Neoadjuvant therapy does not adversely affect the short-term outcome of critically ill cancer patients who underwent surgery
Introduction
Neoadjuvant therapy (NT) has been increasingly used in many potential operable solid tumors such as gastric cancer, colon cancer, lung cancer, and it leads to improved long-term survival (1-3). It was not associated with increased postoperative complications and deaths in esophageal cancer, and even decreased the incidence of some morbidities of pancreatic cancer surgeries (4,5). However, other reports had found that NT conferred no survival benefit over adjuvant therapy in lung cancer and pancreatic cancer patients (6,7). In some studies, NT was even related to the occurrence of pulmonary embolism (8), and reduced cardiopulmonary reserve (9), which may have adverse effect on the short term outcomes of cancer patients who underwent surgery.
Currently there are no studies regarding the effect of NT on the short-term outcome in critically ill cancer patients who underwent surgeries. Therefore, we performed this study in an academic cancer center which aims to investigate whether NT affects the short-term outcome in critically ill cancer patients who underwent surgery.
Methods
This was a retrospective study which enrolled all critically ill cancer patients who admitted to intensive care unit (ICU) of Cancer Hospital of Chinese Academy of Medical Sciences and Peking Union Medical College between September 2017 and September 2018. The study was compliant with the 1964 ethical Declaration of Helsinki and its revision. Inform consents were waived, owning to the non-interventional nature of the study.
The following data were extracted and analyzed: age, gender, preoperative co-morbidities including a diabetic mellitus, coronary heart disease, history of hypertension, and chronic obstructive pulmonary disease (COPD), body mass index (BMI), type of admission to ICU (planned or unplanned), simplified acute physiology score 3 (SAPS 3) and sequential organ failure assessment (SOFA) on the admission day of ICU, diagnosis of acute kidney injury (AKI) and sepsis during ICU, duration of ventilation, American Joint Committee on Cancer (AJCC) staging, ICU death, in-hospital death, ICU length of stay (LOS), and hospital LOS.
Patients were divided into two groups: NT group and no NT (nNT) group. NT was defined as patients underwent chemotherapy and (or) radiotherapy at least 3 months before surgery. SOFA score was determined as a total of points of six different systems, one each for the coagulation, neurological, hepatic, cardiovascular, renal, and respiratory systems (10). SAPS 3 was determined using variables within 1 hour after patient admitted to ICU (11). Sepsis was defined using the new sepsis definitions, which consisted of sepsis and septic shock (12). AKI was determined according to the absolute of relative change of serum creatinine or the change of urine output (13). AJCC staging was carried out according to AJCC Cancer staging manual (14).
The primary outcome was ICU mortality. Secondary outcomes were duration of mechanical ventilation, ICU LOS, hospital LOS and in-hospital mortality.
We used SPSS software for Windows, version 16.0 (SPSS Inc., Chicago, IL, USA) for statistical analysis. Categorical variables were presented as absolute numbers (percentages of frequency) and χ2 test was used to compare the difference. Continuous variables are reported as mean ± standard deviation and Student’s t-test was used to compare the difference. In order to balance the confounding factors, we conducted propensity score matching analysis by the method proposed by Austin (15). First we did the logistic regression analysis that calculated propensity scores receiving NT as outcome with age, sex, co-morbidities (diabetic mellitus, coronary heart disease, hypertension, and COPD), BMI, AJCC staging and type of admission to ICU. We then excluded patients whose scores were lower than 0.05 (low chance having NT) and higher than 0.90 (high chance having NT). Then we analyzed patients with matching scores. Finally, we used univariable and multivariable logistic analysis to investigate the risk factors of ICU death. A P value less than 0.05 was defined as significant.
Results
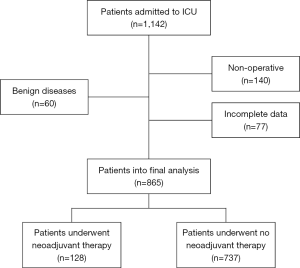
During the study period, there were a total of 1,142 admissions to ICU. After excluding 60 benign diseases, 140 non-operative cases, and 77 incomplete data, there were 865 patients who were enrolled into the final analysis (Figure 1).
General characteristics of 865 patients are presented in Table 1. There were 128 patients who received NT and 737 patients who received nNT. Before propensity score matching, patients in NT group were younger (60.08±10.15 vs. 64.70±12.04, P<0.001), had more stage III to IV disease (68.8% vs. 38.8%, P<0.001) compared with patients in nNT group. There were no significant differences in gender, co-morbidities and BMI between these two groups. There were more ICU deaths in NT group compared with nNT group (3.9% vs. 1.4%, P=0.041) (Table 2). There were more unplanned admissions to ICU (39.8% vs. 28.5%, P=0.01) more mechanical ventilations (52.3% vs. 36.4%, P=0.001) in NT group than those in NT group. Patients in NT group were more severe as reflected by higher SAPS 3 (41.77±13.35 vs. 35.03±12.37, P<0.001) and SOFA (3.60±2.84 vs. 2.61±2.70, P<0.001).
Table 1
| Clinical variables | Before propensity scores matching | After propensity scores matching | |||||
|---|---|---|---|---|---|---|---|
| NT group (n=128) | nNT group (n=737) | P value | NT group (n=118) | nNT group (n=118) | P value | ||
| Age (years) | 60.08±10.15 | 64.70±12.04 | <0.001 | 59.94±10.01 | 60.52±11.35 | 0.680 | |
| Male (%) | 39 (30.5) | 251 (34.1) | 0.676 | 85 (72.0) | 77 (65.3) | 0.262 | |
| Hypertension (%) | 11 (8.6) | 89 (12.1) | 0.427 | 39 (33.1) | 37 (31.4) | 0.781 | |
| Coronary heart disease (%) | 15 (11.7) | 113 (15.3) | 0.255 | 7 (5.9) | 9 (7.6) | 0.605 | |
| Diabetic mellitus (%) | 1 (0.8) | 13 (1.8) | 0.288 | 11 (9.3) | 13 (11.0) | 0.667 | |
| COPD (%) | 39 (30.5) | 251 (34.1) | 0.416 | 0 | 1 (0.8) | 0.316 | |
| BMI (kg/m2) | 24.39±5.22 | 24.03±3.58 | 0.341 | 24.39±5.22 | 24.55±3.78 | 0.780 | |
| AJCC staging (%) | <0.001 | 0.572 | |||||
| Stage 0−II | 40 (31.2) | 451 (61.2) | 34 (28.8) | 38 (32.2) | |||
| Stage III−IV | 88 (68.8) | 286 (38.8) | 84 (71.2) | 80 (67.8) | |||
| Type of ICU admission (%) | 0.010 | 0.166 | |||||
| Planned admission | 77 (60.2) | 527 (71.5) | 74 (62.7) | 84 (71.2) | |||
| Unplanned admission | 51 (39.8) | 210 (28.5) | 44 (37.3) | 34 (28.8) | |||
| SAPS3 score on ICU admission | 41.77±13.35 | 35.03±12.37 | <0.001 | 41.62±13.42 | 33.24±11.35 | <0.001 | |
| SOFA score on ICU admission | 3.60±2.84 | 2.61±2.70 | <0.001 | 3.42±2.55 | 2.58±2.79 | 0.017 | |
| Septic shock (%) | 6 (4.7) | 31 (4.2) | 0.804 | 5 (4.2) | 4 (3.4) | 0.734 | |
| Acute kidney injury (%) | 5 (3.9) | 22 (3.0) | 0.580 | 5 (4.2) | 5 (4.2) | 1.000 | |
| Mechanical ventilation (%) | 67 (52.3) | 268 (36.4) | 0.001 | 59 (50.0) | 49 (41.5) | 0.191 | |
NT, neoadjuvant therapy; nNT, no neoadjuvant therapy; COPD, chronic obstructive pulmonary disease; BMI, body mass index; AJCC, American Joint Committee on Cancer; SAPS 3, simplified acute physiology score; SOFA, sequential organ failure assessment.
Table 2
| Clinical variables | Before propensity scores matching | After propensity scores matching | |||||
|---|---|---|---|---|---|---|---|
| NT group (n=128) | nNT group (n=737) | P value | NT group (n=118) | nNT group (n=118) | P value | ||
| Duration of mechanical ventilation | 0.94±1.90 | 0.75±1.91 | 0.315 | 0.75±1.32 | 0.93±2.30 | 0.467 | |
| ICU length of stay (d) | 3.12±3.16 | 3.12±3.63 | 0.982 | 3.11±2.94 | 3.28±4.36 | 0.727 | |
| Hospital length of stay (d) | 17.59±9.33 | 16.70±12.24 | 0.438 | 17.93±9.20 | 17.06±15.7 | 0.603 | |
| ICU death (%) | 5 (3.9) | 10 (1.4) | 0.041 | 4 (3.4) | 2 (1.7) | 0.408 | |
| Hospital death (%) | 5 (3.9) | 10 (1.4) | 0.041 | 4 (3.4) | 2 (1.7) | 0.408 | |
NT, neoadjuvant therapy; nNT, no neoadjuvant therapy; ICU, intensive care unit.
After matching, the general characteristics of patients including age, gender, co-morbidities, BMI, unplanned admissions to ICU and tumor staging were similar between two groups (Table 1). There were no significant differences in secondary outcomes including duration of mechanical ventilation, ICU mortality, in-hospital mortality, ICU LOS and hospital LOS between NT group and nNT group (Table 2).
For all 865 patients, univariable logistic analysis demonstrated that a history of coronary heart disease (P=0.008), NT (P=0.041), unplanned admission to ICU (P<0.001), SAPS 3 on ICU admission (P<0.001), SOFA on ICU admission (P<0.001), AKI (P<0.001), and mechanical ventilation (P<0.001 were risk factors of ICU death (Table 3). Multivariable logistic regression analysis demonstrated that a history of coronary heart disease (P=0.010; OR =9.614; 95% CI, 1.731–53.405), SAPS 3 on ICU admission (P=0.026; OR =1.070; 95% CI, 1.008–1.135) and SOFA on ICU admission (P=0.031; OR =1.289; 95% CI, 1.024–1.622) were independent risk factors of ICU death, while NT was not (P=0.118).
Table 3
| Clinical variables | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| ICU death (n=15) | ICU alive (n=850) | P value | P value | RR (95% CI) | ||
| Age (years) | 60.87±9.02 | 64.00±11.94 | 0.781 | |||
| Male (%) | 9 (60.0) | 565 (66.5) | 0.599 | |||
| Hypertension (%) | 6 (40.0) | 284 (33.4) | 0.592 | |||
| Coronary heart disease (%) | 5 (33.3) | 95 (11.2) | 0.008 | 0.010 | 9.614 (1.731–53.405) | |
| Diabetic mellitus (%) | 0 | 128 (15.1) | 0.103 | |||
| COPD (%) | 0 | 14 (1.6) | 0.616 | |||
| BMI (kg/m2) | 23.63±3.11 | 24.09±3.87 | 0.674 | |||
| AJCC staging (%) | 0.799 | |||||
| Stage 0-II | 9 (60.0) | 482 (56.7) | ||||
| Stage III-IV | 6 (40.0) | 368 (43.3) | ||||
| Neoadjuvant therapy (%) | 5 (33.3) | 123 (14.5) | 0.041 | 0.118 | 3.502 (0.728–16.852) | |
| Type of ICU admission (%) | <0.001 | 0.990 | ||||
| Planned admission | 0 | 604 (71.1) | ||||
| Unplanned admission | 15 (100.0) | 246 (28.9) | ||||
| SAPS3 on ICU admission | 71.20±15.07 | 35.40±11.80 | <0.001 | 0.026 | 1.070 (1.008–1.135) | |
| SOFA on ICU admission | 10.53±3.94 | 2.62±2.51 | <0.001 | 0.031 | 1.289 (1.024–1.622) | |
| Septic shock (%) | 2 (13.3) | 35 (4.1) | 0.080 | |||
| Acute kidney injury (%) | 3 (20.0) | 24 (2.8) | <0.001 | 0.169 | 3.373 (0.597–19.059) | |
| Mechanical ventilation (%) | 15 (100.0) | 320 (37.6) | <0.001 | 0.991 | ||
ICU, intensive care unit; COPD, chronic obstructive pulmonary disease; BMI, body mass index; AJCC, American Joint Committee on Cancer; SAPS 3, simplified acute physiology score; SOFA, sequential organ failure assessment.
Discussion
In this study, we used two statistical methods to analyze the data and found that in critically ill cancer patients who underwent surgery, NT was not a risk factor for ICU death.
In our study, ICU mortality was increased in patients who received NT before propensity score matching analysis. Sabra et al. found that NT was associated with the occurrence of pulmonary embolism in esophageal cancer patients (8). Yendamuri et al. demonstrated that 30- and 90-day mortality were increased in advanced staged non-small cell lung cancer. In their study, the risk of 30- and 90-day mortality in stage II was 1.11- and 1.28-fold respectively compared with stage I lung cancer, and was 1.19- and 1.53-fold respectively in stage III, and 1.72- and 2.99-fold in stage IV (16). In our study, more patients in NT group had stage III−IV cancer (68.8% vs. 38.8%), which may account for increased ICU mortality and hospital mortality in patients who received NT compared with patients who did not receive NT. After controlling confounder factors including age, tumour staging, the ICU mortality was similar between NT group and nNT group in this study.
In addition, other postoperative complications such as septic shock, AKI were also similar between two groups, and there were no significant differences in secondary outcomes including duration of mechanical ventilation, ICU LOS and hospital LOS. Combined literatures and our results, we concluded that NT was safe, and it did not lead to increment of postoperative complications rates or 30-day mortality (4).
Although the propensity scores matching analysis had advantages over conventional regression modeling, and it is a well option for the analysis of data of non-randomized intervention trials (17), regression model is still a commonly used statistical method to control confounder factors. In this study, we also used the regression model to investigate whether NT was a risk factor of ICU death. In our study, although univariable logistic analysis showed that NT was associated with increased odds of ICU death, multivariable logistic regression analysis demonstrated that a history of coronary heart disease, SAPS 3 score on ICU admission and SOFA on ICU admission were risk factors of ICU death, while NT was not. Our results were consistent with Sabra et al., that NT did not adversely affect 30-day death in cancer patients after esophagectomy. In Sabra et al. study, they also used multivariable logistic regression by data of American College of Surgeons National Surgical Quality Improvement Program (8).
However, controversy exists regarding the benefit of NT on the short-term outcome of cancer patients who underwent surgeries. Yendamuri et al. examined the impact of NT on the short-term and long-term survival in lung cancer patients with the National Cancer Database (16). They found that 30-day (3% vs. 2.6%; P<0.01) and 90-day mortality (6.5% vs. 4.9%; P<0.01) was higher in patients who received NT than patients who underwent upfront surgery after univariable and multivariable logistic analysis. However, follow up of the study demonstrated superior long-term survival in NT group than that in upfront surgery group. This paradox phenomenon deserves further study.
Several limitations should be noted in this study. First, the results were from a single center, and the sample is relatively small, further multicenter large sample studies are in need to clear up the role of NT on short-term outcome in critically ill cancer patients who underwent surgeries. Second, there is a high heterogeneities in patients in this study. There were cancer patients of different sites including cancer of head and neck, cancer of thorax, caner of abdomen. However, the main endpoint was ICU death in our study, and short-term outcome of cancer patients mainly depend on the disease severity, but not the type of primary tumor, because the nature of cancer biologics might not significantly affect short-term outcomes but long-term survival (18). Third, intraoperative variables were not included in this study, as intraoperative esophagectomy surgical Apgar score is a risk factor of major morbidity in patients who underwent open esophagectomy in our previous study (19).
In conclusion, we found that NT was not a risk factor of ICU death in critically ill cancer patients who underwent surgery. Owing to the beneficial effect of NT on long term survival in cancer patients, intensivists should make every effort to treat each critically ill cancer patient who underwent NT.
Acknowledgments
Funding: This study is supported by special fund of
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.12.78). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Investigational review board of Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, China approved the research and informed consent was waived owning to the observational nature of this study. The ethical number is NCC-2019C-162.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Miao ZF, Liu XY, Wang ZN, et al. Effect of neoadjuvant chemotherapy in patients with gastric cancer: a PRISMA-compliant systematic review and meta-analysis. BMC Cancer 2018;18:118. [Crossref] [PubMed]
- Dehal A, Graff-Baker AN, Vuong B, et al. Neoadjuvant Chemotherapy Improves Survival in Patients with Clinical T4b Colon Cancer. J Gastrointest Surg 2018;22:242-9. [Crossref] [PubMed]
- Blumenthal GM, Bunn PA Jr, Chaft JE, et al. Current Status and Future Perspectives on Neoadjuvant Therapy in Lung Cancer. J Thorac Oncol 2018;13:1818-31. [Crossref] [PubMed]
- Mungo B, Molena D, Stem M, et al. Does neoadjuvant therapy for esophageal cancer increase postoperative morbidity or mortality?. Dis Esophagus 2015;28:644-51. [Crossref] [PubMed]
- Marchegiani G, Andrianello S, Nessi C, et al. Neoadjuvant Therapy Versus Upfront Resection for Pancreatic Cancer: The Actual Spectrum and Clinical Burden of Postoperative Complications. Ann Surg Oncol 2018;25:626-37. [Crossref] [PubMed]
- Tao X, Yuan C, Zheng D, et al. Outcomes comparison between neoadjuvant chemotherapy and adjuvant chemotherapy in stage IIIA non-small cell lung cancer patients. J Thorac Dis 2019;11:1443-55. [Crossref] [PubMed]
- Sohal DPS. Adjuvant and neoadjuvant therapy for resectable pancreatic adenocarcinoma. Chin Clin Oncol 2017;6:26. [Crossref] [PubMed]
- Sabra MJ, Smotherman C, Kraemer DF, et al. The effects of neoadjuvant therapy on morbidity and mortality of esophagectomy for esophageal cancer: American college of surgeons national surgical quality improvement program (ACS-NSQIP) 2005-2012. J Surg Oncol 2017;115:296-300. [Crossref] [PubMed]
- Thomson IG, Wallen MP, Hall A, et al. Neoadjuvant therapy reduces cardiopulmunary function in patients undegoing oesophagectomy. Int J Surg 2018;53:86-92. [Crossref] [PubMed]
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707-10. [Crossref] [PubMed]
- Moreno RP, Metnitz PG, Almeida E, et al. SAPS 3--From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med 2005;31:1345-55. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Kellum JA, Lameire NKDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013;17:204. [Crossref] [PubMed]
- Amin MB, Edge S, Greene F. editors. AJCC cancer staging manual. Springer, 2017.
- Austin PC. Propensity-score matching in the cardiovascular surgery literature from 2004 to 2006: a systematic review and suggestions for improvement. J Thorac Cardiovasc Surg 2007;134:1128-35. [Crossref] [PubMed]
- Yendamuri S, Groman A, Miller A, et al. Risk and benefit of neoadjuvant therapy among patients undergoing resection for non-small-cell lung cancer. Eur J Cardiothorac Surg 2018;53:656-63. [Crossref] [PubMed]
- Benedetto U, Head SJ, Angelini GD, et al. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg 2018;53:1112-7. [Crossref] [PubMed]
- Kiehl MG, Beutel G, Böll B, et al. Consensus statement for cancer patients requiring intensive care support. Ann Hematol 2018;97:1271-82. [Crossref] [PubMed]
- Xing XZ, Wang HJ, Qu SN, et al. The value of esophagectomy surgical apgar score (eSAS) in predicting the risk of major morbidity after open esophagectomy. J Thorac Dis 2016;8:1780-7. [Crossref] [PubMed]