Association between Chlamydia pneumoniae infection and lung cancer: a meta-analysis
Introduction
Lung cancer is the most common diagnosed cancer, accounting for 11.6% (2,093,876 new cases) of new carcinomatosis cases and 18.4% (1,761,007 deaths) of all cancer deaths in 2018 (1). The 1- and 5-year survival rates were 42% and 15%, respectively, and it is poor while compared with those in high incidence of other cancer (2). The mechanism of lung cancer has not been fully understood. Smoking status was identified as the most crucial independent risk element for lung cancer (3,4). Some literatures also proved that both genetic and environment factors were related to the risk of lung carcinoma, such as exposure to radon and asbestos, air pollution, second-hand smoking and chronic bacterial infection and parasitic infections [Chlamydia pneumoniae (C. pneumoniae)] (5,6).
C. pneumoniae, a gram-negative bacterium, has been present as an individual species since 1989, is a common respiratory pathogen that causes the chronic and persistent respiratory infections (7,8). C. pneumoniae infection not only lead to worldwide widespread respiratory infections such as pneumonia, pharyngitis, bronchitis, and sinusitis, but also associated with asthma, chronic obstructive pulmonary disease, and atherosclerosis (9). Kuo et al. have reported that C. pneumoniae infection causes an average of 7–10% of community-acquired pneumonia (CAP) and 5% of bronchitis and sinusitis cases among adults (10). Laurila et al. (11) firstly discovered that C. pneumoniae infection might be an independent hazards for lung carcinoma in 1997 according to the relevant observation case-control research. Since then, the potential risk of C. pneumoniae and lung cancer has been vividly studied (12,13), but the results have been inconsistent. In order to comprehensively evaluate the association between C. pneumonia is infection and lung carcinoma, and to provide scientific basis for the etiology study, clinical treatment of lung cancer, we performed the meta-analysis from all eligible researches to explore the relationship between C. pneumoniae infection and lung carcinoma risk.
Methods
Search strategy
A systematic search was performed conducted on PubMed, Embase, Ovid, Wanfang and China National Knowledge Infrastructure (CNKI) databases. The search terms were as follows: “Chlamydia pneumoniae”, “lung cancer” and their synonyms or similar words (from their inception to February, 2019). Searches were limited to English and Chinese literature and were first screened by two independent reviewers. Furthermore, reference lists of all included articles and related comments were searched manually to find other potentially eligible articles.
Inclusion and exclusion criteria
For inclusion, articles were selected on the basis of the following criteria: (I) evaluating the relationship between C. pneumoniae infection and lung carcinoma risk; (II) study design was limited to prospective cohort studies or retrospective case-control studies; (III) clinical pathology confirmed lung cancer patients; (IV) the control group was relative healthy people with no diagnosis of any cancer; (V) the C. pneumoniae infection rate can be extracted from the included individual studies.
Assessment of methodological quality of included articles
All articles that met the inclusion criteria were evaluated to assess the risk of bias in each outcome. The evaluation was conducted independently by two comments using the Cochrane Collaboration’s risk of bias tool as depictive in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions, version 5.1.0. 2011, http://handbook.cochrane.org/). If there were any disagreements in the evaluation study, we have discussed it. The results of the assessment measured the following areas: random sequence generation, allocation concealment, blinding, incomplete outcome data addressed, free of selective outcome reporting, and other possible sources of bias. The consequences of the meta-analysis were comprehended as the results of the study on the risk of bias.
Data extraction
Data collection and analysis were carried out in accordance with the standard Cochrane protocol (14). Two authors independently reviewed and extracted the following data from every study: study design, study year, participants number, the positivity or negativity for C. pneumoniae (IgA, IgG) antibody.
We attempted to find and exclude duplicate data from different studies. For multiple studies of repeated or overlapping data (by population, time, location, and results), we followed the PRISMA reporting guidelines when submitting manuscripts.
Statistical analysis
Meta-analysis was performed with the Cochrane Collaboration’s Review Manager Software (RevMan, version 5.1). Odds ratio (OR) with 95% CI (confidence interval) was performed to evaluate the potential relationship between chronic C. pneumoniae infection and lung carcinoma risk. Heterogeneity was evaluated by I square test. Random effects model was used if heterogeneity was significant (I2>50%). When heterogeneity was not detected or the heterogeneity was relatively small, fixed effects model was performed.
Results
Literature selection and bias
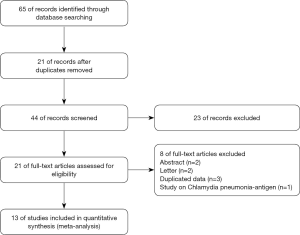
Totally of 65 potentially related researches and abstracts were identified (Figure 1). After removal of repeats (n=21) and filtration of abstracts (n=23), 21 full-text researches were evaluated for eligibility. Eight studies were excluded for the following: abstract (n=2), letter (n=2), duplicated data (n=3), study on Chlamydia pneumonia-antigen (n=1). Thirteen publications (11,15-26) were ultimately eligible for final meta-analysis. No more citations were found from the reference review.
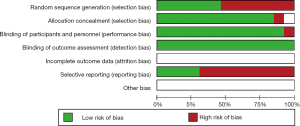
The detail of the risk-of-bias evaluation of included researches was summarized in Figure 2. All studies were evaluated as low risk according to the appropriate randomization sequence. However, many relative information in the studies wasn’t available, such as allocation concealment and blinding of participants and personnel, blinding of outcome assessment. Nevertheless, the overall methodological quality was generally fair.
Intervention characteristics
The included articles were printed between 1997 and 2013, involving 2,553 lung cancer cases and 2,460 controls. Controls were predominantly healthy people and matched for age, sex and/or smoking status. Of the 13 included articles (11,15-26), 6 were published in Chinese and 7 papers were published in English. For the study design, three articles were nest case-control and other 10 were case-control studies. Sample sizes ranged from 103 to 1,264. The characteristics of the studies is presented in Table 1.
Table 1
| Included studies | Studies design | Sample size | Age, years | Sex (male/female) | Case (positive/all) | Control (positive/all) | |||
|---|---|---|---|---|---|---|---|---|---|
| IgA | IgG | IgA | IgG | ||||||
| Yuqing 2001 | Case-control | 80:80 | 58±17:57±19 | 64/16:64/16 | 69/80 | 57/80 | |||
| Yanbin 2004 | Case-control | 50:108 | NA:40.8±8.5 | NA:63/45 | 28/50 | 22/108 | |||
| Meichun 2004 | Case-control | 128:70 | 67.8:52.3 | 99/29:46/24 | 3/128 | 1/70 | |||
| Yanbin 2005 | Case-control | 87:108 | 50.9±11:48.1±10.1 | 51/36:63/45 | 56/87 | 62/87 | 22/108 | 51/108 | |
| Xiumei 2010 | Case-control | 36:67 | NA | NA:40/27 | 26/36 | 6/67 | |||
| Fei 2014 | Case-control | 185:190 | 58.57±9.49:57.96±9.28 | 133/52:135/55 | 49/185 | 110/185 | 13/190 | 65/190 | |
| Laurila 1997 | Nest case-control | 230:230 | 60.3:60.3 | NA | 129/230 | 225/230 | 106/230 | 219/230 | |
| Jackson 2000 | Case-control | 143:147 | 59.8:59.4 | NA | 67/143 | 114/143 | 56/147 | 118/147 | |
| Koyi 2001 | Case-control | 198:68 | 128/70: NA | 116/198 | 88/198 | 11/68 | 13/68 | ||
| Kocazeybek 2003 | Case-control | 123:123 | 55:55:00 | 101/22:101/22 | 62/123 | 98/123 | 25/123 | 62/123 | |
| Littman 2004 | Nest case-control | 508:508 | 59:59:00 | 254/254:254/254 | 281/508 | 324/508 | 261/508 | 326/508 | |
| Chaturvedi 2010 | Nest case-control | 593:671 | NA | 407/186:437/234 | 174/593 | 293/593 | 201/671 | 356/671 | |
| Liu 2010 | Case-control | 192:90 | 54.6±10.4:53.6±9.4 | 0/192:0/90 | 119/192 | 26/90 | |||
Relationship between C. pneumoniae IgA antibody and lung carcinoma
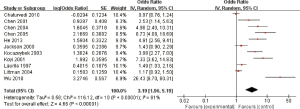
Eleven studies reported the relationship between C. pneumoniae infection and lung carcinoma risk by using the serum IgA. Among them, significant heterogeneity was scanned (I2=91%; heterogeneity P<0.00001; Figure 3). Random effect model was performed and the result showed that the C. pneumoniae infection significantly improved the risk of lung carcinoma (OR =3.19; 95% CI, 1.96–5.19; P<0.00001).
Relationship between C. pneumoniae IgG antibody and lung carcinoma
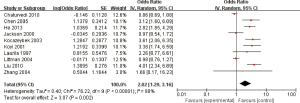
Ten studies reported the relationship between C. pneumoniae infection and lung carcinoma risk by using the serum IgG. Among them, significant heterogeneity was scanned (I2=88%; heterogeneity P<0.00001; Figure 4). Random effect model was performed and the result showed that the C. pneumoniae infection significantly improved the risk of lung carcinoma (OR =2.02; 95% CI, 1.29–3.16; P=0.002).
Discussion
Lung carcinoma is reported to be the most common cancer among women and men, representing huge social and economic burdens in both developing and developed countries (27). However, the risk factors for its occurrence has not been fully understood. In recent years, studies have reported that the pulmonary inflammatory disease is significantly related to the risk of lung carcinoma. C. pneumonia, which is closely related to chronic lung inflammation and may act an significant part in progression of lung carcinoma (28). Therefore, we performed the meta-analysis of all published articles to determine the relationship between C. pneumoniae infection and lung carcinoma risk. Our meta-analysis included 13 studies, including 2,553 lung carcinoma cases and 2,460 controls. Results showed that C. pneumoniae infection was significantly related to the risk of lung carcinoma, with a 3.19-fold increased risk compared to a negative titre (95% CI, 1.96–5.19) for IgA and 2.02 times (95% CI, 1.29–3.16) for IgG.
The association between C. pneumoniae infection and lung carcinoma is reasonable in biology, but the mechanism is still not clear. There are three possible reasons for this mechanism.
Firstly, chronic inflammation played an important part in development of malignant transformation (11). Medicaments that induce inflammation, such as infectious substances, can induce stretched-out stimulation, leading to cell death and increased mitotic activity. Subsequent cell division that happens during the repair of the damaged tissue possibly enhance the risk of cancer in the affected area (29). For instance, several researches have linked chronic infection with Helicobacter pylori to an enhanced the risk of gastric adenocarcinoma (30,31). Chumduri et al. reported that Chlamydia trachomatis infection perturb host chromatin, DNA double-strand breaks (DSBs) repair, and cell-cycle regulation, thus promoting DNA double strand breaks in host cells, inducing genomic instability and leading to cancer (32). C. pneumoniae may act a similar part in the occurrence and progress of lung carcinoma. C. pneumoniae promotes the delivery of inflammatory mediators, such as tumor necrosis factor (TNF), interleukin-10, and interleukin-8 (33). Chronic infection mediators, especially interleukin-8, may lead to genetic damage. Interleukin-8 also promotes the growth of human non-small cell lung carcinoma (NSCLC) by its angiogenic characteristics. In addition, C. pneumoniae may damage or even block apoptosis in infected cells via inducting interleukin-10 (34), leading to chronic infection and increasing the risk of vicious transformation of infected cells.
On the other hand, molecular simulation theory. Persistent C. pneumoniae infection could cause the release of endotoxin-like substance chlamydial heat shock protein-60 (CHSP-60). CHSP-60 is expressed throughout the life cycle of C. pneumoniae infection and may act a major part in the pathogenesis of lung carcinoma (35). In addition, Mayer et al. also demonstrated that C. pneumoniae infection may cause the release of nitric oxide (36), the mutagenicity of nitric oxide and other metabolites has been confirmed elsewhere (37).
Last but not least, some studies have reported that C. pneumoniae infection promotes the liberation of inflammatory mediators, such as nuclear factor-kappa B (NF-κB), TNF-α, and interleukin-8 (33), triggering the abnormal inflammatory response. Overexpression of inflammatory mediators and inadequate production of anti-inflammatory mediators can cause inflammatory reactions in the body, which in turn lead to overexpression of toll-like receptor (TLR) on the cell surface. However, the TLR signaling pathway may act a part in the carcinogenesis and progression of tumors. Bauer et al. revealed that TLR4-mediated gene expression pathways, which can be used as prognostic marks for predicting lung cancer susceptibility in mice (38).
The present study has several limitations that ought to be considered. Firstly, heterogeneity is an underlying conundrum when explanation all the studies of meta-analyses. Although we carefully searched the published articles, using explicit research inclusion criteria, strictly performed data collection and analysis, the significant heterogeneity between researches still existed. The existence of heterogeneity could arise from differences in the choice of controls, age distribution, prevalence rates and so on. Secondly, the inconsistency of the study population may lead to uncertainty in the research results.
In summary, the results of this meta-analysis demonstrate that the C. pneumoniae infection may increase the risk of lung carcinoma. Future prospective studies with extensive people are required to validate the connection of C. pneumoniae infection and lung carcinoma.
Acknowledgments
The author thanked all the commentators, they reviewed and extracted the relevant data from every study.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.10.35). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Zhang X, Yang Q. Association between serum copper levels and lung cancer risk: A meta-analysis. J Int Med Res 2018;46:4863-73. [Crossref] [PubMed]
- Ou SH. Lung cancer in never-smokers. Does smoking history matter in the era of molecular diagnostics and targeted therapy? J Clin Pathol 2013;66:839-46. [Crossref] [PubMed]
- Islami F, Torre LA, Jemal A. Global trends of lung cancer mortality and smoking prevalence. Transl Lung Cancer Res 2015;4:327-38. [PubMed]
- Liu C, Cui H, Gu D, et al. Genetic polymorphisms and lung cancer risk: Evidence from meta-analyses and genome-wide association studies. Lung Cancer 2017;113:18-29. [Crossref] [PubMed]
- Papadopoulos D, Papadoudis A, Kiagia M, et al. Nonpharmacologic Interventions for Improving Sleep Disturbances in Patients With Lung Cancer: A Systematic Review and Meta-analysis. J Pain Symptom Manage 2018;55:1364-81.e5. [Crossref] [PubMed]
- Radouani F, El Yazouli L, Elyazghi Z, et al. Chlamydia pneumoniae sero-prevalence in Moroccan patients with cardiovascular diseases. Infect Dis Health 2019;24:67-74. [Crossref] [PubMed]
- Schmidt SM, Muller CE, Bruns R, et al. Bronchial Chlamydia pneumoniae infection, markers of allergic inflammation and lung function in children. Pediatr Allergy Immunol 2001;12:257-65. [Crossref] [PubMed]
- Burillo A, Bouza E. Chlamydophila pneumoniae. Infect Dis Clin North Am 2010;24:61-71. [Crossref] [PubMed]
- Kuo CC, Jackson LA, Campbell LA, et al. Chlamydia pneumoniae (TWAR). Clin Microbiol Rev 1995;8:451-61. [Crossref] [PubMed]
- Laurila AL, Anttila T, Läärä E, et al. Serological evidence of an association between Chlamydia pneumoniae infection and lung cancer. Int J Cancer 1997;74:31-4. [Crossref] [PubMed]
- Smith JS, Kumlin U, Nyberg F, et al. Lack of association between serum antibodies of Chlamydia pneumoniae infection and the risk of lung cancer. Int J Cancer 2008;123:2469-71. [Crossref] [PubMed]
- Konstantopoulou S, Karachalios S, Mourikis A, et al. Chronic Chlamydia pneumoniae infection and risk of lung cancer. Clin Microbiol Infect 2003;203.
- Furlan AD, Pennick V, Bombardier C, et al. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976) 2009;34:1929-41. [Crossref] [PubMed]
- Chaturvedi AK, Gaydos CA, Agreda P, et al. Chlamydia pneumoniae infection and risk for lung cancer. Cancer Epidemiol Biomarkers Prev 2010;19:1498-505. [Crossref] [PubMed]
- Fei H, Cai L. Association of Chlamydia pneumoniae infection with risk of lung cancer. Chinese Journal of Public Health 2014;30:70-3.
- Jackson LA, Wang SP, Nazar-Stewart V, et al. Association of Chlamydia pneumoniae immunoglobulin A seropositivity and risk of lung cancer. Cancer Epidemiol Biomarkers Prev 2000;9:1263-6. [PubMed]
- Kocazeybek B. Chronic Chlamydophila pneumoniae infection in lung cancer, a risk factor: A case-control study. J Med Microbiol 2003;52:721-6. [Crossref] [PubMed]
- Koyi H, Branden E, Gnarpe J, et al. An association between chronic infection with Chlamydia pneumoniae and lung cancer. A prospective 2-year study. APMIS 2001;109:572-80. [Crossref] [PubMed]
- Littman AJ, White E, Jackson LA, et al. Chlamydia pneumoniae infection and risk of lung cancer. Cancer Epidemiol Biomarkers Prev 2004;13:1624-30. [PubMed]
- Liu Z, Su M, Yu SC, et al. Association of Chlamydia pneumoniae immunoglobulin G antibodies with the risk of lung cancer among non-smoking women in Liaoning, China. Thorac Cancer 2010;1:126-9. [Crossref] [PubMed]
- Meichun Z, Qoing C, Chengping H, et al. Chlamydia pneumoniae infection in patients with lung cancer and chronic obstructive pulmonary disease. China Journal of Modern Medicine 2004;14:31-4,40.
- Xiumei W. Investigation of chlamydia pneumoniae infection in respiratory inpatients. Chinese Community Doctors 2010;12:81.
- Yanbin C, Yueduo T, Chunhua L, et al. Association between chlamydia pneumoniae infection and lung cancer. Jiangsu Medical Journal 2005;31:306.
- Yanbin C, Yueduo T, Chunhua L, et al. Serological study of an association between chronic Chlamydia pneumoniae infection and respiratory tract diseases in the elderly. Practical Geriatrics 2004;18:303-4,7.
- Yuqing C, Liming L, Fengchao W, et al. Study on Chlamydia pneumoniae infection in patients with Lung Cancer. Chinese Journal of Zoonoses 2001;17:46-9.
- Minguet J, Smith KH, Bramlage P. Targeted therapies for treatment of non-small cell lung cancer--Recent advances and future perspectives. Int J Cancer 2016;138:2549-61. [Crossref] [PubMed]
- Qunxia C, Lin C. Association of Cpn infection with lung cancer: a meta-analysis of serological studies. Chinese Journal of Public Health 2015;31:1095-9.
- Preston-Martin S, Pike MC, Ross RK, et al. Increased cell division as a cause of human cancer. Cancer Res 1990;50:7415-21. [PubMed]
- Björkholm B, Falk P, Engstrand L, et al. Helicobacter pylori: resurrection of the cancer link. J Intern Med 2003;253:102-19. [Crossref] [PubMed]
- Sipponen P. Gastric cancer: pathogenesis, risks, and prevention. J Gastroenterol 2002;37:39-44. [Crossref] [PubMed]
- Chumduri C, Gurumurthy RK, Zadora PK, et al. Chlamydia infection promotes host DNA damage and proliferation but impairs the DNA damage response. Cell Host Microbe 2013;13:746-58. [Crossref] [PubMed]
- Gaydos CA. Growth in vascular cells and cytokine production by Chlamydia pneumoniae. J Infect Dis 2000;181:S473-8. [Crossref] [PubMed]
- Geng Y, Shane RB, Berencsi K, et al. Chlamydia pneumoniae inhibits apoptosis in human peripheral blood mononuclear cells through induction of IL-10. J Immunol 2000;164:5522-9. [Crossref] [PubMed]
- Biasucci LM, Liuzzo G, Ciervo A, et al. Antibody response to chlamydial heat shock protein 60 is strongly associated with acute coronary syndromes. Circulation 2003;107:3015-7. [Crossref] [PubMed]
- Mayer J, Woods ML, Vavrin Z, et al. Gamma interferon-induced nitric oxide production reduces Chlamydia trachomatis infectivity in McCoy cells. Infect Immun 1993;61:491-7. [PubMed]
- Arroyo PL, Hatch-Pigott V, Mower HF, et al. Mutagenicity of nitric oxide and its inhibition by antioxidants. Mutat Res 1992;281:193-202. [Crossref] [PubMed]
- Bauer AK, Fostel J, Degraff LM, et al. Transcriptomic analysis of pathways regulated by toll-like receptor 4 in a murine model of chronic pulmonary inflammation and carcinogenesis. Mol Cancer 2009;8:107. [Crossref] [PubMed]