
miR-486 as an unfavorable prognostic biomarker for patients with non-small cell lung cancer
Introduction
The morbidity and mortality of lung cancer, in which non-small cell lung cancer (NSCLC) accounts for approximately 85%, ranks first among all human malignant tumors in many countries (1,2). The morbidity of lung adenocarcinoma (LUAD) has gradually increased in the past decades. Nowadays, LUAD has replaced lung squamous cell carcinoma (LUSC), becoming the most common type of lung cancer in China (2). In recent years, although the surgical techniques, strategies of chemoradiotherapy and target therapy have been developed, the 5-year overall survival (OS) rate of lung cancer is still very low (3). It’s well known that early detection, early diagnosis and early treatment are the most effective strategies to improve the survival rate of lung cancer patients (4). However, there is no efficient clinical biomarker for early diagnosis, treatment and prognosis prediction of lung cancer. Therefore, it’s urgent to locate the diagnosis, prognosis markers and drug targets of lung cancer.
A microRNA (miRNA) is a small non-coding RNA molecule (containing about 22 nucleotides) that functions in RNA silencing and post-transcriptional regulation of gene expression (5). It is widely believed that miRNA plays an important role in tumorigenesis. miR-486 located on chromosome Chr8 was first discovered in fetal liver tissue in 2005 (6). As a member of miRNAs, miR-486 also has been reported to be associated with many tumors. Previous studies have shown that the expression profile and mechanism of miR-486 in NSCLC were controversial (7-10). In present study, we mainly explore the expression profile and prognostic value of miR-486 in NSCLC.
Methods
Ethics
This study was reviewed and approved by the Research Ethics Committee of The affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University. Written informed consent was obtained from all the patients. All specimens were processed and anonymized according to the ethical and legal standards.
Tissue microarray (TMA)
A total of 150 tissue samples (140 NSCLC tissue samples and 10 normal lung tissue samples) were obtained from patients who were operated at the Thoracic Surgical Division in year 2005. Patients with NSCLC were staged and graded according to the international staging system or the 2004 World Health Organization criteria. Patients included in this study received no preoperative chemotherapy or radiotherapy and had no other malignant tumors within 5 years prior to diagnosis. According to the method described in our previous study (11), a TMA containing 150 tissue samples was constructed.
In situ hybridization (ISH)
A detection kit named miR-486 ISH Kit (Boster, Pleasanton, CA, USA) was used to detect miR-486 in TMA. Antisense miR-486 probes were as follows: 5’-CTCGGGGCAGCTCAGTACAGGA-3’. Briefly, the ISH was performed as follows (11): the sections were deparaffinized in xylene, rehydrated in graded ethanol, digested in pepsin solution and then postfixed in 1% paraformaldehyde (PFA) for 10 min. Hybridization was then carried out with a mixture containing target probes to miR-486 at 37 °C overnight. After wash with saline sodium citrate (SSC), the sections were incubated at 37 °C for 30 min with blocking buffer. The slides were visualized using DAB (3,3'-diaminobenzidine) and counterstaining with hematoxylin.
ISH evaluation
Evaluation of miR-486 expression was semi-quantitatively performed by two independent investigators in a blinded fashion. The intensity of cytoplasmic staining was graded as absent, weak, moderate, or strong. The extent of cytoplasmic staining was expressed as the percentage of positive cancer cells from 0% to 100%. As previously described (12), tumors were scored (score 1–4) according to the intensity and extent of staining. In this study, tumors with score 3/4 were defined as positive miR-486 staining, whereas tumors with score 1/2 was defined as negative miR-486 staining.
Statistical analysis
The data were presented as mean ± standard deviation (SD). OS time was defined as the date of primary surgery to the date of death or latest follow-up. Student’s t-test, χ2 test, Kaplan-Meier survival analysis, and Cox regression analysis were performed. SPSS 23.0 software (IBM, NY, USA) was used to process the data and draw the graphs. P<0.05 was considered statistically significant.
Results
Characteristics of the patients
Table 1 shows the clinicopathological characteristics of the 140 NSCLC patients. The median age of the patients was 60 years (range from 26 to 79 years). The overwhelming majority of NSCLC patients are male. Fifty-seven percent of the tumors were LUSC and forty-two percent of the tumors were poorly differentiated. And, seventy-nine percent patients were in stage I or II. Clinical follow-up was recorded until July 2013. The median follow-up time was 45 months (range from 3–101 months). Ninety-one patients died at the end of follow-up.
Table 1
| Patient demographics | Number of patients | % of total |
|---|---|---|
| Agea | 60 [26–79] | |
| Sex | ||
| Male | 112 | 80 |
| Female | 28 | 20 |
| Location | ||
| Left | 64 | 46 |
| Right | 76 | 54 |
| Pathological type | ||
| Squamous cell carcinoma | 80 | 57 |
| Adenocarcinoma | 46 | 33 |
| Adenosquamous cell carcinoma | 5 | 4 |
| Bronchiolo-alveolar carcinoma | 3 | 2 |
| Sarcomatoid carcinoma | 1 | 1 |
| Neuroendocrine carcinoma | 3 | 2 |
| Mucoepidermoid carcinoma | 2 | 1 |
| Differentiation | ||
| Poorly | 59 | 42 |
| Moderate | 49 | 35 |
| Well | 32 | 23 |
| T stage | ||
| 1 | 15 | 11 |
| 2 | 105 | 75 |
| 3 | 20 | 14 |
| N stage | ||
| 0 | 82 | 59 |
| 1 | 31 | 22 |
| 2 | 27 | 19 |
| NSCLC stage | ||
| I | 67 | 48 |
| II | 44 | 31 |
| III | 29 | 21 |
| Follow-up perioda | 45 [3–101] |
a, median (range). NSCLC, non-small cell lung cancer.
Expression of miR-486 in NSCLC patients
The in situ miR-486 mRNA were stained and the representative images are shown in Figure 1. miR-486 was mainly expressed in the cytoplasm of lung cancer cells, but was less expressed in the cytoplasm of normal or para-tumor cells. The staining of miR-486 varied among tumors. Similar to lung cancer cells, miR-486 also mainly expressed in the cytoplasm of renal clear cell carcinoma cells and breast cancer cells. According to the scoring criteria described above, positive miR-486 staining was observed in 51.43% cases (N=140, positive vs. negative: 51.43% vs. 48.57%).
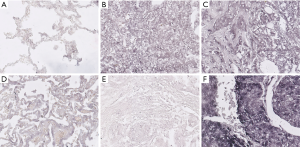
miR-486 expression and clinicopathological characteristics
For investigating the correlation between miR-486 and the clinicopathological features, the staining score 2 was selected as the cut-off value. Then, the patients were divided into two groups: low miR-486 expression group (staining score ≤2) and high miR-486 expression group (staining score >2). As shown in Table 2, miR-486 expression was correlated with tumor differentiation (P=0.011). However, no statistically significant correlations were found between miR-486 and tumor size (P=0.683), lymph node status (P=0.640), tumor stage (P=0.425) or any other clinicopathological characteristics (all P>0.05) (Table 2).
Table 2
| Variable | miR-486-5p | P value | |
|---|---|---|---|
| Low | High | ||
| Age | 59.1±10.2 | 61.0±10.0 | 0.276 |
| Sex | 0.866 | ||
| Male | 54 | 58 | |
| Female | 14 | 14 | |
| Location | 0.084 | ||
| Left | 26 | 38 | |
| Right | 42 | 34 | |
| Histological typea | 0.770 | ||
| Squamous | 38 | 42 | |
| Non squamous | 30 | 30 | |
| Differentiation | 0.011* | ||
| Well | 11 | 21 | |
| Moderate | 32 | 17 | |
| Poorly | 25 | 34 | |
| pT | 0.683 | ||
| 1 | 7 | 8 | |
| 2 | 53 | 52 | |
| 3 | 8 | 12 | |
| pN | 0.640 | ||
| 0 | 42 | 40 | |
| 1 | 15 | 16 | |
| 2 | 11 | 16 | |
| TNM stage | 0.425 | ||
| I | 35 | 32 | |
| II | 22 | 22 | |
| III | 11 | 18 | |
a, some tumors such as adenocarcinoma, adenosquamous cell carcinoma, bronchiolo-alveolar carcinoma, sarcomatoid carcinoma, neuroendocrine carcinoma, and mucoepidermoid carcinoma were included in the non squamous cancer data; *, significant correlation.
miR-486 expression and patients’ OS
The Kaplan-Meier survival curves were plotted to elaborate the correlation between miR-486 and the patients’ OS. Figure 2A showed that in all the 140 patients (stage I–III), high expression of miR-486 was correlated strongly with shortened OS (P=0.001). Further, the survival of patients with stage I or II–III NSCLC was also analyzed. We discovered that the difference in survival between the low and high miR-486 groups was statistically significant in stage I patients (P=0.005, Figure 2B). However, miR-486 expression was not found to be correlated to OS in patients with stage II–III NSCLC (P=0.090, Figure 2C).

Finally, univariate and multivariate analysis were made. As shown in Table 3, miR-486 and lymph node status had an independent prognostic meaning (P=0.002 and 0.024, respectively) for the whole group of 140 patients for OS.
Table 3
| Parameters | B | SE | Wald | P value | Exp(B) | 95% CI for Exp(B) | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Univariate analysis | |||||||
| Age (≤60 vs. >60) | 0.423 | 0.213 | 3.936 | 0.047* | 1.526 | 1.005 | 2.316 |
| Sex (male vs. female) | −0.240 | 0.275 | 0.762 | 0.383 | 0.786 | 0.458 | 1.349 |
| Location (left vs. right) | −0.216 | 0.210 | 1.059 | 0.303 | 0.806 | 0.534 | 1.216 |
| Histological type (LUSC vs. non-LUSC) | −0.123 | 0.213 | 0.337 | 0.562 | 0.884 | 0.583 | 1.341 |
| Differentiation (poorly vs. moderate vs. well) | −0.014 | 0.139 | 0.010 | 0.922 | 0.987 | 0.752 | 1.295 |
| pT (T1 vs. T2–3) | 0.839 | 0.423 | 3.940 | 0.047* | 2.314 | 1.011 | 5.298 |
| pN (N0 vs. N1–2) | 0.825 | 0.212 | 15.166 | 0.000* | 2.281 | 1.506 | 3.454 |
| Stage (stage I vs. stage II–III) | 0.655 | 0.215 | 9.275 | 0.002* | 1.926 | 1.263 | 2.937 |
| miR-486 (low vs. high) | 0.713 | 0.220 | 10.526 | 0.001* | 2.040 | 1.326 | 3.138 |
| Multivariate analysis | |||||||
| Age (≤60 vs. >60) | 0.300 | 0.216 | 1.934 | 0.164 | 1.350 | 0.884 | 2.061 |
| pT (T1 vs. T2–3) | 0.695 | 0.430 | 2.609 | 0.106 | 2.004 | 0.862 | 4.656 |
| pN (N0 vs. N1–2) | 0.870 | 0.386 | 5.078 | 0.024* | 2.388 | 1.120 | 5.091 |
| Stage (stage I vs. stage II–III) | −0.193 | 0.396 | 0.236 | 0.627 | 0.825 | 0.380 | 1.793 |
| miR-486 (low vs. high) | 0.686 | 0.222 | 9.553 | 0.002* | 1.986 | 1.285 | 3.069 |
*, significant correlation. LUSC, lung squamous cell carcinoma.
Discussion
NSCLC is a highly malignant and aggressive tumor type, with a poor 5-year OS rate (1,2). Similar to many other malignant tumors, NSCLC is initiated by either the activation of oncogenes or the inactivation of tumor suppressor genes (13). Thus, in the past decades, many attempts have been made to identify the key biomarker for diagnosis, treatment and prognosis prediction of NSCLC. Since the discovery of the first miRNA in 1993 (14), more and more miRNAs have been discovered, and the role of miRNAs has been gradually revealed. The first miRNA found to be associated with NSCLC was let-7 (15). Since then, more miRNAs were found to be associated with NSCLC. Such as, Zheng et al. found that miR-155, miR-197 and miR-182 were significantly higher expressed in NSCLC, and the combined detection of the three miRNAs had a diagnostic value for lung cancer (16). A study, in which the CT scan and miRNA detection were combined, revealed that after a low-dose spiral CT scan, the detection of 34 miRNAs expression level in the serum could be used to identify benign pulmonary nodules, high-risk lung cancer and malignant tumors (17). Yet, another study discovered that miR-205 and miR-21 can be used to identify LUAD and LUSC (18). By using mouse NSCLC model, miR-34 and miR-7 were found could affect the tumor growth (19,20). It is reported that miR-192, miR-135a, miR-133b and miR-9 were associated with chemotherapy resistance in NSCLC (21-23). miR-195, let-7, etc. were found to be associated with NSCLC prognosis (24,25).
miR-486 was first found and named in 2005 (6). In recent years, with the advancement of research, the roles of miR-486 played in tumors are becoming clear. A study found that miR-486 was lower expressed in esophageal cancer (EC) and inhibited growth and metastasis of EC cells by targeting CDK4/BCAS2 (26). It is reported that miR-486 was higher expressed and had a diagnosis value in pancreatic cancer (PC) and could promote the development of PC by targeting CD40 (27,28). The role miR-486 played in prostatic cancer was controversial. Yang et al. believed that miR-486 acted as an oncogene in prostatic cancer while Song et al. and Zhang et al. hold the opposite view (29-31). Similar to prostatic cancer, the role miR-486 played in NSCLC was also controversial. It was reported that miR-486 was down-regulated and its downregulation correlated to stages and lymph metastasis of patients with lung cancer (7,8). However, Hu et al. revealed that up-regulated miR-486 level in plasma was correlated with shorten OS (9). Sromek et al. reported that miR-486 was significantly high expressed in NSCLC patients’ plasma (10). Wang et al. further reported that miR-486 was high expressed and had a diagnostic value in NSCLC patients (32).
In this study, we mainly investigated the prognostic value of miR-486. We found that miR-486 was high expressed in majority of NSCLC tissues (mainly expressed in the cytoplasm of tumor cells). Also, miR-486 expression was found to be correlated with tumor differentiation. Further, miR-486 was demonstrated to have a prognostic value in NSCLC patients, especially in stage I patients. And, miR-486 had an independent prognostic meaning for NSCLC patients. Combining our previous study results (26,33), we have more reasons to believe that miR-486 functions as an oncogene in NSCLC. However, this study still has some shortcomings, such as too few normal lung tissue samples.
In conclusion, miR-486 is an unfavorable prognostic biomarker for NSCLC patients. These findings provide additional evidence for clarifying the role of miR-486 in NSCLC.
Acknowledgments
Funding: This research was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.11.19). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the Research Ethics Committee of The affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University. Written informed consent was obtained from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Cao M, Chen W. Epidemiology of lung cancer in China. Thorac Cancer 2019;10:3-7. [Crossref] [PubMed]
- Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271-89. [Crossref] [PubMed]
- Ambros V. The functions of animal microRNAs. Nature 2004;431:350-5. [Crossref] [PubMed]
- Fu H, Tie Y, Xu C, et al. Identification of human fetal liver miRNAs by a novel method. FEBS Lett 2005;579:3849-54. [Crossref] [PubMed]
- Peng Y, Dai Y, Hitchcock C, et al. Insulin growth factor signaling is regulated by microRNA-486, an underexpressed microRNA in lung cancer. Proc Natl Acad Sci U S A 2013;110:15043-8. [Crossref] [PubMed]
- Wang J, Tian X, Han R, et al. Downregulation of miR-486-5p contributes to tumor progression and metastasis by targeting protumorigenic ARHGAP5 in lung cancer. Oncogene 2014;33:1181-9. [Crossref] [PubMed]
- Hu Z, Chen X, Zhao Y, et al. Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J Clin Oncol 2010;28:1721-6. [Crossref] [PubMed]
- Sromek M, Glogowski M, Chechlinska M, et al. Changes in plasma miR-9, miR-16, miR-205 and miR-486 levels after non-small cell lung cancer resection. Cell Oncol (Dordr) 2017;40:529-36. [Crossref] [PubMed]
- Yuan K, Gao ZJ, Yuan WD, et al. High expression of SLC6A10P contributes to poor prognosis in lung adenocarcinoma. Int J Clin Exp Pathol 2018;11:720-6.
- Gao ZJ, Wang Y, Yuan WD, et al. HIF-2alpha not HIF-1alpha overexpression confers poor prognosis in non-small cell lung cancer. Tumour Biol 2017;39:1010428317709637. [Crossref] [PubMed]
- Cooper WA, Lam DC, O'Toole SA, et al. Molecular biology of lung cancer. J Thorac Dis 2013;5:S479-90. [PubMed]
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993;75:843-54. [Crossref] [PubMed]
- Takamizawa J, Konishi H, Yanagisawa K, et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res 2004;64:3753-6. [Crossref] [PubMed]
- Zheng D, Haddadin S, Wang Y, et al. Plasma microRNAs as novel biomarkers for early detection of lung cancer. Int J Clin Exp Pathol 2011;4:575-86. [PubMed]
- Lin PY, Yang PC. Circulating miRNA signature for early diagnosis of lung cancer. EMBO Mol Med 2011;3:436-7. [Crossref] [PubMed]
- Boeri M, Verri C, Conte D, et al. MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proc Natl Acad Sci U S A 2011;108:3713-8. [Crossref] [PubMed]
- Wiggins JF, Ruffino L, Kelnar K, et al. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res 2010;70:5923-30. [Crossref] [PubMed]
- Rai K, Takigawa N, Ito S, et al. Liposomal delivery of MicroRNA-7-expressing plasmid overcomes epidermal growth factor receptor tyrosine kinase inhibitor-resistance in lung cancer cells. Mol Cancer Ther 2011;10:1720-7. [Crossref] [PubMed]
- Wei X, Shen X, Ren Y, et al. The Roles of microRNAs in Regulating Chemotherapy Resistance of Non-Small Cell Lung Cancer. Curr Pharm Des 2018;23:5983-8. [Crossref] [PubMed]
- Lin C, Xie L, Lu Y, et al. miR-133b reverses cisplatin resistance by targeting GSTP1 in cisplatin-resistant lung cancer cells. Int J Mol Med 2018;41:2050-8. [PubMed]
- Xiong K, Shao LH, Zhang HQ, et al. MicroRNA-9 functions as a tumor suppressor and enhances radio-sensitivity in radio-resistant A549 cells by targeting neuropilin 1. Oncol Lett 2018;15:2863-70. [PubMed]
- Castro D, Moreira M, Gouveia AM, et al. MicroRNAs in lung cancer. Oncotarget 2017;8:81679-85. [Crossref] [PubMed]
- Zhao Y, Wei Q, Hu L, et al. Polymorphisms in MicroRNAs are associated with survival in non-small cell lung cancer. Cancer Epidemiol Biomarkers Prev 2014;23:2503-11. [Crossref] [PubMed]
- Lang B, Zhao S. miR-486 functions as a tumor suppressor in esophageal cancer by targeting CDK4/BCAS2. Oncol Rep 2018;39:71-80. [PubMed]
- Xu J, Cao Z, Liu W, et al. Plasma miRNAs Effectively Distinguish Patients With Pancreatic Cancer From Controls: A Multicenter Study. Ann Surg 2016;263:1173-9. [Crossref] [PubMed]
- Mees ST, Mardin WA, Sielker S, et al. Involvement of CD40 targeting miR-224 and miR-486 on the progression of pancreatic ductal adenocarcinomas. Ann Surg Oncol 2009;16:2339-50. [Crossref] [PubMed]
- Yang Y, Ji C, Guo S, et al. The miR-486-5p plays a causative role in prostate cancer through negative regulation of multiple tumor suppressor pathways. Oncotarget 2017;8:72835-46. [PubMed]
- Song C, Chen H, Wang T, et al. Expression profile analysis of microRNAs in prostate cancer by next-generation sequencing. Prostate 2015;75:500-16. [Crossref] [PubMed]
- Zhang X, Zhang T, Yang K, et al. miR-486-5p suppresses prostate cancer metastasis by targeting Snail and regulating epithelial-mesenchymal transition. Onco Targets Ther 2016;9:6909-14. [Crossref] [PubMed]
- Wang X, Zhi X, Zhang Y, et al. Role of plasma MicroRNAs in the early diagnosis of non-small-cell lung cancers: a case-control study. J Thorac Dis 2016;8:1645-52. [Crossref] [PubMed]
- Li W, Wang Y, Zhang Q, et al. MicroRNA-486 as a Biomarker for Early Diagnosis and Recurrence of Non-Small Cell Lung Cancer. PLoS One 2015;10:e0134220. [Crossref] [PubMed]


