes of high-intensity focused ultrasound for localized prostate cancer in 803 patients. Eur Urol 2010;58:559-66. [PubMed]
- Uchida T, Ohkusa H, Yamashita H, et al. Five years experience of transrectal high-intensity focused ultrasound using the Sonablate device in the treatment of localized prostate cancer. Int J Urol 2006;13:228-33. [PubMed]
- Zacharakis E, Ahmed HU, Ishaq A, et al. The feasibility and safety of high-intensity focused ultrasound as salvage therapy for recurrent prostate cancer following external beam radiotherapy. BJU Int 2008;102:786-92. [PubMed]
- Catane R, Beck A, Inbar Y, et al. MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases--preliminary clinical experience. Ann Oncol 2007;18:163-7. [PubMed]
- Gianfelice D, Gupta C, Kucharczyk W, et al. Palliative treatment of painful bone metastases with MR imaging--guided focused ultrasound. Radiology 2008;249:355-63. [PubMed]
- Liberman B, Gianfelice D, Inbar Y, et al. Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: a multicenter study. Ann Surg Oncol 2009;16:140-6. [PubMed]
- Candiano G, Russo G, Stefano A, et al. Metabolic changes after MRgFUS treatment of a bone metastasis using PET/CT: a case report. 10th International Symposium on Therapeutic Ultrasound. AIP Conf Proc 2012;1503:168.
- Hynynen K, Pomeroy O, Smith DN, et al. MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: a feasibility study. Radiology 2001;219:176-85. [PubMed]
- Gianfelice D, Khiat A, Amara M, et al. MR imaging-guided focused US ablation of breast cancer: histopathologic assessment of effectiveness-- initial experience. Radiology 2003;227:849-55. [PubMed]
- Gianfelice D, Khiat A, Amara M, et al. MR imaging-guided focused ultrasound surgery of breast cancer: correlation of dynamic contrast-enhanced MRI with histopathologic findings. Breast Cancer Res Treat 2003;82:93-101. [PubMed]
- Furusawa H, Namba K, Thomsen S, et al. Magnetic resonance-guided focused ultrasound surgery of breast cancer: reliability and effectiveness. J Am Coll Surg 2006;203:54-63. [PubMed]
- Kennedy JE, Wu F, ter Haar GR, et al. High-intensity focused ultrasound for the treatment of liver tumours. Ultrasonics 2004;42:931-5. [PubMed]
- Ross JC, Tranquebar R, Shanbhag D. Real-time liver motion compensation for MRgFUS. Med Image Comput Comput Assist Interv 2008;11:806-13. [PubMed]
- Anzidei M, Marincola BC, Bezzi M, et al. Magnetic Resonance-Guided High-Intensity Focused Ultrasound Treatment of Locally Advanced Pancreatic Adenocarcinoma: Preliminary Experience for Pain Palliation and Local Tumor Control. Invest Radiol 2014; [Epub ahead of print]. [PubMed]
- Illing RO, Kennedy JE, Wu F, et al. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer 2005;93:890-5. [PubMed]
- Geiger D, Napoli A, Conchiglia A, et al. MR-guided focused ultrasound (MRgFUS) ablation for the treatment of nonspinal osteoid osteoma: a prospective multicenter evaluation. J Bone Joint Surg Am 2014;96:743-51. [PubMed]
- Fry WJ, Fry FJ. Fundamental neurological research and human neurosurgery using intense ultrasound. IRE Trans Med Electron 1960;ME-7:166-81. [PubMed]
- Lele PP. A simple method for production of trackless focal lesions with focused ultrasound: physical factors. J Physiol 1962;160:494-512. [PubMed]
- Clement GT, White PJ, King RL, et al. A magnetic resonance imaging-compatible, large-scale array for trans-skull ultrasound surgery and therapy. J Ultrasound Med 2005;24:1117-25. [PubMed]
- Hynynen K, McDannold N, Clement G, et al. Pre-clinical testing of a phased array ultrasound system for MRI-guided noninvasive surgery of the brain--a primate study. Eur J Radiol 2006;59:149-56. [PubMed]
- Hynynen K, Jolesz FA. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med Biol 1998;24:275-83. [PubMed]
- Meyers R, Fry WJ, Fry FJ, et al. Early experiences with ultrasonic irradiation of the pallidofugal and nigral complexes in hyperkinetic and hypertonic disorders. J Neurosurg 1959;16:32-54. [PubMed]
- Bauer R, Martin E, Haegele-Link S, et al. Noninvasive functional neurosurgery using transcranial MR imaging-guided focused ultrasound. Parkinsonism Relat Disord 2014;20:S197-9. [PubMed]
- Buldakov MA, Hassan MA, Zhao QL, et al. Influence of changing pulse repetition frequency on chemical and biological effects induced by low-intensity ultrasound in vitro. Ultrason Sonochem 2009;16:392-7. [PubMed]
- Padilla F, Puts R, Vico L, et al. Stimulation of bone repair with ultrasound: a review of the possible mechanic effects. Ultrasonics 2014;54:1125-45. [PubMed]
- Guha C, Huagang Z, Chen W, et al. Immune System Modulation with LOFU And HIFU Treatment of Prostate Cancer. 10th International Symposium on Therapeutic Ultrasound. AIP Conf Proc 2011;1359:227-82.
- Furusawa Y, Fujiwaraa Y, Zhaoa Q, et al. Ultrasound induced DNA damage and signal transductions indicated by gammaH2AX. 10th International Symposium on Therapeutic Ultrasound. AIP Conf Proc 2011;1359:322-25.
- Harada Y, Ogawa K, Irie Y, et al. Ultrasound activation of TiO2 in melanoma tumors. J Control Release 2011;149:190-5. [PubMed]
- Buldakov MA, Feril LB Jr, Tachibana K, et al. Low-intensity pulsed ultrasound enhances cell killing induced by X-irradiation. Ultrason Sonochem 2014;21:40-2. [PubMed]
- Feril LB Jr, Kondo T, Zhao QL, et al. Enhancement of hyperthermia-induced apoptosis by non-thermal effects of ultrasound. Cancer Lett 2002;178:63-70. [PubMed]
- Tabuchi Y, Takasaki I, Zhao QL, et al. Genetic networks responsive to low-intensity pulsed ultrasound in human lymphoma U937 cells. Cancer Lett 2008;270:286-94. [PubMed]
- Lentacker I, De Cock I, Deckers R, et al. Understanding ultrasound induced sonoporation: definitions and underlying mechanisms. Adv Drug Deliv Rev 2014;72:49-64. [PubMed]
- Schroeder A, Kost J, Barenholz Y. Ultrasound, liposomes, and drug delivery: principles for using ultrasound to control the release of drugs from liposomes. Chem Phys Lipids 2009;162:1-16. [PubMed]
- Zhong W, Sit WH, Wan JM, et al. Sonoporation induces apoptosis and cell cycle arrest in human promyelocytic leukemia cells. Ultrasound Med Biol 2011;37:2149-59. [PubMed]
- Suzuki R, Takizawa T, Negishi Y, et al. Tumor specific ultrasound enhanced gene transfer in vivo with novel liposomal bubbles. J Control Release 2008;125:137-44. [PubMed]
- Dromi S, Frenkel V, Luk A, et al. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin Cancer Res 2007;13:2722-7. [PubMed]
- Tashjian JA, Dewhirst MW, Needham D, et al. Rationale for and measurement of liposomal drug delivery with hyperthermia using non-invasive imaging techniques. Int J Hyperthermia 2008;24:79-90. [PubMed]
- Ponce AM, Vujaskovic Z, Yuan F, et al. Hyperthermia mediated liposomal drug delivery. Int J Hyperthermia 2006;22:205-13. [PubMed]
- Kooiman K, Emmer M, Foppen-Harteveld M, et al. Increasing the endothelial layer permeability through ultrasound-activated microbubbles. IEEE Trans Biomed Eng 2010;57:29-32. [PubMed]
- Seip R, Chin CT, Hall CS, et al. Targeted ultrasound-mediated delivery of nanoparticles: on the development of a new HIFU-based therapy and imaging device. IEEE Trans Biomed Eng 2010;57:61-70. [PubMed]
- Dayton PA, Zhao S, Bloch SH, et al. Application of ultrasound to selectively localize nanodroplets for targeted imaging and therapy. Mol Imaging 2006;5:160-74. [PubMed]
- Kinoshita M, McDannold N, Jolesz FA, et al. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci U S A 2006;103:11719-23. [PubMed]
- Yang FY, Lin GL, Horng SC, et al. Pulsed high-intensity focused ultrasound enhances the relative permeability of the blood-tumor barrier in a glioma-bearing rat model. IEEE Trans Ultrason Ferroelectr Freq Control 2011;58:964-70. [PubMed]
- Jordão JF, Ayala-Grosso CA, Markham K, et al. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer’s disease. PLoS One 2010;5:e10549 [PubMed]
- Sapareto SA, Raaphorst GP, Dewey WC. Cell killing and the sequencing of hyperthermia and radiation. Int J Radiat Oncol Biol Phys 1979;5:343-7. [PubMed]
- Palorini R, Cammarata FP, Balestrieri C, et al. Glucose starvation induces cell death in K-ras-transformed cells by interfering with the hexosamine biosynthesis pathway and activating the unfolded protein response. Cell Death Dis 2013;4:e732 [PubMed]
- Pandita TK, Pandita S, Bhaumik SR. Molecular parameters of hyperthermia for radiosensitization. Crit Rev Eukaryot Gene Expr 2009;19:235-51. [PubMed]
- Scola L, Giacalone A, Marasà L, et al. Genetic determined downregulation of both type 1 and type 2 cytokine pathways might be protective against pancreatic cancer. Ann N Y Acad Sci 2009;1155:284-8. [PubMed]
- Wu F, Wang ZB, Lu P, et al. Activated anti-tumor immunity in cancer patients after high intensity focused ultrasound ablation. Ultrasound Med Biol 2004;30:1217-22. [PubMed]
- Xu ZL, Zhu XQ, Lu P, et al. Activation of tumor-infiltrating antigen presenting cells by high intensity focused ultrasound ablation of human breast cancer. Ultrasound Med Biol 2009;35:50-7. [PubMed]
- Bravatà V, Cammarata FP, Forte GI, et al. “Omics” of HER2-positive breast cancer. OMICS 2013;17:119-29. [PubMed]


