
Pre-expansion of posterior gastric fascia during laparoscopic radical gastrectomy for gastric cancer prevents injuries to upper short gastric vessels
Introduction
Due to the presence of the gastrosplenic ligament, the branches of the short gastric vessel are challenging adequately expose during the radical surgery for gastric cancer, especially in obese patients. Furthermore, bleeding from the injured branches will contaminate the surgical field and thus, increase the difficulty of surgery. The lymph node dissection in the perigastric mesentery should be based on its anatomical layers and distribution. Although the laparoscopic radical gastrectomy and the traditional open surgery for gastric cancer share the same surgical principles, their surgical approaches and operation methods dramatically differ. Most operators have limited knowledge of the anatomical features of this area under laparoscope. This study aimed to illustrate whether the expansion of the posterior gastric fascia during radical gastrectomy helps prevent injuries to the upper short gastric vessels.
Clinical data
A 52-year-old male patient experienced intermittent upper abdominal pain, discomfort, and a feeling of fullness for about 20 days, but without nausea, vomiting, or fever. He visited a local hospital, where gastroscopy revealed a 3 cm × 2 cm mucosal defect at the fundus of the stomach. Pathology suggested the possibility of a poorly-differentiated adenocarcinoma at the fundus of the stomach. Contrast-enhanced computed tomography (CT) showed abnormal enhancement at the fundus side of the cardia. The patient has smoked for 35 years (about 20 cigarettes or 1 pack per day). The patient denied a history of alcohol abuse. Blood tests for cancer markers showed no abnormalities. Left ventricular ejection fraction (EF%) was 66%. Arterial blood gas analysis showed the PO2 was 86.8 mmHg. On August 3, 2018, he received laparoscopy-assisted total gastrectomy + D2 lymph node dissection under general anesthesia.
Surgical process
Establishment of an operating platform
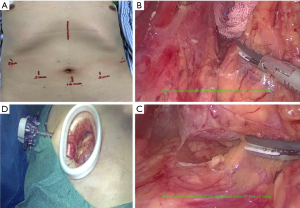
After successful tracheal intubation under general anesthesia, the patient was placed in the supine position, with two legs open. Disinfection and draping were routinely performed in the surgical field. A transverse incision about 1.2 cm in length was established at infraumbilical fold along skin line. The skin and abdominal wall were cut open before the abdominal cavity was accessed with a 12 mm optical trocar (Kangji Medical, Hangzhou, China). Neumoperitoneum was created, and the intra-abdominal pressure was maintained at 13 to 15 mmHg. A 30-degree laparoscope (Karl Storz, Shanghai, China) was then inserted. Under laparoscopic monitoring, 12- and 5-mm trocars were placed 2 cm below the rim of the left rectus abdominis and at the umbilical level, respectively, as the main and auxiliary working ports, during which any injury to the inferior epigastric artery was carefully avoided. Two 5-mm trocars were placed at the right symmetry points, as the auxiliary working ports (Figure 1A).
Intra-abdominal exploration
There were no ascites in the abdominal cavity. Peritoneum, omentum, visceral surface, liver, spleen, and intestine showed no abnormalities. After the transverse colon was lifted, examinations showed that there was no swollen lymph node around the abdominal aorta, around the middle collateral artery, and at the mesenteric root. The tumor was located at the fundus, about 4 cm in diameter. It invaded the serosal surface, but there was no abnormality in the duodenal bulb and antrum. The gastrocolic ligament was divided at the upper edge of the transverse colon to access the omental bursa. The posterior wall of the stomach was smooth, without tumor penetration. The pancreas was normal, without being affected by the tumor. The intraoperative diagnosis was carcinoma of gastric fundus and cardia, and radical surgery was scheduled. Total gastrectomy + D2 gastric lymph node dissection and Rou-en-Y esophagojejunostomy were planned.
Mobilization of the greater omentum, anterior lobe of the transverse mesocolon, and capsule of pancreas
Beginning with the splenic flexure of the descending colon, the mobilization continued rightwards along the upper edge of the transverse colon. After the gastrocolic ligament, hepatocolic ligament, and anterior lobe of transverse mesocolon were divided, the omental bursa was accessed till the hepatic flexure of the colon, and thus the upper and descending parts of the duodenum were exposed. The anterior lobe of the transverse mesocolon and the pancreatic capsules were detached to the cephalic side until reaching the upper edge of the pancreas.
Handling of left gastro-omental blood vessel
The greater omentum was mobilized, and the lymph nodes at the greater curvature of the stomach were removed (No. 4sb, 4d). The greater curvature of the stomach was pulled towards the lower right side. With the splenic artery at the upper edge of the pancreas as a marker, the branch of the left gastro-omental blood vessel was found at the junction between the gastrocolic ligament and gastrosplenic ligament. It was clamped with hemostatic clips (SinoLinks, Changzhou, China) and transected at the distal end with high-intensity focused ultrasound (HIFU) (Johnson & Johnson, Shanghai, China). The greater omentum was divided to the right side along the greater curvature of the stomach till the lower edge of the pylorus; meanwhile, the lymph nodes at the greater curvature of the stomach were completely removed (No. 4sb, 4d).
Handling of right gastro-omental blood vessel and removal of the infrapyloric lymph nodes (station 6)
The stomach was pulled to the cephalic side, and the transverse colon was pulled downwards. The fusion fascia where the mesentery of the stomach was attached to the transverse mesocolon was divided at the lower margin of the pancreatic neck and the front of the horizontal segment of the duodenum. After the superior mesenteric vein was found in the transverse mesocolon, the Henle’s gastrocolic trunk was located along the vein. Subsequently, the right gastroepiploic vein was traced to the cephalic side till the surface of the pancreas, where the vein was horizontally clamped and transected. The right gastroepiploic artery on the left side of the vein was found and then transected, along with the dissection of infrapyloric lymph node (No. 6).
Transaction of the duodenum
The connective tissues among the pyloric canal, the upper part of the duodenum, and the pancreas were further removed to lift the upper part of the duodenum adequately. The distal side was about 2 cm away from the pylorus. The duodenum was closed and transfected with an endoscopic linear cutter/stapler (Echelon Flex 60, Johnson & Johnson, Shanghai, China).
Exposure of all the branches of the celiac trunk and dissection of lymph node stations 5 (suprapyloric lymph nodes), 12a (lymph nodes along hepatoduodenal ligament), 8a (common hepatic artery nodes anterosuperior group), 7 (left gastric lymph nodes), 9 (celiac lymph nodes), and 11p (lymph nodes around the splenic artery)
The stomach was rolled over to the cephalic side. The gastroduodenal artery was located in the groove between the gastric antrum and pancreatic head; with this artery as a marker, the bifurcation of common hepatic artery and the gastroduodenal artery was located at the left side, and the proper hepatic artery was located at the upper side. Then, the left gastric vein (coronary vein), which runs vertically upwards, was located at the highest point of the “back of the arch” of the pancreas. Behind it, the root of the splenic artery at the celiac artery was found. After the splenic vein below the splenic artery was exposed, the left gastric vein was skeletonized, and the root of the left gastric vein at the splenic vein was located. The left gastric artery originating in the celiac artery was found in the left posterior side of the left gastric vein, and both the left gastric artery and vein were transected at their roots. The posterior pancreatic space behind the splenic artery and the upper edge of the pancreas was cut open, and the dissection continued along the splenic artery, which was skeletonized for about 5 cm to the distal end. The lymph node stations 7, 9, and 11p were removed. Then, the vascular sheath was opened along the common hepatic artery and was mobilized to the right side along the common hepatic artery inside the sheath. After the three bifurcations of the common hepatic artery were reconfirmed and localized, the proper liver artery (to the liver) and the right gastric artery (to the stomach wall) were skeletonized. The right gastric artery was transected at its root and the lymph node stations 5, 8a and 12a were removed. The skeletonization of the proper hepatic artery continued; the anterior wall of the portal vein and the common bile duct were exposed at the posterior and right sides of the hepatoduodenal ligament, respectively, enabling the further dissection of the lymph node station 12a. The vessels near the lesser curvature of the stomach were skeletonized towards the cardia till the diaphragmatic crura at the right side to facilitate the dissection of lymph node stations 1 and 3.
Mobilization of lesser omentum and dissection of lymph node stations 1 and 3
The stomach was repositioned and pulled downwards. The hepatogastric ligament was cut open along the lower edge of the left hepatic lobe on the left edge of the hepatoduodenal ligament. The omental bursa was reaccessed, and the dissection continued leftwards till the right side of the cardia at the right diaphragmatic crura, during which the adhesions at the gastric antrum, the body of the stomach, and the visceral surface of the left lobe of the liver were divided. The lesser omentum was divided distally along the right side of the cardia to the upper edge of the upper segment of the duodenum. Meanwhile, lymph node stations 1 and 3 were dissected. The fascia gap behind the stomach was further mobilized and extended along the posterior wall of the stomach (Figure 1B) till the upper pole of the spleen (video clip, 08:17–09:58), during which the posterior gastric vessels were transected and ligated. The assistant grasped the greater curvature of the stomach near gastric fundus with the left hand and the middle and lower parts of greater curvature with the left hand. The stomach was pulled to the abdominal side and right side to expose the short gastric vessels (video clip, 10:12–10:56). These short gastric vessels were transected, along with the dissection of lymph node station 4Sa. The mobilization continued to the cephalic side to expose the gastrosplenic ligament. All the short gastric vessels were transected with HIFU one by one leftwards (video clip, 10:56–11:52) (Figure 1C). Vessels in the greater curvature of the stomach were skeletonized towards the cardia till the left diaphragmatic crura, and the lymph node station 2 was removed.
Thus, the distal segment of esophagus, the whole stomach, and the duodenal bulb, as well as the attached greater and lesser omenta, were mobilized entirely.
Roux-en-Y anastomosis
A 5-cm incision into the abdominal cavity was made layer by layer in the left costal margin, and an incision-protecting sleeve (Beijing Aerospace Kadi Technology Development Institute, Beijing, China) was placed (Figure 1D). The jejunum was transected about 20 cm away from the ligament of Treitz. The site 3 cm below the distal stump of jejunum was marked with a suture, and a circular stapler (Johnson & Johnson, Shanghai, China) was placed to connect with the anvil in the esophageal stump. After the end-to-side anastomosis of the esophagus to jejunum was completed, the circular stapler was withdrawn. The jejunal stump was closed with a linear cutter&stapler about 3 cm away from the esophagojejunal anastomosis. An incision about 50 cm away from the distal end of the stump was made in the same size as the proximal jejunal stump, and an end-to-side anastomosis was made at the proximal end of the jejunal stump. Contralateral suturing was performed. After embedding of the serosa and muscle tissue, the anastomosis was checked for patency. The bilateral incision margins in the circular stapler were checked for continuity and integrity. The esophagus-jejunum anastomosis was 50 cm away from the proximal end of the jejunal end-to-side anastomosis. The esophagus-jejunum anastomosis and the jejunal end-to-side anastomosis were checked for patency, which was defined as allowing the passing of two fingers and without apparent stenosis, leakage, or ischemia. Areas around the hepatoduodenal ligament and the head of the pancreas were checked for bile leakage, pancreatic leakage, and hemorrhage. Also, the abdominal cavity was checked for any visible bleeding. The jejunum and transverse colon were placed in the right position, and hemostatic gauzes were applied to cover the anastomosis and the stump of the small intestine. A rubber drainage tube was placed near the esophagus-jejunum anastomosis and the splenic hilum, and it was introduced via the left abdominal wall incision and then fixed. After the surgical instruments and gauzes were counted, the abdomen was closed layer by layer.
Discussion
Gastric cancer is highly prevalent worldwide (1), and the prevalence and mortality of gastric cancer remain high in China (2). Laparoscopic radical gastrectomy has been widely applied globally. However, organ exposure and intraoperative bleeding remain challenging problems during laparoscopic surgery. During the laparoscopy-assisted D2 radical gastrectomy for gastric cancer (3,4), the transaction of upper short gastric vessels in the traditional surgical procedure may lead to the tearing and bleeding of the spleen due to tissue traction. Once the bleeding becomes uncontrollable, a splenectomy may be required, which not only prolongs hospital stay but also increase postoperative complications. In our practice, when the upper short gastric vessels were handled, we firstly mobilized the posterior fascia of the stomach to left diaphragmatic crura, during which the upper pole of the spleen was naturally exposed (video clip, 10:56–11:52). The adequate mobilization of the posterior fascia of the stomach also increased the operating space for the surgery. The short gastric vessels can be easily exposed and identified, which effectively prevents secondary injuries. Also, the mesenteric gaps formed by perigastric mesentery around the pancreas, spleen, and other organs are mostly potential fusion gaps filled with loose connective tissue and a small amount of adipose tissue. They have an apparent boundary with adjacent mesentery or fascia, which allows the complete dissociation of the mesentery; in particular, en bloc resection of the mesentery and the lymphatic vessels traveling inside it can be easily performed, which is more in line with the principles of radical treatment for tumors.
Acknowledgments
Funding:
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.09.14). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All procedures performed in this study involving patient was in accordance with the ethical standards of Bengbu Medical College Ethics Committee (No. 2019143). Informed consent was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines. 5th ed. Tokyo: Kanehara Shuppan, 2018.
- Kinami S, Nakamura N, Tomita Y, et al. Precision surgical approach with lymph-node dissection in early gastric cancer. World J Gastroenterol 2019;25:1640-52. [Crossref] [PubMed]


