Matrine reverses the drug resistance of K562/ADM cells to ADM and VCR via promoting autophagy
Introduction
Leukemia is a common malignant tumor in human (1). In clinical practice, chemotherapy is the main treatment strategy for Leukemia. However, multidrug resistance of leukemia cells greatly limits the outcomes of leukemia patients, and high recurrence has become one of the main manifestation of treatment failure (2,3). Multidrug resistance refers to the fact that tumor cells are not only resistant to one anti-tumor drug, but also resistant to other anti-tumor drugs with different molecular structures and effects. Multidrug resistance greatly reduces the treatment efficacy of anti-tumor drugs (4,5). At present, the multidrug resistance of tumors to different chemotherapeutic drugs is still the main cause of chemotherapy failure, which is also a major problem in cancer treatment, including leukemia. Thus, in order to improve the therapeutic efficacy, inhibiting the multidrug resistance of leukemia cells is urgently needed.
Matrine (MAT) is an alkaloid extracted from the root of Sophora flavescens (6). It had been proved that MAT exerts obvious anti-tumor effects, as well as anti-inflammatory effects (7). For example, MAT suppresses the progression of prostate cancer through stimulating the expression of GADD45B (8). MAT enhances the apoptosis, and weakens the invasion ability of cervical cancer cells (9). MAT inhibits the invasion and epithelial-mesenchymal transition of pancreatic cells through regulating ROS/NF-κB/MMPs pathway (10). MAT exerts significant anti-tumor activity against gastric cancer, and both apoptosis and autophagy are activated in gastric cancer by MAT (11). MAT is also able to accelerate the apoptosis of leukemia cells (12). Note worthily, MAT has also reported to be involved in the regulation of multidrug resistance in tumors. It has been reported that MAT reverses the multidrug resistance of breast cancer cells via regulating PI3K/AKT signal pathway (13). However, the effect of MAT on multidrug resistance of leukemia cells is still unclear. Since multidrug resistance of leukemia is still a difficult problem in clinical practice, this article was devoted to investigate the specific effects of MAT on multidrug resistance of leukemia cells. Our findings may reveal a novel multidrug resistance inhibitor on leukemia cells.
Methods
Cell culture
Human leukemia cell line K562/S and multidrug-resistant cell line K562/ADM were both provided by Tianjin Institute of Hematology, Chinese Academy of Medical Sciences. Cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS) in an incubator at 37 °C with 5% CO2. RPMI 1640 medium containing 1.0 µg/mL adriamycin (ADM) (Doxorubicin hydrochloride, C27H29NO11·HCl, Sigma, St Louis, USA) was used to maintain the drug-resistance of K562/ADM cells. Two weeks before experiments, K562/ADM cells were maintained in normal RPMI 1640 medium without ADM.
Cell treatments
K562/S and K562/ADM cells in logarithmic growth phase were used for treatments. K562/S and K562/ADM cells were incubated in 96-well plates (5×105 cells/mL, 100 µL per well), and treated with 0, 0.5, 1, 2, and 4 mg/mL MAT (Matrine, C15H24N2O, Baiyunshan Mingxing, Guangzhou, China) for 48 h. Another part of K562/S and K562/ADM cells were treated with 0, 0.01, 0.1, 1.0, and 10 µg/mL vincristine (VCR) (Vincristine sulfate, C46H56N4O10·H2SO4, Wanle, Shenzhen, China) or ADM for 48 h, respectively. In addition, 0.5 mg/mL MAT per-treated K562/ADM cells (30 min of MAT treatment) were treated with VCR or ADM for another 48 h (MAT + VCR, MAT + ADM). Another part of MAT per-treated K562/ADM cells were treated with chloroquine (CQ) (a lysosomal inhibitor) combined with VCR or ADM for 48 h.
Cell viability assay by MTT
The cell viability was determined by MTT assay. Briefly, after 48 h of treatment, MTT solution (20 µL, 5 mg/mL) was added into each well, and incubated for 4 h at 37 °C. Dimethyl sulfoxide (DMSO) at a volume of 100 µL was used to replace the residual liquid in each well. Ten min later, the absorbance value at 490 wavelength (A490 value) of each well was measured on an automatic microplate reader. Cell viability was obtained with the following formula: Viability (%) = (A490 value of treated cells/A490 value of control) ×100%. The half-inhibitory concentration (IC50) was calculated by the drug concentration-viability curve. Furthermore, drug-resistant multiple (IC50 value of drug-resistant cells/IC50 value of drug-sensitive cells) and drug-resistant reversal multiple (IC50 value before reversal of drug-resistant cells/IC50 value after reversal of drug-resistant cells) was calculated.
Apoptosis assay by flow cytometry
The cell apoptosis was detected by flow cytometry. Briefly, after 48 h of treatment, cells were washed with pre-cooled PBS (1 mL) for twice. Then cells were resuspended in a mixture of 500 µL binding buffer and 5 µL Annexin V-FITC. After 20 min incubation at room temperature in darkness, the apoptosis rate was detected on flow cytometry.
Cell cycle assay by flow cytometry
The cell cycle was also detected by flow cytometry. Briefly, after 48 h of treatment, cells were washed with pre-cooled PBS for twice, and resuspended in 75% pre-chilled ethanol for 12 h at −20 °C. After 5 min of centrifugation at 1,000 r/min, cells were resuspended in 10 mL pre-cooled PBS, and then incubated in PBS containing 200 µL of propidium iodide (PI) for 30 min at 4 °C in darkness. The cell cycle was detected on flow cytometry.
Detection of autophagic vacuoles by transmission electron microscope
MAT-treated K562/ADM cells were washed with PBS, and fixed in glutaraldehyde for 12 h at 4 °C. Then cells were incubated with tannic acid for 1.5 h at 4 °C, washed with PBS for 3 times (5 min each time), and dehydrated with gradient ethanol (50%, 70%, 90% and 100%) and 100% acetone. After incubated in embedding agent and acetone (1:1) for 2 h at 25 °C, cells were embedded in epoxy resin at 35 °C overnight, cured in an oven for 24 h at 45 °C, and sectioned. Followed by 12 h of staining with uranyl acetate and lead citrate at 66 °C, cells were observed under transmission electron microscope.
Observation of autophagy by GFP-LC3 staining
K562/ADM cells were transfected with GFP-LC3 (Invitrogen) for 48 h by using lipofectamine 3000 (Invitrogen). Then these cells were treated with MAT (0, 0.5, 1, 2 mg/mL) for 48 h. After washed with PBS, the LC3+ punctate fluorescence was observed under a fluorescence microscope.
Detection of autophagy-related protein levels by Western blot
MAT-treated K562/ADM cells were lysed in RIPA Lysis buffer (Biyuntian, Shanghai, China). Total proteins were quantified by using BCA kit (Biyuntian), separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to PVDF membrane. Then the membrane was blocked with 5% skim milk for 2 h, and incubated with primary antibodies, anti-LC3 and anti-P62 (mouse anti-human, 1:1,000, Abcam, Cambridge, UK) for 12 h at 4 °C. Followed by 1 h of incubation with alkaline phosphatase-labeled secondary antibody (goat anti-mouse, 1:5,000, Zhongshan Jinqiao, Beijing, China) at 25 °C, protein bands were visualized with enhanced chemiluminescence (ECL) (Pierce, Rockford, IL, USA). β-actin was served as the internal control.
Statistical analysis
All data were processed using SPSS 21.0 (SPSS Inc., Chicago, IL, USA) statistical software. Measurement data were expressed as mean ± standard deviation. T-test was used for comparison between two groups. One-way analysis of variance was used for comparison among multiple groups. P<0.05 meant statistically significant difference.
Results
MAT reversed the drug-resistance of K562/ADM cells to ADM and VCR
MAT reduced the viability of K562/S and K562/ADM cells in a dose-dependent manner. No significant difference on the cell viability was revealed between K562/S cells and K562/ADM cells at the same concentrations of MAT (Figure 1A). The IC50 value of MAT on K562/S cells and K562/ADM cells was 2.33 and 2.50 mg/mL, respectively. The concentration of MAT at 0.5 mg/mL with a cell inhibition rate of less than 5% was set as the non-cytotoxic concentration.
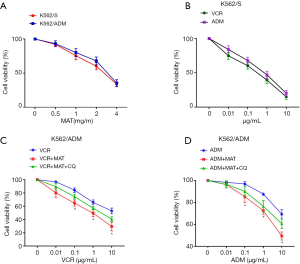
Both ADM and VCR could significantly reduce the viability of K562/S cells in a dose-dependent manner (Figure 1B). MAT eliminated the drug resistance of K562/ADM cells to ADM and VCR. The cell viability was significantly lower in VCR + MAT-treated K562/ADM cells than in VCR-treated K562/ADM cells, and significantly lower in ADM + MAT-treated K562/ADM cells than in ADM-treated K562/ADM cells (P<0.05). Noteworthy, the intervention of CQ significantly reversed the inhibitory effect of MAT on the viability of K562/ADM cells (P<0.05) (Figure 1C,D).
The drug resistance multiple of K562/ADM cells to VCR and ADM was 39.2 and 77.23, respectively. The drug resistance reversal multiple of MAT to VCR and ADM was 10.12 and 4.91, respectively (Table 1). The intervention of CQ significantly reduced the drug resistance reversal multiples of MAT to VCR and ADM (P<0.05). All these results indicated that MAT reversed the drug-resistance of K562/ADM cells to VCR and ADM, and CQ reversed the effects of MAT.
Table 1
| Drugs | K562/S | K562/ADM | K562/ADM + MAT | K562/ADM + MAT + CQ | DR multiple | DR reversal multiplea | DR reversal multipleb |
|---|---|---|---|---|---|---|---|
| VCR | 0.24±0.065 | 9.414±3.95 | 0.93±0.77* | 2.83±1.08*# | 39.2 | 10.12 | 3.32& |
| ADM | 0.59±0.113 | 45.57±2.67 | 9.28±3.23* | 24.17±5.15*# | 77.23 | 4.91 | 1.89& |
Note: DR multiple = IC50 (K562/ADM cells)/IC50 (K562/S cells). DR reversal multiplea = IC50 (K562/ADM cells)/IC50 (K562/ADM + MAT cells). DR reversal multipleb = IC50 (K562/ADM cells)/IC50 (K562/ADM + MAT + CQ cells). *, P<0.05 when compared with K562/S and K562/ADM cells; #, P<0.05 when compared with K562/ADM + MAT cells; &, P<0.05 when compared with DR reversal multiplea.
MAT promoted the apoptosis of K562/ADM cells through arresting the cell cycle at G0/G1 phase
As shown in Figure 2A, the MAT treatment significantly increased the apoptosis rate of K562/ADM cells in a dose-dependent manner (P<0.05). The apoptosis rate of K562/ADM cells reached 60% at the concentration of 2 mg/mL. It could also be noticed in Figure 2B that, MAT significantly increased the proportion of K562/ADM cells in G0/G1 phase and decreased the proportion of K562/ADM cells in S phase in a dose-dependent manner (P<0.05) (Figure 2B). These results indicated that MAT induced the apoptosis of K562/ADM cells through arresting the cell cycle at G0/G1 phase in a dose-dependent manner.
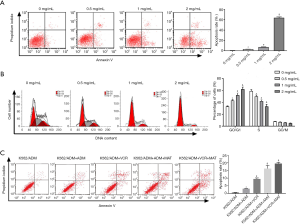
Noteworthy, the apoptosis rate was significantly higher in VCR + MAT-treated K562/ADM cells than in VCR-treated K562/ADM cells, and significantly lower in ADM + MAT-treated K562/ADM cells than in ADM-treated K562/ADM cells (P<0.05). These results further indicated that MAT eliminated the drug resistance of K562/ADM cells to ADM and VCR (Figure 2C).
MAT induced autophagy in K562/ADM cells
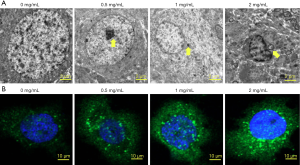
The autophagy of K562/ADM cells was observed by transmission electron microscopy and GFP-LC3 staining. With the increasing of MAT concentration, the autophagic vacuoles in K562/ADM cells was gradually increased (Figure 3A), and the LC3+ punctate fluorescence around the nucleus was also gradually increased (Figure 3B). These results indicated that MAT induced the autophagy of K562/ADM cells in a dose-dependent manner.
MAT regulated autophagy-related protein levels in K562/ADM cells
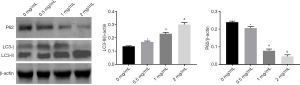
The effects of MAT on autophagy-related protein levels were monitored in K562/ADM cells by Western blot. With the increasing of MAT concentration, relative LC3 II protein level was remarkably increased (P<0.05), whereas relative P62 protein level was decreased gradually (P<0.05) (Figure 4). These results indicated that MAT promoted the conversion of LC3I to LC3II, as well as the degradation of P62 in a dose-dependent manner.
Discussion
MAT is an alkaloid complex extracted from the traditional Chinese medicine Sophora flavescens, which exerts diverse biological activities with low toxicity (14,15). MAT exhibits obvious anti-tumor effects through inhibiting the proliferation and inducing the differentiation and apoptosis in diverse cancers, such as lung cancer, esophageal cancer, gastric cancer, breast cancer, colon cancer, ovarian cancer, lymphoma, and multiple myeloma (16,17). It has been proved that MAT can reduce the adverse reactions of chemotherapy and radiotherapy, thereby improving the life quality of patients. MAT can also synergize with radiotherapy and chemotherapy by regulating immune function, thereby further improving the therapeutic effect (18). However, it is still unclear whether MAT can alleviate the multidrug resistance of leukemia cells to chemotherapy. In this article, the effect of MAT on multidrug resistance of leukemia cells was studied, and we found that MAT reversed the drug resistance of K562/ADM cells to VCR and ADM through promoting apoptosis and autophagy.
MAT stimulated the apoptosis of K562/ADM cells in a dose-dependent manner in this research. Inactivation of apoptosis contributes to the survival and therapeutic resistance of cancer cells. MAT can suppress the tumor progression by promoting apoptosis in multiple tumors. Wang et al. (19) showed that, declined Bcl-2 and increased Bax are observed in human hepatocellular carcinoma HepG2 cells treated by MAT, thereby interfering the metabolism of lipid proteins and destroying cell structure. Xu et al. (20) revealed that MAT promotes the apoptosis of osteosarcoma cells, as well as apoptotic factor Bax expression in a dose dependent manner. MAT can also induce the apoptosis of cisplatin-resistant lung cancer cells in vitro (21). However, there is no literature documenting the effect of MAT on the apoptosis of multidrug resistant leukemia cells. This paper discovered for the first time that MAT could induce the apoptosis of multidrug resistance K562/ADM cells in a dose-dependent manner.
MAT is also proved to be able to affect the cell cycle of tumor cells in previous researches. It is well known that the cell cycle is one of the important indicators for monitoring the proliferation of tumor cells. Zhang et al. (22) proved that MAT blocks gastric cancer cells in G1 phase by down-regulating the expression of G1/S essential protein Cyclin E. P21 protein is a cell cycle negative regulator with the broadest range of kinase inhibitory activity. It has been proved that MAT is able to block retinoblastoma cells in the G0/G1 phase by up-regulating p21 protein expression (23). Zhou et al. (24) suggested that, after being treated by MAT for 48 h, human hepatocellular carcinoma HepG2 cells are arrested in G0/G1 phase, accompanied by obvious morphological changes, including cell atrophy, increased nuclear heterochromatin and perinuclear space, as well as differentiation and even lysis. Moreover, MAT induces the differentiation and apoptosis of tumor cells by up-regulating the levels of proapoptotic proteins (such as Caspase-3) and surface differentiation antigen CD11b. Our results showed that MAT blocked K562/ADM cells in G0/G1 phase in a dose-dependent manner, which further illustrated that MAT regulated the cell cycle of K562/ADM cells.
Autophagy is a process that lysosomes degraded the damaged organelles and macromolecules to maintain normal cell biological function (25,26). Recent studies have been shown that autophagy plays an important role in the pathogenesis and treatment of leukemia, and is also involved in multidrug resistance mechanisms. Auberger et al. (27) pointed out that, suppression of autophagy accelerates the progression of acute myelogenous leukemia. Kaewpiboon et al. (28) proved that, Feroniellin A enhances the apoptosis of multidrug resistant lung cancer cells through promoting autophagy. The pharmacological regulation of autophagy is considered as a promising cancer treatment strategy. It has been reported that MAT enhances the autophagy of human hepatoma cells through regulating p53/AMPK signaling pathway (29). Wu et al. (30) demonstrated that MAT acts as a tumor suppressor in acute myelocytic leukaemia by promoting autophagy. In this study, MAT promoted the conversion of LC3 I to LC3 II, as well as the degradation of P62 in a dose-dependent manner. It is known that, the conversion of LC3 I to LC3 II indicates the enhancement of autophagy. P62 protein can inhibit autophagy flux, and is considered as a treatment target for suppression of autophagy in tumors (31-33). Our findings illustrated that MAT induced the autophagy of K562/ADM cells.
Conclusions
In conclusion, MAT reversed the multidrug resistance of K562/ADM cells to ADM and VCR through promoting autophagy. Thus, in clinical practice, MAT may use as a promising pharmaceutical formulation to enhance the sensitivity of multidrug resistant leukemia to chemotherapeutic drugs. This article provided a novel therapeutic strategy against multidrug resistant leukemia.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.12.11). The authors have no conflicts of interest to declare.
Ethical Statement The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Human leukemia cell line K562/S and multidrug-resistant cell line K562/ADM were both provided by Tianjin Institute of Hematology, Chinese Academy of Medical Sciences.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Koh JW, Chin F, Teh YH, et al. Second neoplasms in survivors of childhood acute lymphoblastic leukemia treated with both chemotherapy and radiotherapy. J Clin Oncol 2016;119:S658.
- Du Y, Chen B. Detection approaches for multidrug resistance genes of leukemia. Drug Des Devel Ther 2017;11:1255-61. [Crossref] [PubMed]
- Chen JR, Jia XH, Wang H, et al. Timosaponin A-III reverses multi-drug resistance in human chronic myelogenous leukemia K562/ADM cells via downregulation of MDR1 and MRP1 expression by inhibiting PI3K/Akt signaling pathway. Int J Oncol 2016;48:2063-70. [Crossref] [PubMed]
- Shankaraiah N, Nekkanti S, Ommi O, et al. Diverse targeted approaches to battle multidrug resistance in cancer. Curr Med Chem 2019;26:7059-80. [Crossref] [PubMed]
- Yu DH, Liu YR, Luan X, et al. IF7-Conjugated Nanoparticles Target Annexin 1 of Tumor Vasculature against P-gp Mediated Multidrug Resistance. Bioconjug Chem 2015;26:1702-12. [Crossref] [PubMed]
- Xu Y, Lin H, Zheng W, et al. Matrine ameliorates adriamycin-induced nephropathy in rats by enhancing renal function and modulating Th17/Treg balance. Eur J Pharmacol 2016;791:491-501. [Crossref] [PubMed]
- Liu L, Ren W, Chen K. MiR-34a Promotes Apoptosis and Inhibits Autophagy by Targeting HMGB1 in Acute Myeloid Leukemia Cells. Cell Physiol Biochem 2017;41:1981-92. [Crossref] [PubMed]
- Huang H, Wang Q, Du T, et al. Matrine inhibits the progression of prostate cancer by promoting expression of GADD45B. Prostate 2018;78:327-35. [Crossref] [PubMed]
- Qin X, Chen J, Wu L, et al. MiR-30b-5p acts as a tumor suppressor, repressing cell proliferation and cell cycle in human hepatocellular carcinoma. Biomed Pharmacother 2017;89:742-50. [Crossref] [PubMed]
- Huang M, Xin W. Matrine inhibiting pancreatic cells epithelial-mesenchymal transition and invasion through ROS/NF-κB/MMPs pathway. Life Sciences 2018;192:55-61. [Crossref] [PubMed]
- Li Y, Zhang J, Ma H, et al. Protective role of autophagy in matrine-induced gastric cancer cell death. Int J Oncol 2013;42:1417-26. [Crossref] [PubMed]
- Ma L, Zhu Z, Jiang L, et al. Matrine suppresses cell growth of human chronic myeloid leukemia cells via its inhibition of the interleukin-6/Janus activated kinase/signal transducer and activator of transcription 3 signaling cohort. Leukemia & Lymphoma 2015;56:2923-30. [Crossref] [PubMed]
- Zhou BG, Wei CS, Zhang S, et al. Matrine reversed multidrug resistance of breast cancer MCF-7/ADR cells through PI3K/AKT signal pathway. J Cell Biochem 2018;119:3885-91. [Crossref] [PubMed]
- Chen F, Huang K. Effects of the Chinese medicine matrine on experimental C. parvum infection in BALB/c mice and MDBK cells. Parasitol Res 2012;111:1827-32. [Crossref] [PubMed]
- Yang Y, Xiu J, Zhang X, et al. Antiviral effect of matrine against human enterovirus 71. Molecules 2012;17:10370-6. [Crossref] [PubMed]
- Sun M, Cao H, Sun L, et al. Antitumor Activities of Kushen: Literature Review. Evid Based Complement Alternat Med 2012;2012:373219. [Crossref] [PubMed]
- Wang Q, Du H, Geng G, et al. Matrine inhibits proliferation and induces apoptosis via BID-mediated mitochondrial pathway in esophageal cancer cells. Mol Biol Rep 2014;41:3009-20. [Crossref] [PubMed]
- Liu Y, Xu Y, Ji W, et al. Anti-tumor activities of matrine and oxymatrine: literature review. Tumour Biol 2014;35:5111-9. [Crossref] [PubMed]
- Wang L, Gao C, Yao S, et al. Blocking Autophagic Flux Enhances Matrine-Induced Apoptosis in Human Hepatoma Cells. Int J Mol Sci 2013;14:23212-30. [Crossref] [PubMed]
- Xu GP, Zhao W, Zhuang JP, et al. Matrine inhibits the growth and induces apoptosis of osteosarcoma cells in vitro by inactivating the Akt pathway. Tumor Biology 2015;36:1653-9. [Crossref] [PubMed]
- Wang HQ, Jin JJ, Wang J. Matrine induces mitochondrial apoptosis in cisplatin-resistant non-small cell lung cancer cells via suppression of β-catenin/survivin signaling. Oncol Rep 2015;33:2561-6. [Crossref] [PubMed]
- Zhang Z, Wang X, Wu W, et al. Effects of Matrine on Proliferation and Apoptosis in Gallbladder Carcinoma Cells (GBC-SD). Phytother Res 2012;26:932-7. [Crossref] [PubMed]
- Zhao B, Li B, Shen L, et al. Effects of matrine on proliferation and apoptosis of cultured retinoblastoma cells. Graefes Arch Clin Exp Ophthalmol 2012;250:897-905. [Crossref] [PubMed]
- Zhou W, Xu X, Gao J, et al. TCM matrine induces cell arrest and apoptosis with recovery expression of the hepato-specific miR122a in human hepatocellular carcinomaHep G2cell line. Int J Clin Exp Med 2015;8:9004-12. [PubMed]
- Eskelinen EL, Saftig P. Autophagy: a lysosomal degradation pathway with a central role in health and disease. Biochim Biophys Acta 2009;1793:664-73. [Crossref] [PubMed]
- Suzuki H, Osawa T, Fujioka Y, et al. Structural biology of the core autophagy machinery. Curr Opin Struct Biol 2017;43:10-7. [Crossref] [PubMed]
- Auberger P, Puissant A. Autophagy, a key mechanism of oncogenesis and resistance in leukemia. Blood 2017;129:547-52. [Crossref] [PubMed]
- Kaewpiboon C, Surapinit S, Malilas W, et al. Feroniellin A-induced autophagy causes apoptosis in multidrug-resistant human A549 lung cancer cells. Int J Oncol 2014;44:1233-42. [Crossref] [PubMed]
- Xie SB, He XX, Yao SK. Matrine-induced autophagy regulated by p53 through AMP-activated protein kinase in human hepatoma cells. Int J Oncol 2015;47:517-26. [Crossref] [PubMed]
- Wu J, Gang H, Dong Y, et al. Matrine induces Akt/mTOR signalling inhibition-mediated autophagy and apoptosis in acute myeloid leukaemia cells. J Cell Mol Med 2017;21:1171-81. [Crossref] [PubMed]
- Kuang XL, Liu Y, Chang Y, et al. Inhibition of store-operated calcium entry by sub-lethal levels of proteasome inhibition is associated with STIM1/STIM2 degradation. Cell Calcium 2016;59:172-80. [Crossref] [PubMed]
- Wang L, Kim D, Wise JTF, et al. p62 as a therapeutic target for inhibition of autophagy in prostate cancer. Prostate 2018;78:390-400. [Crossref] [PubMed]
- Jiang X, Huang Y, Xue L, et al. Metastatic prostate cancer-associated P62 inhibits autophagy flux and promotes epithelial to mesenchymal transition by sustaining the level of HDAC6. Prostate 2018;78:426-34. [Crossref] [PubMed]



