
Correction of spurious low platelet counts by optical fluorescence platelet counting of BC-6800 hematology analyzer in EDTA-dependent pseudo thrombocytopenia patients
Introduction
Pseudo thrombocytopenia (PTCP) remains a pre-analytical challenge in hematological analysis with multiple etiologies, including improper venipuncture, inadequate mixing blood sample, sample clotting, PLT satellitism, giant or large platelets, and anticoagulant-related platelet agglutination, etc. (1,2). Among different kinds of anticoagulant-related platelet agglutination, ethylene diamine tetraacetic acid-dependent pseudo thrombocytopenia (EDTA-PTCP) is the most frequent cause (3). It is reported that the incidence of EDTA-PTCP is 0.07–0.21% (4). Currently, the widely used workflow to manage EDTA-PTCP samples usually is as follows: the alarm of “platelet aggregation” or “low platelet count” from the hematology analyzer initiated the suspect of EDTA-PTCP, and a blood smear was prepared and stained to confirm the existence of platelet aggregates. In the case of blood smear with positive platelet clumps, patients were asked to consent to the drawing of additional blood samples with citrates or another anticoagulant (5). Except for extra blood samples with other anticoagulants, several approaches have been proposed to prevent this type of spurious platelet count, such as pre-warming blood samples or using addictives to revert aggregation and correct platelet counts (5,6). Nevertheless, none of them have always been effective, so that more approaches need to be developed. The Mindray BC-6800 hematology analyzer's optical fluorescence platelet count is obtained from the reticulocyte channel (7). Accidentally, we found that the optical fluorescence platelet counts were significantly higher than impedance platelet counts when using BC-6800 Hematology Analyzer to count platelets of EDTA-PTCP samples, and the optical fluorescence platelet counts were comparable with the platelet counts of additionally drawn citrate anticoagulated blood sample (without platelet aggregates). This is the first research reporting that optical fluorescence platelet counting on BC-6800 analyzer is useful to correct spurious low platelet counts in EDTA-PTCP patients.
Methods
Patients and samples
EDTA anticoagulated whole blood samples for routine hematological analysis were collected in this study. If the “platelet aggregation” flag was presented and the impedance platelet count was <100×109/L, blood smears were made, and platelet aggregation was confirmed by microscopic examination. Once the above rules were fulfilled, patients were asked for written consent to obtain additional two tubes of whole blood samples anticoagulated with EDTA and citrate (8). As a control, 30 non-platelet aggregation blood samples were collected, which neither triggered the “platelet aggregation” flag nor presented with platelet aggregation on the blood smear. The Clinical Research Ethics Committee of Zhejiang Cancer Hospital approved this study.
Methodology
Mindray BC-6800 Automatic Hematology Analyzer (Shenzhen Mindray Biomedical Electronics Co. Ltd., China), matched reagents & controls and Sysmex XE-2100 Automatic Hematology Analyzer (Sysmex Co., Japan), matched reagents & controls were routinely used in the clinical lab. Daily maintenance and daily quality control were performed according to the user’s manual to ensure that the instrument was in a stable state. All samples in EDTA tubes were performed platelet counting both in impedance channel and reticulocyte channel within 30 minutes. All samples re-collected in citrate tubes were performed platelet counting and blood smear making, as the previous paper suggested (8). Platelet counts in Citrate tubes were multiplied with a correction factor to correct the volume impact of liquid citrate anticoagulant. A blood smear was prepared and stained with Wright-Giemsa according to the standard operating procedures of “National Guide to Clinical Laboratory Procedures” (9).
Statistical analysis
SPSS 20.0 was used for statistical analysis of data. Measurement data were expressed as and paired-sample t-test was performed for comparisons. A P value <0.05 indicated that the difference was statistically significant.
Results
Identification and characteristics of EDTA-PTCP samples
Twenty-three samples were finally identified as EDTA dependent pseudo thrombocytopenia. All of them triggered the “PLT aggregation” flag on the hematology analyzer. The platelet histogram showed typical serrated irregularity and a zigzag tail (Figure 1), and platelet clumps could be seen under microscopy in all these 23 samples, with no presence of platelet satellitism or giant platelets (Figure 1). All these samples had an impedance platelet count lower than 100×109/L, with an average platelet level of (52.61±21.90)×109/L. These EDTA-PTCP patients were re-collected for blood samples into EDTA and citrate anticoagulated tubes. Platelet aggregation reoccurred in tubes containing EDTA anticoagulant, but not in tubes containing citrate anticoagulant. All Samples in citrate anticoagulant tubes had normal impedance platelet histogram without “PLT aggregation” flags. No signs or symptoms of platelet disorders were found on these 23 EDTA-PTCP patients.

Correction of spurious low platelet counts by optical fluorescence platelet counting of BC-6800 hematology analyzer
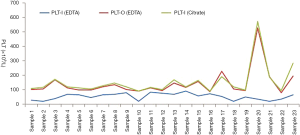
Except for impedance platelet counting, optical fluorescence platelet counting is also available on BC-6800 hematology analyzer, where platelets are stained with a fluorescent dye and counted in reticulocyte channel. These 23 blood samples in EDTA tubes (with platelet aggregation) were also tested in the reticulocyte channel of Mindray BC-6800, and the optical fluorescence platelet counts were significantly higher than the impedance platelet counts (Figure 2, t=4.33, P=0.00). Interestingly, the optical fluorescence platelet counts were comparable with the platelet counts of re-collected samples in tubes containing citrate anticoagulant (Figure 2), which was proved without platelet aggregation under a microscope.
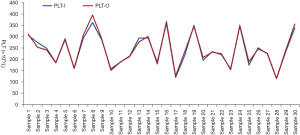
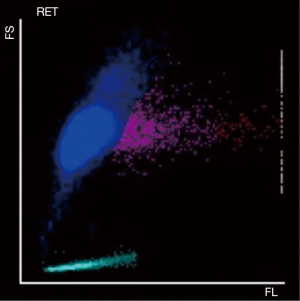
As a control, 30 normal samples (without platelet aggregation in EDTA anticoagulant tubes) were tested for platelet counts in impedance channel and reticulocyte channel, and there was no significant difference between the optical fluorescence platelet counts and impedance platelet counts (Figure 3, t=1.25, P=0.22). Further investigating the scatter plots of the reticulocyte channel of these 23 EDTA-PTCP samples, non-platelet particles such as red blood cell fragments or white blood cell fragments were not found in their scatter plots (Figure 4). The significantly higher optical fluorescence platelet counts were not pseudo thrombocytosis but a real reflection of dissociated platelets.
The dissociation effect of optical fluorescence platelet counting on these 23 EDTA-PTCP samples was not homogeneous. The dissociation rate was defined as optical fluorescence platelet counts in EDTA tubes/impedance platelet counts in citrate tubes ×100% to evaluate the dissociation effect of optical fluorescence platelet counting of EDTA-PTCP samples on BC-6800 hematology analyzer. The results showed that the dissociation rate of 22 EDTA-PTCP samples was greater than 80%, with an average dissociation rate of 93% among all these 23 EDTA-PTCP samples (Figure 2).
The dissociation effect of optical fluorescence platelet counting on EDTA-PTCP samples was independent of fluorescent dye staining
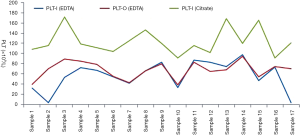
The optical fluorescence platelet counting is available both in Sysmex XN/XE series hematology analyzers and Mindray BC-6800 series hematology analyzers. A fluorescent dye is used to stain nucleic acids of platelets, allowing the recognition of large platelets and the exclusion of interference of non-platelet particles such as erythrocyte debris, micro erythrocytes, leukocyte debris, etc. To verify whether the dissociation effect of optical fluorescence platelet counting was dependent on fluorescent dye staining, 17 of those 23 EDTA-PTCP samples in EDTA tubes were also tested on the reticulocyte channel and impedance channel of Sysmex XE-2100 analyzer, and there was no significant difference between the platelet counts of reticulocyte channel and impedance channel (Figure 5, t=1.86, P=0.08). Furthermore, there was a big gap between XE-2100’s optical fluorescence platelet counts of EDTA anticoagulant EDTA-PTCP samples and impedance platelets of citrate anticoagulant corresponding samples. Only 1 of the 17 EDTA-PTCP samples showed a dissociation rate of more than 80%, with an average dissociation rate of 56% among all these 17 EDTA-PTCP samples (Figure 5).
Discussion
EDTA is a widely used anticoagulant for whole blood routine hematologic examination (10), proposed by the International Council for Standardization in Hematology (ICSH). Since Shreiner and Bell first defined a phenomenon of platelet aggregation directly attributed to EDTA anticoagulant (1), numerous cases of EDTA-PTCP have been reported in patients with immune thrombocytopenic purpura (11), dengue infection (12), scrub typhus infection (13), sepsis (14), gastrectomy (15), neuroendocrine carcinoma (16), etc. The classic pathogenesis hypothesis of EDTA-PTCP is that in the presence of EDTA, the negatively charged phospholipids or membrane receptors such as glycoprotein (Gp) IIb–IIIa turn allosteric, react with autoantibodies, activate the tyrosine kinase pathway and finally induce platelet aggregation (5). Additional phlebotomy is always needed to obtain accurate platelet counts in these EDTA-PTCP cases, and blood specimens are anticoagulated with sodium citrate, ammonium oxalate, or sodium heparin before automated platelet counting (5).
Recently several methods have been proposed to correct platelet counts in EDTA-PTCP patients. Supplementation of Kanamycin or other aminoglycosides within 30 minutes after blood withdrawal dissociated platelet clumps and the platelet counts, as well as the platelet histogram, were close to those examined immediately after phlebotomy (17). The mechanism of aminoglycosides’ dissociation effect on platelet clumps is not clear, but it might be explained by sodium-citrate, which is used as a stabilization additive during the drug preparation (18). A mixture of sodium heparin and calcium chloride with the effect of platelet clumps dissociation was also reported (19). The mechanism of this mixture was based on the pathophysiological mechanism of EDTA-PTCP: calcium chloride re-association of glycoprotein IIb–IIIa and sodium heparin anticoagulation of blood samples (19). In some labs, magnesium salt was recommended as an anticoagulant for platelet counting in patients with known or suspected EDTA-PTCP (18). However, all these remedies are not always effective and extra in vitro preparation is needed before automatically platelet counting. In this research, a novel method based on an automated hematology analyzer was found with a dissociation effect on EDTA-PTCP samples.
Optical fluorescence platelet counting on reticulocyte channel is an alternative method to count platelets on the BC-6800 hematology analyzer. Optical fluorescence platelet counting is generally acknowledged to recognize large platelets and exclude non-platelet particles such as erythrocyte debris, micro erythrocytes, leukocyte debris, etc. On BC-6800 hematology analyzer, optical fluorescence platelet counts of 23 EDTA-PTCP patients were significantly higher than impedance platelet counts (Figure 2) and comparable with the platelet counts of re-collected samples in tubes containing citrate anticoagulant (Figure 2). Among these 23 EDTA-PTCP samples, 22 samples showed a dissociation rate greater than 80%, and the average dissociation rate of all these 23 EDTA-PTCP samples was 93%. The dissociation effect was higher than that of in vitro supplementation of Kanamycin (8) or calcium chloride (19).
To investigate the mechanism of BC-6800 hematology analyzer’s dissociation effect on EDTA-PTCP samples, a case-by-case study was designed and the XE-2100 hematology analyzer was used to test 17 EDTA-PTCP samples, which was another hematology analysis platform with an optical fluorescence platelet counting. Only one sample showed a dissociation rate greater than 80%, and the average dissociation rate of all these 17 EDTA-PTCP samples was 56% on the XE-2100 hematology analyzer, indicating that the dissociation effect of optical fluorescence platelet counting on EDTA-PTCP samples was independent of fluorescent dye staining. Further discussing the methodology of reticulocyte channel of BC-6800 hematology analyzer with its manufacturer’s R&D engineers, we suppose there may be a combination of physical and chemical factors contributing to this dissociation effect. Firstly, blood samples and reagent were pre-warmed up to 42 °C in the chamber before platelets being counted, while the optimal reaction temperature of EDTA-PTCP is 0–4 °C (5), and it has been reported that pre-warming blood samples in vitro increased platelet counts (20).
Moreover, the high-speed of the vortex in the chamber for mixing blood samples and reagent will also benefit the dissociation of platelet clumps. The previous report has shown a successful methodology to obtain correct platelet count by mixing EDTA-anticoagulated blood via vortex before rerunning the specimen through an automated analyzer (21). After comparing the reagent composition using in reticulocyte channel between BC-6800 and XE-2100 hematology analyzer (data not shown), we suppose that the additives in the reagent of BC-6800 hematology analyzer may also contribute to the dissociation effect of platelet clumps, although fluorescence staining has been excluded in the mechanism of BC-6800 hematology analyzer’s dissociation effect in this study. Further investigation is needed to confirm which additive takes this role for dissociation of platelet clumps in EDTA-PTCP samples.
Taking into account the dissociation effect of BC-6800’s optical fluorescence platelet counting, we reset the review rules to alarm EDTA-PTCP more sensitively and correct the spurious low platelet counts in EDTA-PTCP samples without a second venous or capillary phlebotomy. Impedance platelet counts less than 100/µL or “platelet aggregation” flag triggers an additional optical fluorescence platelet counting as well as blood smear making/staining. Big gaps between impedance platelet counts and optical fluorescence platelet counts strongly indicate PTCP. In these cases, the optical fluorescence platelet counts are more reliable. After the platelet clumps were confirmed in blood smear, they could be reportable if the optical fluorescence platelet counts are higher than 100/µL and much higher than impedance platelet counts. This approach offers a novel automated way to obtain reliable platelet counts in EDTA-PTCP patients without any in vitro preparation or second collection of blood samples.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.12.58). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Clinical Research Ethics Committee of Zhejiang Cancer Hospital approved the study. Informed consent was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shreiner DP, Bell WR. Pseudothrombocytopenia: Manifestation of a new type of platelet agglutinin. Blood 1973;42:541-9. [Crossref] [PubMed]
- Zandecki M, Genevieve F, Gerard J, et al. Spurious counts and spurious results on haematology analysers: A review. Part I: Platelets. Int J Lab Hematol 2007;29:4-20. [PubMed]
- Salignac S, Latger-Cannard V, Schlegel N, et al. Platelet counting. Methods Mol Biol 2013;992:193-205. [Crossref] [PubMed]
- Yoneyama A, Nakahara K. EDTA-dependent pseudothrombocytopenia-differentiation from true thrombocytopenia. Nippon Rinsho 2003;61:569-74. [PubMed]
- Lippi G, Plebani M. EDTA-dependent pseudothrombocytopenia further insights and recommendations for prevention of a clinically threatening artifact. Clin Chem Lab Med 2012;50:1281-5. [Crossref] [PubMed]
- Jeon IS, Yang SW. Prevention and Dissociation of the Platelet Aggregation in a Patient with EDTA-dependent Pseudothrombocytopenia by Supplementation of Kanamycin: A Case Report. Korean Journal of Pediatrics 2005;48:675-7.
- Fuster Ó, Andino B, Laiz B. Performance evaluation of low platelet count and platelet clumps detection on Mindray BC-6800 hematology analyzer. Clin Chem Lab Med 2016;54:e49-51. [Crossref] [PubMed]
- Lin J, Luo Y, Yao S, et al. Discovery and Correction of Spurious Low Platelet Counts due to EDTA-Dependent Pseudothrombocytopenia. J Clin Lab Anal 2015;29:419-26. [Crossref] [PubMed]
- Shang H, Wang Y, Shen Z. National Guide to Clinical Laboratory Procedures (Forth edition). Beijing, China: People’s Medical Publishing House, 2015:123.
- Mehmood R, Muhammed RK, Hussain S, et al. Evaluation of di-potassium and tri-potassium EDTA evacuated tubes for routine haematological testing. J Clin Lab Anal 2018; [Crossref] [PubMed]
- Fozza C, Pardini S, Marras T, et al. Pseudothrombocytopenia in a patient receiving romiplostim for immune thrombocytopenic purpura. Ann Hematol 2014;93:899-900. [Crossref] [PubMed]
- Vaidya P, Venkataraman R. Pseudothrombocytopenia in a Child with Dengue. Indian J Pediatr 2014;81:1395-6. [Crossref] [PubMed]
- Lamech TM, Chellaraj M, Penchalaiah R, et al. Pseudothrombocytopenia in Patients with Scrub Typhus Infection. Indian Journal of Hematology and Blood Transfusion 2019; [Crossref]
- Mori M, Kudo H, Yoshitake S, et al. Transient EDTA-dependent pseudothrombocytopenia in a patient with sepsis. Intensive Care Medicine 2000;26:218-20. [Crossref] [PubMed]
- Wenzel F, Lasshofer R, Rox J, et al. Transient appearance of postoperative EDTA-dependent pseudothrombocytopenia in a patient after gastrectomy. Platelets 2011;22:74-6. [Crossref] [PubMed]
- Kim Han J. Ethylenediaminetetraacetic acid-dependent pseudothrombocytopenia associated with neuroendocrine carcinoma: A case report. Oncol Lett 2012;4:86-8. [Crossref] [PubMed]
- Sakurai S, Shiojima I, Tanigawa T, et al. Aminoglycosides prevent and dissociate the aggregation of platelets in patients with EDTA-dependent pseudothrombocytopenia. Br J Haematol 1997;99:817-23. [Crossref] [PubMed]
- Schuff-Werner P, Steiner M, Fenger S, et al. Effective estimation of correct platelet counts in pseudothrombocytopenia using an alternative anticoagulant based on magnesium salt. Br J Haematol 2013;162:684-92. [Crossref] [PubMed]
- Chae H, Kim M, Lim J, et al. Novel method to dissociate platelet clumps in EDTA-dependent pseudothrombocytopenia based on the pathophysiological mechanism. Clin Chem Lab Med 2012;50:1387-91. [Crossref] [PubMed]
- Williams TL, Archer J. Effect of prewarming EDTA blood samples to 37 °C on platelet count measured by Sysmex XT-2000iV in dogs, cats, and horses. Vet Clin Pathol 2016;45:444-9. [Crossref] [PubMed]
- Gulati GL, Asselta A, Chen C. Using a Vortex To Disaggregate Platelet Clumps. Laboratory Medicine 1997;28:665-7. [Crossref]


