
The clinicopathological study of lung cancer concealed in end-stage of interstitial lung disease
IntroductionOther Section
Interstitial lung disease (ILD) is also known as diffuse parenchymal lung disease (DPLD). It is a kind of lung disease that involves alveolar wall and alveolar cavity and has different grade of inflammation and fibrosis. At present, the general respiratory disease community adopts the classification of ILD and idiopathic ILD recommended by the American Thoracic Society (ATS) and the European Respiratory Disease Society (ERS) (1).
The pathological characteristics of patients with lung cancer (LC) are malignant proliferation of tumor cells, but the etiology of ILD is only a few known. However, no matter what the cause of ILD, lung fibroblasts will show abnormal or malignant proliferation, which is similar to the pathological characteristics of LC patients.
When ILD develops to the end stage, lung transplantation becomes an effective method for the treatment of end-stage lung disease (2). While, the incidence of LC in ILD patients is increasing. Hitherto, the relationship between ILD and the pathogenesis of LC is not clear. Because most of the clinical manifestations of ILD complicated with LC (ILD-LC) are non-specific, when ILD-LC is diagnosed, it often progresses to the advanced stage of LC. Consequently, how to diagnose the disease in the early stage of ILD-LC has become a serious problem in clinical practice.
With the progress of medical technology, the strategy of diagnosis and treatment of LC has changed from cellular level to precise medical treatment based on molecular level (3). Drive genes are essential genes related to tumorigenesis and development, which play a crucial role in the induction and proliferation of LC. Epidermal growth factor receptor (EGFR) gene, kirsten rat sarcoma viral oncogene (KRAS) gene, B-type Raf kinase (BRAF) gene, human epidermal growth factor receptor-2 (Her-2) gene, and other genes are the most commonly used LC drive genes. It is beneficial to develop the best possible treatment option LC by detecting the driving genes in these ILD-LC.
After routine diagnostic procedures, the cases of LC do not occur in the lungs removed from lung transplants. However, 154 cases of lung transplantation were completed in Sino-Japanese Friendship Hospital within two years, of which 7 cases were not found or could not be diagnosed before operation. However, in the lung transplanted out of the lung, pathological examination found that it was finally diagnosed as terminal qualitative disease complicated with LC. This kind of case can be called lung cancer concealed in end-stage of interstitial lung disease (LC-CES-ILD). We retrospectively studied these rare cases and discussed the clinicopathological features.
MethodsOther Section
Patients
We reviewed all cases of lung transplantation in China-Japan Friendship Hospital from March 2017 to December 2018. According to the criteria of the ATS (4), patients with severe lung diseases diagnosed by imaging or pathology and recommended for lung transplantation (2). A total of 154 patients were recruited. Inclusion criteria: (I) patients who met the criteria of ATS and ERS for lung transplantation; (II) cardiac function of the New York Heart Association (NYHA) III or IV; (III) the experimental treatment of intravenous administration of vasocyclin or similar equivalent preparation is ineffective; (IV) cardiac index (CI) <2 L/(min.m2). Exclusion criteria: (I) the receptor has a history of malignant tumors in the past 2 years, but localized malignant tumors; (II) the receptor has other primary organ dysfunction that can’t be treated (such as heart, liver, kidney, and brain); (III) the patient does not agree to a lung transplant, or mental or psychological problems are unable to cooperate with the medical team and do not comply with the doctor’s diagnosis and treatment.
These cases, who are not diagnosed with a tumor before the operation, but LC was found in the transplanted lung, was screened from 154 lung transplant cases for inclusion in this study. The clinical data, preoperative imaging data, laboratory examination, and pathological data of these patients were collected.
Preoperative pathological examination
The fasting venous blood of 3 mL was taken and the serum was separated for examination. Microparticle enzyme immunoassay (MEIA) was used. The experimental operation was carried out in strict accordance with the relevant guidelines and instructions, and the changes of serum tumor marker carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA 125), CA-153, neuron-specific enolase (NSE), cytokeratin 19-fragments (CYFRA21-1), CA72-4, CA199 in the samples were analyzed. Positive reference values: CEA (<5 ng/mL), CA125 (<35 U/mL), CA-153 (<25 U/mL), NSE (<16.3 ng/mL), CYFRA21-1 (<3.3 ng/mL), CA72-4 (<6.9 U/mL), CA199 (<27 U/mL).
Treatment and observation of lung transplantation samples
The diseased lungs resected after lung transplantation were treated with routine pathological specimens. The specimens were perfused with 10% neutral formalin and fixed overnight. The pathologist scrutinized the specimen according to the standard of lung tissue sampling. Each lung lobe was taken separately, and the number of samples was determined according to the sample situation. The volume of material was at least three tissue blocks/lung lobes. Thus, the suspicious tumor tissue found in naked eye examination, and the number of tissue blocks was increased. Besides, the severed bronchial end, severed vascular end and lymph nodes in the specimen were routinely taken.
Hematoxylin-eosin stain (HE) and immunohistochemical detection
The tissue was embedded in paraffin and cut into sections of 4 µm thickness. The sections were stained with HE and observed under the microscope. Immunohistochemistry was performed by EnVision method. The primary antibodies (TTF-1, p63, Ki67, and p53) were purchased from Beijing Zhongshan Jinqiao Biotechnology Co., Ltd. The second antibody and chromogenic agent were purchased from Roche Diagnostic products (Shanghai) Co., Ltd. The immunohistochemical experiment was carried out according to the reagent instructions, and the corresponding negative and positive controls were established. All pathological sections were read independently by two senior pathologists.
Molecular biological examination
Ten paraffin specimens of tumor with a thickness of 5 µm were selected for each case. Human LC polygene combined detection kit (Eddard Company) was used to extract DNA, and the fluorescence PCR method was used to detect the LC drive gene. The genes tested included EGFR, reactive oxygen species ROS1, BRAF, anaplastic lymphoma kinase (ALK), KRAS, receptor tyrosine kinase gene (RET), neuroblastoma RAS viral oncogene homolog (NRAS), Her-2, α-isoform of phosphatidylinositol 3-kinase (PIK3Ca), Methoprene-tolerant (Met). The detailed information of these genes was shown in Table S1.
Statistical analysis
SPSS 22.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The proportion of patients is represented by n%.
ResultsOther Section
Clinical information of 154 patients undergoing lung transplantation
As shown in Table 1, the male patients accounted for 87% of the 154 lung transplant patients, and 78.57% of the patients were 50–69 years old. The primary type of disease was idiopathic pulmonary fibrosis (IPF), which accounted for 44.81% of the transplanted patients. There was no significant difference in the proportion of bilateral lung sequential transplantation, left single lung transplantation and right single lung transplantation among the three groups.
Table 1
| Item | Number of cases (n) | Proportion |
|---|---|---|
| Male/female | 134/20 | 87%/13% |
| Age (years) | ||
| 20–29 | 4 | 2.60% |
| 30–39 | 11 | 7.14% |
| 40–49 | 11 | 7.14% |
| 50–59 | 40 | 25.97% |
| 60–69 | 81 | 52.60% |
| 70–79 | 7 | 4.55% |
| The type of disease | ||
| IPF | 69 | 44.81% |
| CTD-ILD | 19 | 12.34% |
| NSIP | 12 | 7.79% |
| CHP | 8 | 5.19% |
| CPFE | 6 | 3.90% |
| Vasculitis | 4 | 2.60% |
| Pneumoconiosis | 4 | 2.60% |
| PPFE | 3 | 1.95% |
| BOS | 1 | 0.65% |
| Pulmonary hypertension. | 5 | 3.25% |
| Bronchiectasis | 9 | 5.84% |
| COPD | 13 | 8.44% |
| Unclassified type | 1 | 0.65% |
| Lung transplantation indication. | ||
| Bilateral lung sequential transplantation | 53 | 34.42% |
| Left one lung transplantation | 44 | 28.57% |
| Right one lung transplantation | 57 | 37.01% |
IPF, idiopathic pulmonary fibrosis; CTD-ILD, connective tissue disease-associated interstitial lung disease; NSIP, non-specific interstitial pneumonia; CHP, chronic hypersensitivity pneumonia; CPFE, combined pulmonary fibrosis and emphysema; PPFE, pleuroparenchymal fibroelastosis; COPD, chronic obstructive pulmonary disease; BOS, obliterative bronchiolitis syndrome.
LC-CES-ILD patient clinical information
A total of 7 cases of LC were diagnosed pathologically after operation. These ILD patients were not diagnosed as LC before transplantation, but they were found to belong to LC-CES-ILD after transplantation. The clinical information is shown in Table 2.
Table 2
| Item | Case number (No.) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Gender | Male | Male | Male | Male | Male | Male | Male | |
| Age | 59 | 46 | 63 | 64 | 52 | 61 | 58 | |
| Smoking index (bag/year) | 20 | 90 | 70 | 20 | 30 | 40 | 15 | |
| Lung transplantation indication | ||||||||
| IPF | + | + | + | + | ||||
| COPD | + | |||||||
| CTD-ILD | + | + | ||||||
| Serum tumor biomarkers detected before operation (nit of marker, range of normal value) | ||||||||
| CEA (ng/mL, <5) | 21.49 | 1.56 | 5.09 | 19.59 | – | 6.07 | 8.6 | |
| CA125 (U/mL, <35) | 127 | 48.6 | 82.45 | 948.2 | 79.19 | 74.88 | 127.2 | |
| CA-153 (U/mL, <25) | 193.2 | 6.44 | 34.26 | 221.3 | 58.26 | 74.61 | 38.73 | |
| NSE (ng/mL, <16.3) | 18.27 | 13.37 | – | 12.94 | 16.95 | – | – | |
| CYFRA21-1 (ng/mL, <3.3) | 7.3 | 2.87 | 4.73 | 6.16 | 6.42 | 3.41 | 5.22 | |
| CA72-4 (U/mL, <6.9) | 9.94 | 2.45 | 10.01 | 17.03 | 17.57 | – | – | |
| CA199 (U/mL, <27) | – | – | 51.11 | 583.4 | 28.93 | 525.8 | 284.1 | |
| The results of the last CT examination before operation | ||||||||
| Interstitial pneumonia | + | + | + | + | + | |||
| Mediastinal lymphadenopathy | + | |||||||
| Pulmonary infection | + | |||||||
| Interstitial fibrosis | + | + | + | |||||
| The time between last CT examination and operation (days) | 2 | 12 | 29 | 6 | 7 | 16 | 15 | |
| Preoperative diagnosis of tumor | ||||||||
| Suspicious | + | + | + | + | + | |||
| No tumor | + | + | ||||||
| Transplantation type | ||||||||
| Bilateral lung sequential | + | + | ||||||
| Right single lung | + | + | + | |||||
| Left single lung | + | + | ||||||
IPF, idiopathic pulmonary fibrosis; CTD-ILD, connective tissue disease-associated interstitial lung disease; COPD, chronic obstructive pulmonary disease.
Before transplantation, the clinical diagnosis suspected the five patients of having tumors, but it was not ensured before the operation. However, only No. 2 and No. 7 patients were confirmed to be LC by pathological examination after the surgery. All seven patients were male, with an average age of 57.57 years (46–64 years) and a history of severe smoking (15–90 package years). The primary diseases of lung transplantation were IPF (4 cases), connective tissue disease-associated interstitial lung disease (CTD-ILD) (2 cases), and chronic obstructive pulmonary disease (COPD) (1 case). Two of the patients underwent bilateral sequential lung transplantation, while the others underwent unilateral lung transplantation.
The interval between the last computed tomography (CT) examination and operation was 2–29 days. None of these patients was diagnosed as tumors by CT. Alternatively, positron emission tomography-CT (PET-CT) screening was performed in with No. 3 and No. 6 patients before operation, but the cancer could not be confirmed. Serum tumor biomarkers were screened in 7 patients before the surgery, and CEA, CA125, CA-153, NSE, CYFRA21-1, CA72-4, CA199 was significantly increased in 6 patients. However, the serum biomarkers of No. 2 patients were only slightly elevated in CA125, and the other serum tumor biomarkers were normal. Therefore, this patient was diagnosed as tumor-free before operation.
Detection of tumor tissue in postoperative LC-CES-ILD patients
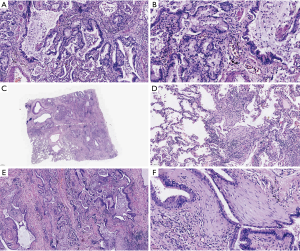
The tumor tissue of 5 patients with LC-CES-ILD involved the upper lobe, while two patients involved two lobes of the lung. The lung tissue structure of 7 patients was destroyed, the texture was medium to tight, and honeycomb appearance could be seen in some areas. In some cases, the presence of mucus is prominent, and the boundary of the surrounding lung tissue is unclear (for example, No. 5 patients, Figure 1A).

Three cases (patient: No. 1, No. 3, No. 4) with tumor symptoms were found by accident during experiential sampling. Moreover, it is still impossible to accurately determine the location and size of the tumor with the naked eye at the time of re-examination of the specimen (for example, patient No. 4, Figure 1B). Four cases were single tumor lesions and three cases where two tumor lesions (Table 3).
Table 3
| Item | Case number (No.) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Histological types | |||||||
| Invasive adenocarcinoma | + | + | + | + | |||
| Squamous cell carcinoma | + | ||||||
| Invasive mucinous adenocarcinoma | + | + | |||||
| Histological subtype | |||||||
| Acinar type | + 80%) | + | + (70%) | + | + (60%) | + (80%) | |
| Solid type | + (20%) | + (20%) | |||||
| Keratinized type | + | ||||||
| Lepidic type | + (15%) | ||||||
| Papillary type | + (25%) | + (20%) | |||||
| Number of tumor lesions | 1 | 2 | 2 | 1 | 1 | 1 | 2 |
| Tumor size (cm) | 1.5 | 0.8/0.15 | 1.2/0.3 | 0.7 | 7.5 | 10 | 0.8/1.5 |
| Location of tumor in lung | Upper left | Upper left | Upper left | Lower left | Upper right and middle right | Upper left | Lower right |
| Identification of tumor by naked eye | |||||||
| Unrecognizable | + | + | + | + | |||
| Visible | + | + | + | ||||
| Pleural involvement | |||||||
| Affected | + | + | + | ||||
| Unaffected | + | + | + | + | |||
| Lymph node involvement | 7/19 | 0/7 | 0/6 | 0/16 | 1/5 | 0/10 | 1/11 |
| Stage of pTNM | T2aN1M0 | T3N0M0 | T3N0M0 | T1aN0M0 | T4N1M0 | T4N0M0 | T3N1M0 |
| IIb | IIb | IIb | Ia1 | IIIa | IIIa | IIIa | |
| Immunohistochemical results | |||||||
| TTF-1 | + | + | + | − | + Partial | − | + |
| P63 | − | − | − | + | − | − | − |
| Ki-67 | + (10%) | + (50%) | + (20%) | + (15%) | + (50%) | + (20%) | + (40%) |
| P53 | + Partial | + | − | + Partial | − | + Partial | + Partial |
HE staining showed that the alveolar structure of lung tissue in LC-CES-ILD patients almost disappeared. The interstitial fibrosis was high, and honeycomb lung and fibroblast hyperplasia lesions were formed in some areas of the lung. Multifocal alveolar epithelial hyperplasia, squamous or mucinous epithelialization, and atypical hyperplasia occurred. Infiltration and aggregation of lymphocytes and thickening of pleura could be seen in some areas of the lung. Histologically, most of the patients were lung adenocarcinoma (6/7), 4 cases were acinar type, and 2 cases were mucinous adenocarcinoma (Figure 2). Patients with No. 6 have a small number of pleomorphic cancer components. Microscopically, the tumor cells were fusiform, which has obvious heteromorphism and more mitosis. Only the tumor cells in No. 4 patients were keratinizing squamous cell carcinoma (Figure 3).
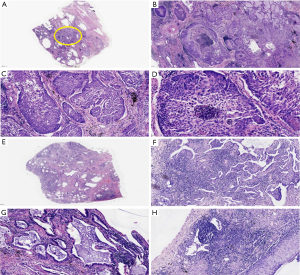
Therefore, we can find that the boundaries of tissue between LC and ILD is not clear, as the tumor center or edge is often visible with the radical lesions of the base cells, squamous cell carcinoma surrounding the phenomenon of epidermal squamous, mucus adenocarcinoma surrounding visible dermalbiosis phenomenon, which suggests that the occurrence of tumor stoma has a certain relationship with the metastasis and proliferation of ILD.
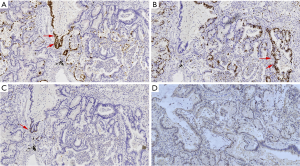
Three of the patients were involved the visceral pleura, and 3 patients detected lymph node metastasis. Postoperative pathology stages show 1 case in stage Ia1, 3 cases in stage IIb, and 3 cases in stage IIIa. Immunohistochemistry showed that TTF-1 was positive (Figure 4A) and p63 was negative (Figure 4B) in adenocarcinoma, a few cells were TTF-1 positive and did not express p63 in mucinous adenocarcinoma, p63 was diffusely positive, and TTF-1 was negative in squamous cell carcinoma. The expression of Ki-67(Figure 4C) was more than 10% in all tumor tissues and p53 positive (Figure 4D) in some tumor tissues (Table 3).
The results of the molecular pathological examination showed that only one case of mucinous adenocarcinoma had KRAS mutation, and no mutation of LC drive gene was detected in the rest of the patients (Table S2). Follow-up indicated that two patients died of infection after transplantation; the rest of the patients were followed up for 68–347 days, and three patients had tumor metastasis (Table 4).
Table 4
| Item | Case number (No.) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Follow-up (days) | 68 | 9 | 90 | 54 | 182 | 300 | 347 |
| Follow-up results | |||||||
| No recurrence of tumor | + | + | |||||
| Tumor metastasis | + | + | + | ||||
| Death | + | + | |||||
| Causes of death | Infected | Infected | |||||
DiscussionOther Section
LC and ILD also have many similarities in the pathogenesis, in addition, the two diseases have many similar risk factors, including smoking, advanced age, and so on (5). When ILD occurs, cytokines, chemokines, and growth factors promote the fibrosis process, and then lead to the destruction of the lung tissue structure, thus resulting in a series of reactions, and eventually develop into neoplastic lesions (6,7).
It is also different for the incidence of LC induced by different types of ILD. The incidences of LC were 1.43% in ILD-only groups (8), however, the incidence of LC was 4.4% to 48% in patients with IPF (9), while the prevalence of LC was as high as 8.9% in patients with pulmonary fibrosis complicated with emphysema (CPFE) (10). Therefore, it is confirmed that the incidence of ILD-LC may be underestimated because it is not detected from the LC-CES-ILD patients in our study, and the actual prevalence of ILD-LC may be higher than the data reported in the literature. Therefore, it is challenging to discover LC based on ILD, which is also a difficult point in the diagnosis and treatment of interstitial diseases.
It has been reported that the probability of accidental detection of LC in transplanted resected lungs is 0.8–2.2% (11), compared with a slightly higher proportion. Seven patients performed high-resolution CT screening before transplantation. The interval was only 2–29 days, which was significantly lower than the range in reference (12), but still failed to reduce the removal of ILD-LC from the lungs effectively. It is suggested that more effective methods are needed for the preoperative diagnosis of ILD-LC.
It is the key to confirming ILC-CES-LC by careful naked eye observation of the diseased lungs resected in end-stage ILD transplantation. Adenocarcinoma was the most common histological type of ILD-LC, accounting for 43% (11), followed by squamous cell carcinoma. Most of our cases were adenocarcinoma. The differentiation of mucinous adenocarcinoma was found in several cases, and a rare pleomorphic cancer component was found in one case (12). Hata et al. (13) summarized the surgical specimens and found that ILD-LC had a higher proportion of pleural invasion than LC alone. Three/seven cases in this study also confirmed the destruction of visceral pleura, for the prognostic significance of this phenomenon, more cases are needed to accumulate summary further.
The pathological diagnosis of LC from ILD, especially invasive tumors, is not difficult, but squamous epithelial metaplasia and hyperplasia of squamous epithelium or mucinous epithelium often occur at the same time of interstitial fibrosis, which is similar to the carcinomatous interstitial reaction of tumor. This needs to be differentiated from invasive tumors. The deletion of p63 positive basal cells can assist in the diagnosis of invasive adenocarcinoma. The expression of p53 and Ki-67 was significantly increased in tumors (14,15), which has a particular significance in differential diagnosis.
Detecting the ILD-LC drive gene, we discovered the mutation of KRAS in only one case of mucinous adenocarcinoma, and the results were consistent with those reported in the literature (16,17). The mutation rate of EGFR in ILD-LC was significantly lower than that in LC patients without ILD, but the mutation of KRAS was relatively common, indicating that ILD-LC may be different from LC without ILD in pathogenesis and molecular phenotype. At the same time, the effect of targeted drugs on ILD-LC is minimal, therefore, the difference between the two should be studied, and the effective treatment for ILD-LC should be studied further.
ConclusionsOther Section
The incidence of ILD-LC should not be ignored, and be more aware of the development of this disease and follow-up of ILD patients in clinical practice. Before lung transplantation in ILD end-stage patients, a variety of methods are needed to find the hidden LC. This study reveals that ILD combined LC differs from LC in clinical manifestations, pathological characteristics and molecular phenotypes, and this finding has a guiding effect on future clinical practice.
Table S1
| Detection gene | Exon/codon | Mutation type |
|---|---|---|
| EGFR gene | Exon-19 | 19-del |
| Exon-21 | L858R | |
| Exon-20 | T790M | |
| Exon-18 | G719X | |
| Exon-20 | S768I | |
| Exon-21 | L861Q | |
| KRAS gene | Exon-2 | G12D/S, G12A/V/R/C, G13C |
| BRAF gene | Exon-15 | V600E/K/R/D |
| NRAS gene | Exon-2 | G12C/A/V, G13R/C |
| Exon-3 | Q51R/K/L/H | |
| HER2 gene | Exon-20 | 20-ins/ G776>VC (1) |
| PIK3CA gene | Exon-20 | H1047R |
| Exon-9 | E545K | |
| ALK Fusion gene | ALK-Exon-20 | E13; A20, E6ins33; A20, E20; A20, E18; A20, E2; A20, E17; ins68A20, E2; ins117A20, E13; ins69A20, E6; A20, E6; A19, E6; ins18A20, E20; ins18A20, E17del58; ins39A20, E17ins65; A20, E17; ins30A20, E17ins61; ins34A20, E3; ins53A20, KI24; A20, KI17; A20, KL9; A20, T4; A20 |
| ROS1Fusion gene | ROS1-Exon-32/34/35 | SLC34A2 Exon-4/14 |
| CD74 Exon-6, SDC4 Exon-2/4 | ||
| SLC34A2 Exon-4/14, EZR Exon-10 | ||
| CD74 Exon-6, SDC4 Exon-4 | ||
| TPM3 Exon-8 | ||
| LRIG3 Exon-16, GOPC Exon-8 | ||
| RET Fusion gene | RET-Exon-12 | CCDC6 exon 1; RET exon 12 |
| NCOA4 exon6; RET exon 12 | ||
| KIF5B exon 15; RET exon 12 | ||
| KIF5B exon 16; RET exon 12 | ||
| KIF5B exon 23; RET exon 12 | ||
| KIF5B exon 22; RET exon 12 |
Table S2
| Item | Case number | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| NRAS | − | − | − | − | − | − | − |
| EGFR | − | − | − | − | − | − | − |
| ALK | − | − | − | − | − | − | − |
| ROS1 | − | − | − | − | − | − | − |
| Braf | − | − | − | − | − | − | − |
| Her-2 | − | − | − | − | − | − | − |
| PI3KCA | − | − | − | − | − | − | − |
| RET | − | − | − | − | − | − | − |
| KRAS | − | − | − | − | − | + | − |
− means that the results of examination are negative and + means that the results of examination are positive. The test results in
AcknowledgmentsOther Section
Funding: This work was supported by
FootnoteOther Section
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.11.36). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This work was approved by the ethics committee at China-Japan Friendship Hospital (No. 2012-60). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- American Thoracic Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS executive committee, June 2001. Am J Respir Crit Care Med 2002;165:277-304. [PubMed]
- Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014—an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2015;34:1-15. [Crossref] [PubMed]
- Sheikine Y, Kuo FC, Lindeman NI. Clinical and Technical Aspects of Genomic Diagnostics for Precision Oncology. J Clin Oncol 2017;35:929-33. [Crossref] [PubMed]
- Wallis A, Spinks KJB. The diagnosis and management of interstitial lung diseases. BMJ 2015;350:h2072. [Crossref] [PubMed]
- Naccache JM, Gibiot Q, Monnet I, et al. Lung cancer and interstitial lung disease: a literature review. J Thorac Dis 2018;10:3829-44. [Crossref] [PubMed]
- Tzouvelekis A, Gomatou G, Bouros E, et al. Common pathogenic mechanisms between idiopathic pulmonary fibrosis and lung cancer. Chest 2019;156:383-91. [Crossref] [PubMed]
- Sucre JMS, Deutsch GH, Jetter CS, et al. A Shared Pattern of beta-Catenin Activation in Bronchopulmonary Dysplasia and Idiopathic Pulmonary Fibrosis. Am J Pathol 2018;188:853-62. [Crossref] [PubMed]
- Choi WI, Lee DY, Choi HG, et al. Lung Cancer development and mortality in interstitial lung disease with and without connective tissue diseases: a five-year Nationwide population-based study. Respir Res 2019;20:117. [Crossref] [PubMed]
- Yoo H, Jeong BH, Chung MJ, et al. Risk factors and clinical characteristics of lung cancer in idiopathic pulmonary fibrosis: a retrospective cohort study. BMC Pulm Med 2019;19:149. [Crossref] [PubMed]
- Girard N, Marchand-Adam S, Naccache JM, et al. Lung cancer in combined pulmonary fibrosis and emphysema: a series of 47 Western patients. J Thorac Oncol 2014;9:1162-70. [Crossref] [PubMed]
- Chatron E, Dégot T, Salvaterra E, et al. Lung cancer after lung transplantation: An analysis of 25 years of experience in a single institution. Clin Transplant 2019;33:e13446. [Crossref] [PubMed]
- Ahmad U, Hakim AH, Tang A, et al. Patterns of Recurrence and Overall Survival in Incidental Lung Cancer in Explanted Lungs. Ann Thorac Surg 2019;107:891-896. [Crossref] [PubMed]
- Hata A, Suzuki H, Nakajima T, et al. Concomitant Interstitial Lung Disease Is a Risk Factor for Pleural Invasion in Lung Cancer. Ann Thorac Surg 2017;103:967-74. [Crossref] [PubMed]
- Zhu WY, Hu XF, Fang KX, et al. Prognostic value of mutant p53, Ki-67, and TTF-1 and their correlation with EGFR mutation in patients with non-small cell lung cancer. Histol Histopathol 2019;34:1269-78. [PubMed]
- Folescu R, Levai CM, Grigoras ML, et al. Expression and significance of Ki-67 in lung cancer. Rom J Morphol Embryol 2018;59:227-33. [PubMed]
- Fujimoto D, Tomii K, Otoshi T, et al. Preexisting interstitial lung disease is inversely correlated to tumor epidermal growth factor receptor mutation in patients with lung adenocarcinoma. Lung Cancer 2013;80:159-64. [Crossref] [PubMed]
- Masai K, Tsuta K, Motoi N, et al. Clinicopathological, Immunohistochemical, and Genetic Features of Primary Lung Adenocarcinoma Occurring in the Setting of Usual Interstitial Pneumonia Pattern. J Thorac Oncol 2016;11:2141-9. [Crossref] [PubMed]


