Zero ischemia laparoscopic microwave ablation assisted enucleation vs. laparoscopic partial nephrectomy in clinical T1a renal tumor: a randomized clinical trial
Introduction
During the last decade, there has been a steady increasing use of various imaging modalities, which contributed to an increasing number of smaller size renal masses early diagnosed incidentally (1). Nephron sparing surgery (NSS) has turned be the most reliable way of treating small renal masses due to it could provide better cardiovascular outcomes and kidney function preservation which seems to be related to better cancer specific survival (2-4). Comparing with open partial nephrectomy (PN), laparoscopic partial nephrectomy (LPN) has demonstrated corresponding oncological and renal functional effects over both the short and long term since its first publications (5). However, warm ischemia injury is a major concern for conventional LPN because every minute of ischemia may have deleterious effects on renal function (6,7).
In recent years, alternative local ablative techniques, such as microwave ablation (MWA) and radiofrequency ablation (RFA), have been used in NSS due to short learning curve and low complication rate (8,9). Jacomides et al. reported a technique in which laparoscopic RFA was combined with NSS without clamping the renal pedicle could be a safe method of managing small enhancing renal masses (10). After that, numerous studies have demonstrated that compared to conventional NSS, MWA and RFA assisted NSS has a certain advantage in small incision, less bleeding and lower complication, as well as better oncology outcomes (11-13). Muto et al. reported their first preliminary experience of laparoscopic MWA and enucleation of small renal masses in 2011, but there is still insufficient high-level evidence for this technique (12).
Since 2011, zero ischemia laparoscopic RFA and MWA assisted tumor enucleation has been performed in the treatment of the renal tumor patients. Therefore, we hypothesized that this novel technique, zero ischemia laparoscopic microwave ablation assisted tumor enucleation (LMWATE), could lead to the preservation of more renal unit and achieve comparable oncological outcomes and complication rates in the patients with cT1a renal tumors compared with conventional LPN.
Methods
Patients and study design
In this study, a randomized controlled trial was performed to compare LMWATE with LPN from October 2014 to September 2017, conducted at Ren Ji Hospital, Shanghai, China. This study was planned and performed as a superiority trial. It was approved by the Ethics Committee of Ren Ji Hospital (No. RenJiH-URO-001) and registered in ClinicalTrials.gov (Identifier: NCT02326558). With the institutional review board authorization, individuals who were scheduled for laparoscopic NSS for newly diagnosed sporadic, unilateral, T1aN0M presumed renal cell carcinoma (RCC), were recruited. Before enrollment and surgery, all patients were provided written consent.
The study inclusion criteria were as follows: 15–80 years aged patients, clinical T1aN0M0 renal carcinoma patients, normal contralateral renal function patients (defined as differential renal function greater than 40% as determined by radionuclide scintigraphy.) and those ready to take part in this long-term follow-up study. In addition, exclusion criteria comprised patients who could not tolerate a laparoscopic process, who had history of renal operation or any operational kidney inflammatory conditions, who had renal carcinoma associated to the urinary collecting system and those with other renal diseases (including kidney stone, glomerular nephritis). In this study, every patient was supposed to be followed up for at least a year (12 months).
The sample size of this study was calculated primarily to detect a difference value of 5 in the change of GFR between groups with a P value of less than 0.05, the standard deviation of the change of GFR was 10, based on retrospective review of our prior experience (unpublished data), and the power of the trial was 90%. Therefore, the number needed in each arm ranges from 83 to 95, when 15% of the patients may lost in follow-up was taken into consideration. Moreover, an independent randomization office was called by the investigators after obtaining informed consent from the patients. Two groups of the patients were randomly allocated in a 1:1 ratio using a computer-generated random distribution system.
Surgical interventions
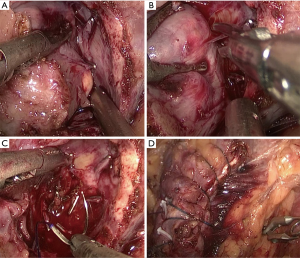
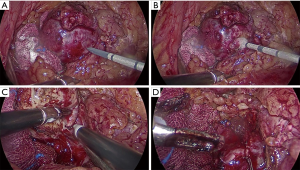
All operations were performed by the same laparoscopic surgeon with extensive LPN experience (Y Chen) The LPN procedure has been presented in detail previously (14), as showed in Figure S1. After the Gerota fascia was opened, the tumor was localized and dissected, sometime with the assistance of intraoperative ultrasonography. The renal artery is identified and dissected in case hilar clamping became necessary. Then MWA was done with a 13-gauge antenna linked to Evident Valleylab generator (Kangyou Medical Instruments, Nanjing, China) capable of producing 45 W at 915 MHz power. MWA was performed at a range of 1 to 3 min per tumor according to size and depth of the tumor for 1 to 3 cycles (Figure S2A,B). The tumor was enucleated without hilar clamping via blunt and sharp dissection (Figure S2C). The unipolar or bipolar coagulation or ablation were used to control tumor bed bleeding (Figure S2D). Subsequently, running/single suture was used to close the calyces when necessary. At the end of operation, the parenchymal defect was maintained open, a drain was placed and the enucleated tumor specimen was sent for pathological assessment.
Outcome measurement
In our study, the primary outcome was the affected kidney’s change of GFR measured by renal scintigraphy at the 3rd month and 12th month postoperatively. The secondary outcomes included changes in estimated blood loss (EBL), estimated glomerular filtration rate (eGFR), operation time, hospital stay, postoperative complications, pathological and oncologic outcome.
The PADUA classification was used to review all the preoperative images of the patients (15). Based on modified Clavien-Dindo classification system, all 30 days postoperatively complications were recorded. Standard hematoxylin and eosin staining was performed to evaluate the histopathological characteristics by using the Fuhrman classification (16).
The serum creatinine levels were used to measure the renal function while eGFR was calculated by using diet adjustment for renal disease study equation (17) and GFR was measured by the 99mTc-DTPA renal scintigraphy. The levels of serum creatinine were assessed preoperatively, and at 3, 6 and 12 months postoperatively. 99mTc-DTPA renal scintigraphy was applied preoperatively, at 3rd and 12th months postoperatively to evaluate the GFR while the renal scan reviewers were blinded to the chosen surgical procedures. The 99mTc-DTPA renal scintigraphy results through γ-camera estimates by the Gates technique was used to calculate GFR and then standardized with surface area of the body (1.73 m2).
All patients in this study were required for follow-up for at least 12 months. Follow-up was conducted at 3 and 6 months after surgery and every 6 months thereafter. It involved plasma chemistry monitoring, chest and abdomen CT scanning or MRI imaging.
The SPSS v21.0 statistical software was used in all analysis. In statistical analysis, the intent method of treatment was adopted. To examine categorized demographic or clinical variables among groups, Pearson Chi-square or Fisher exact test was adopted. In testing variations of non-normal distributed continuous variables the Wilcoxon rank sum was used while in normal distributed continuous variables Student t-test was adopted. For two-sided P value<0.05 it was considered statistically significant.
Results
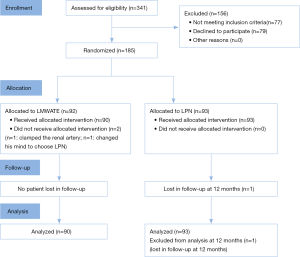
From October 2014 to October 2016, 341 patients with T1aN0M0 renal tumor were assessed against inclusion criteria. Of 264 eligible patients, 185 (70%) patients agreed to participate while 79 (30%) patients declined. The most common reason for declining was patient preference for a specific approach.
One patient who was assigned to LMWATE changed his mind and chose LPN. Another patient was converted to clamp the renal artery due to bleeding and LPN was then performed. In addition, one patient in the LPN group was loss to follow up at 12 months (Figure 1).
Patient demographics and renal function results
No significant difference was found in patients’ demographic characteristics (Table 1). Besides, the data of renal function were presented in Table 2. Comparatively, the LMWATE group patients experienced a smaller decrease in percentage change of the median 99mTc-DTPA GFR of the affected kidney by 3rd month (10.3 vs. 17.4, P<0.001) and 12th month (8.8 vs. 10.2, P=0.023), postoperatively. The LMWATE group had a smaller reduction in median eGFR by 3rd month (7.8 vs. 13.4, P<0.001), 6th month (5.9 vs. 9.2, P=0.003) and 12th month (9.5 vs. 12.5, P=0.016) in comparison to the LPN group.
Table 1
| Variables | LMWATE | LPN | P value |
|---|---|---|---|
| Patients (n) | 90 | 93 | |
| Gender, n (%) | 0.747 | ||
| Male | 56 (62.2) | 60 (64.5) | |
| Female | 34 (37.8) | 33 (35.5) | |
| Median age [range] | 58 [23–80] | 57 [25–79] | 0.63 |
| Left/right side | 47/43 | 44/49 | 0.507 |
| Body mass index (kg/m2) [range] | 25.3 [19.5–35.9] | 24.7 [20–32.8] | 0.068 |
| Hypertension, n (%) | 13 (14.4) | 15 (16.1) | 0.752 |
| Diabetes mellitus, n (%) | 7 (7.8) | 8 (8.6) | 0.839 |
| Median Charlson comorbidity index [range] | 3 [2–7] | 3 [2–7] | 0.772 |
| Median American Society of Anesthesiologists score [range] | 2 [1–3] | 2 [1–3] | 0.370 |
| Median tumor size [range] | 3 [1–4] | 3 [1.5–4] | 0.06 |
| Median PADUA score [range] | 7 [6–10] | 7 [6–10] | 0.403 |
LMWATE, laparoscopic microwave ablation assisted tumor enucleation; LPN, laparoscopic partial nephrectomy.
Table 2
| Variables | LMWATE (interquartile range) | LPN (interquartile range) | P value |
|---|---|---|---|
| Median postoperative change of GFR of affected kidney | |||
| 3 months | 10.3 (8–12.6) | 17.4 (12.7–22.2) | <0.001 |
| 12 months | 8.8 (6.5–10) | 10.2 (7.3–12.8) | 0.023 |
| Median preoperative eGFR | 100.6 (81.9–108.6) | 102.4 (90.4–117.8) | 0.053 |
| Median postoperative change of eGFR | |||
| 3 months | 7.8 (4.6–10.5) | 13.4 (10.8–16.4) | <0.001 |
| 6 months | 5.9 (3.4–9.5) | 9.2 (5.6–13.0) | 0.003 |
| 12 months | 9.5 (5.4–14.0) | 12.5 (8.1–16.0) | 0.016 |
LMWATE, laparoscopic microwave ablation assisted tumor enucleation; LPN, laparoscopic partial nephrectomy; GFR, glomerular filtration rate.
Perioperative data and complications results
One patient assigned to the LMWATE group through random selection, was converted to conventional LPN due to intraoperative bleeding, and excluded from all analysis, while the rest completed laparoscopic successfully without renal hilar clamping or converted to other surgery strategy. The median operative duration was considerably shorter for LMWATE compared with LPN group (91 vs. 112 min, P<0.001). Moreover, in the LMWATE group, the median EBL was less (82.5 vs. 117.5 mL, P<0.001) and hospital stay was shorter (5.5 vs. 6 days, P=0.013) than that in the LPN group.
The LMWATE group cumulative postoperative complication rate was 8.9% (n=8) and 9.7% (n=9) for LPN group, with no statistical significance (P=0.511). Of the eight complications in the LMWATE group, five (5.6%) was Clavien grade 1, two (2.2%) were grade 2, and one (1.1%) was grade 3b. Of the nine complications in the LPN group, four (4.3%) were Clavien grade 1, three (3.2%) were grade 2, and two (2.2%) were grade 3a (Tables 3,4).
Table 3
| Variables | LMWATE | LPN | P value |
|---|---|---|---|
| Median operative time [range] | 91 [40–185] | 112 [55–200] | <0.001 |
| Median warm ischemia time [range] | 0 | 20 [0–33] | |
| Median EBL [range] | 82.5 [20–350] | 117.5 [20–500] | <0.001 |
| Complications, n (%) | 8 (8.9) | 9 (9.7) | 0.511 |
| Clavien grade, n (%) | |||
| 1 | 5 (5.6) | 4 (4.3) | |
| 2 | 2 (2.2) | 3 (3.2) | |
| 3a | 0 | 2 (2.2) | |
| 3b | 1 (1.1) | ||
| Median hospital stay [range] | 5.5 [3–10] | 6 [4–10] | 0.013 |
| Histology, n (%) | 0.682 | ||
| Renal cell carcinoma | 73 (81.1) | 78 (83.9) | |
| Oncocytoma | 2 (2.2) | 3 (3.2) | |
| Benign | 13 (14.4) | 12 (12.9) | |
| Nondiagnostic | 2 (2.2) | 0 |
LMWATE, laparoscopic microwave ablation assisted tumor enucleation; LPN, laparoscopic partial nephrectomy; EBL, estimated blood loss.
Table 4
| Clavien grade | LMWATE | LPN | |||
|---|---|---|---|---|---|
| Complications (n=8, 8.9%) | Treatment | Complications (n=9, 9.7%) | Treatment | ||
| Clavien grade 1 | Atelectasis (n=1) | Physiotherapy | Bleeding (n=1) | Bed rest | |
| Fever (n=2) | Expectant management | Fever (n=2) | Expectant management | ||
| Urine leakage (n=2) | Keep Jackson-Pratt drain in place | confusion (n=1) | Expectant management | ||
| Clavien grade 2 | Wound infection (n=1) | Drainage + oral antibiotics | Bleeding (n=1) | Blood transfusion | |
| Urinary tract infection (n=1) | Oral antibiotics | Pneumonia (n=2) | Intravenous antibiotics | ||
| Clavien grade 3a | – | – | Dehiscent wound (n=1) | Wound closure | |
| – | – | Urine leakage (n=1) | Ureteral Double-J stent+ intravenous antibiotics | ||
| Clavien grade 3b | Urine leakage (n=1) | Ureteral double-J stent under general anesthesia + intravenous antibiotics | – | – | |
LMWATE, laparoscopic microwave ablation assisted tumor enucleation; LPN, laparoscopic partial nephrectomy.
Pathological and oncologic results
In the LMWATE and LPN groups the RCC accounted for 81.2% and 83.9% cases, respectively (Tables 3,5). All patients had negative surgical margins. No patient with local relapse or distant metastasis was found with a median follow-up of 24 months.
Table 5
| Variables | LMWATE, n (%) | LPN, n (%) | P value |
|---|---|---|---|
| Histology | 0.413 | ||
| Clear cell | 60 (80.0) | 65 (83.3) | |
| Papillary | 10 (13.3) | 8 (10.3) | |
| Chromosome | 3 (4.0) | 5 (6.4) | |
| Not specified* | 2 (2.7) | 0 (0.0) | |
| Grade | 0.24 | ||
| 1 | 33 (44.0) | 25 (32.1) | |
| 2 | 31 (41.3) | 41 (52.6) | |
| 3 | 8 (10.7) | 9 (11.5) | |
| 4 | 1 (1.3) | 3 (3.8) | |
| Not specified* | 2 (2.7) | 0 (0.0) |
*, pathological diagnosis of RCC without histologic subtyping or grading. LMWATE, laparoscopic microwave ablation assisted tumor enucleation; LPN, laparoscopic partial nephrectomy; RCC, renal cell carcinoma.
Discussion
NSS has become popular in the management of renal lesions of 4 cm or smaller, since it has been established that local tumor resection without removing the whole kidney is achievable and has been proven effectiveness (18). LPN is now widely popularity as an option in terms of minimally invasive NSS. Two major concerns of LPN are complete removal of renal tumors and obtaining a short WI duration with an efficient tumor bed hemostasis (19). However, conventional LPN requires renal pedicle to remain clamped until complete closure of renal parenchymal (20), which might lead to longer period of WI. Therefore, several techniques have been introduced to reduce or even eliminate WI injury during LPN, such as segmental renal artery clamping, off-clamping, and zero ischemia anatomical PN (21-23). Nevertheless, longer operative time could inevitably increase the risk of bleeding, which required experienced surgeons to use these techniques (22,23).
Thermal ablation, as a less invasive and morbid treatment, has the advantages in the reduction of blood loss, lower incidence of the complication and shorter convalescence during the procedure, which makes it a valid alternative to traditional NSS. In a study of 42 RCC patients, Zhao et al. reported the outstanding oncologic and renal functional outcomes by using laparoscopic RFA assisted tumor enucleation technique of without clamping of the renal pedicle with a median follow-up time of 37.5 months (13). From our previous randomized controlled study, the results also confirmed that laparoscopic RFA assisted tumor enucleation facilitated the excision of tumor with better preservation of renal function in comparison to conventional LPN (11).
Although MWA technology is still in the developmental stages for treating renal cancers, it is one of the most promising minimal invasive approach for nephron sparing. Microwave tissue coagulation plays important role in controlling of parenchymal bleeding for solid and vascular organ, such as liver and spleen (24). Comparing with RFA, MWA has some potential advantages including higher intratumoral temperatures, wider ablation volumes and shorter ablation time (25). Several studies have explored the usefulness of MWA in PN (26-28). In these studies, a microwave tissue coagulator was applied peripheral into the normal parenchyma around the tumor rather than targeting the tumor directly, and circumferential punctures were carried out to produce a conical-shaped portion of coagulated tissue (12).
Recently, Muto et al. assessed that laparoscopic MWA without renal pedicle clamping was applied in small renal tumors (12). Results were revealed from 10 patients with renal tumors of diameter between 1.3 and 4.2 cm. Median operation time was 128.5 min while mean bleeding amount was 60 mL with no haemostatic sutures required in any cases. Moreover, no intraoperative or postoperative complications occurred. It is an innovative PN technique of little ischemia. By utilizing an MWA antenna to carving a relatively avascular plane around the tumor, surgeons perform tumor enucleation instead of wedge resection so that the surrounding healthy parenchyma can be spared as much as possible.
Unlike other mentioned complicated LPN techniques, LMWATE in most case could be easily performed without hilar clamping and suturing during the operation. It might be benefited younger generation of urologists who are lack of experienced in renal parenchymal surgery. Comparing to conventional LPN, LMWATE has unique advantages, such as easier laparoscopic renal tumor enucleation, possible no need of renal pedicle clamping as well as haemostatic sutures, and better renal function preservation.
Nuclear renal scan and functional renal parenchyma volume preservation, which might play an important role in protecting the postoperative renal function in a long-term period were always used to evaluate perioperative renal function (29). Bertolo et al. reported the percentage of loss of renal function was significantly correlated to the loss of renal volume and warm ischemia significantly correlated with the loss of renal volume (30). In our study, LMWATE was not only related with a smaller decline in the affected kidney GFR examined by radionuclide scintigraphy by 3rd month (10.3 vs. 17.4, P<0.001) and 12th month (8.8 vs. 10.2, P=0.023), but also had a small reduction of eGFR (entire renal function) by 3rd month (7.8 vs. 13.4, P<0.001), 6th month (5.9 vs. 9.2, P=0.003) and 12th month (9.5 vs. 12.5, P=0.016). Furthermore, Muto et al. also reported no deterioration of renal function among patients who underwent LMWATE (12). These data all suggest that LMWATE may better preserve renal function compared to LPN. In addition, the use of the semiautomated method for the 3D segmentation of the kidney to get the volumetric assessment and 3D parenchyma measurement system could be a valid tool to support the future use of CT-scan as the tool to pair the oncological and the functional follow-up after PN (30,31).
Comparing to standard MWA which is a single procedure, LMWATE can preserve more renal function due to less energy output and ablation time for ablating the whole tumor. LMWATE also enables surgeons to eradicate the whole tumor tissue to get accurate pathological outcomes with possibility of recognizing positive surgical margins as well as easier follow up. In this study, both LMWATE and LPN groups had favorable oncologic outcomes with no positive surgical margins and no local relapse in a considerable 24 months median follow-up. Meanwhile, Muto et al. also reported no local recurrence at a mean follow-up time of 13 months by using CT scan (12).
With regard to postoperative complications, no significant difference in the overall complication rate was found between the LMWATE and LPN group (8.9% vs. 9.7%, P=0.511). The cases of those tumors involved with the urinary collecting system were excluded in this study to prevent postoperative urine leakage. However, the incidence rate of urine leakage (3.3% vs. 1.1%, P=0.363) in the LMWATE group remained higher than that of conventional LPN. According to published data, complications related to LMWATE include arteriovenous fistula, urine leakage and almost total loss of renal function (27,28). One possible drawback of MWA is unexpected extra damage to surrounding structures including arteries, veins and collecting system. Therefore, it was forcefully recommended that suturing was performed in patients whose tumor was close to urinary collecting system or collecting system were involved by tumor enucleation (11).
This study has a few limitations. Firstly, it was performed at a single high-volume medical center and each group had relatively small sample size, contribute to not fully draw authoritative conclusions on the risk of systematic recurrence free or cancer specific survival rate. Secondly, one problem with recruitment was that a significant number of patients declined randomization because they preferred the LPN approach. However, 70% of eligible patients did participate, indicating that randomization for surgical trails is feasible if patients are well counseled. Thirdly, the patients whose renal tumor was involved in urinary collecting system were excluded. Therefore, the results of this study might not be completely generalized to all cases of T1a renal tumor. What's more, we previously reported the RCT outcomes with RFA treating T1a RCC in 2016 (9), while in the near future a direct comparison of RFA vs. MWA would be conducted to illustrate the difference between the two ablative techniques.
Conclusions
The results of the study revealed that the patients treated with LMWATE for cT1a renal tumors seems attained a better renal function preservation and had a shorter operative time with less blood lost compared to conventional LPN and there was no postoperative complication difference between these two techniques. Moreover, the difference between the two procedures seem to be only with significant statistical difference. Further investigation and long-term follow-up are important and necessary to draw the conclusion about the clinical results. Therefore, this zero ischemia nephron sparing technique was recommended in the selected cases of renal tumor.
Acknowledgments
The abstract of this paper was presented at the SUI conference as a conference talk with interim findings.
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.12.73). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Ren Ji Hospital (No. RenJiH-URO-001). Before enrollment and surgery, each patient provided a written agreement.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v58-68. [Crossref] [PubMed]
- Capitanio U, Larcher A, Cianflone F, et al. Hypertension and Cardiovascular Morbidity Following Surgery for Kidney Cancer. Eur Urol Oncol 2019. doi:
10.1016/j.euo.2019.02.006 . - Veccia A, Autorino R. Is there a relation between preserved renal function and oncological outcomes in patients undergoing partial nephrectomy for renal cell carcinoma? Ann Transl Med 2018;6:S88. [Crossref] [PubMed]
- Antonelli A, Minervini A, Sandri M, et al. Below Safety Limits, Every Unit of Glomerular Filtration Rate Counts: Assessing the Relationship Between Renal Function and Cancer-specific Mortality in Renal Cell Carcinoma. Eur Urol 2018;74:661-7. [Crossref] [PubMed]
- Wang RY, Wang JF, Sun XG, et al. Evaluation of Rex Shunt on Cavernous Transformation of the Portal Vein in Children. World J Surg 2017;41:1134-42. [Crossref] [PubMed]
- Deng W, Liu X, Hu J, et al. Off-clamp partial nephrectomy has a positive impact on short- and long-term renal function: a systematic review and meta-analysis. BMC Nephrol 2018;19:188. [Crossref] [PubMed]
- Thompson RH, Lane BR, Lohse CM, et al. Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol 2010;58:340-5. [Crossref] [PubMed]
- Lin Y, Liang P, Yu XL, et al. Percutaneous microwave ablation of renal cell carcinoma is safe in patients with a solitary kidney. Urology 2014;83:357-63. [Crossref] [PubMed]
- Rimar K, Khambati A, McGuire BB, et al. Radiofrequency Ablation-Assisted Zero-Ischemia Robotic Laparoscopic Partial Nephrectomy: Oncologic and Functional Outcomes in 49 Patients. Adv Urol 2016;2016:8045210. [PubMed]
- Jacomides L, Ogan K, Watumull L, et al. Laparoscopic application of radio frequency energy enables in situ renal tumor ablation and partial nephrectomy. J Urol 2003;169:49-53; discussion 53. [Crossref] [PubMed]
- Huang J, Zhang J, Wang Y, et al. Comparing Zero Ischemia Laparoscopic Radio Frequency Ablation Assisted Tumor Enucleation and Laparoscopic Partial Nephrectomy for Clinical T1a Renal Tumor: A Randomized Clinical Trial. J Urol 2016;195:1677-83. [Crossref] [PubMed]
- Muto G, Castelli E, Migliari R, et al. Laparoscopic microwave ablation and enucleation of small renal masses: preliminary experience. Eur Urol 2011;60:173-6. [Crossref] [PubMed]
- Zhao X, Zhang S, Liu G, et al. Zero ischemia laparoscopic radio frequency ablation assisted enucleation of renal cell carcinoma: experience with 42 patients. J Urol 2012;188:1095-101. [Crossref] [PubMed]
- Verze P, Fedelini P, Chiancone F, et al. Perioperative and renal functional outcomes of laparoscopic partial nephrectomy (LPN) for renal tumours of high surgical complexity: a single-institute comparison between clampless and clamped procedures. World J Urol 2017;35:403-9. [Crossref] [PubMed]
- Ficarra V, Novara G, Secco S, et al. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol 2009;56:786-93. [Crossref] [PubMed]
- Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 1982;6:655-63. [Crossref] [PubMed]
- Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247-54. [Crossref] [PubMed]
- Laganosky DD, Filson CP, Master VA. Surgical Margins in Nephron-Sparing Surgery for Renal Cell Carcinoma. Curr Urol Rep 2017;18:8. [Crossref] [PubMed]
- Zhao PT, Richstone L, Kavoussi LR. Laparoscopic partial nephrectomy. Int J Surg 2016;36:548-53. [Crossref] [PubMed]
- Baumert H, Ballaro A, Shah N, et al. Reducing warm ischaemia time during laparoscopic partial nephrectomy: a prospective comparison of two renal closure techniques. Eur Urol 2007;52:1164-9. [Crossref] [PubMed]
- Greco F, Autorino R, Altieri V, et al. Ischemia Techniques in Nephron-sparing Surgery: A Systematic Review and Meta-Analysis of Surgical, Oncological, and Functional Outcomes. Eur Urol 2019;75:477-91. [Crossref] [PubMed]
- Antonelli A, Veccia A, Francavilla S, et al. On-clamp versus off-clamp robotic partial nephrectomy: A systematic review and meta-analysis. Urologia 2019;86:52-62. [Crossref] [PubMed]
- Veccia A, Antonelli A, Hampton LJ, et al. Near-infrared Fluorescence Imaging with Indocyanine Green in Robot-assisted Partial Nephrectomy: Pooled Analysis of Comparative Studies. Eur Urol Focus 2019; [Crossref] [PubMed]
- Vogl TJ, Nour-Eldin NA, Hammerstingl RM, et al. Microwave Ablation (MWA): Basics, Technique and Results in Primary and Metastatic Liver Neoplasms - Review Article. Rofo 2017;189:1055-66. [Crossref] [PubMed]
- Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics 2005;25:S69-83. [Crossref] [PubMed]
- Higgins LJ, Hong K. Renal Ablation Techniques: State of the Art. AJR Am J Roentgenol 2015;205:735-41. [Crossref] [PubMed]
- Kawai N, Yasui T, Umemoto Y, et al. Laparoendoscopic single-site partial nephrectomy without hilar clamping using a microwave tissue coagulator. J Endourol 2014;28:184-90. [Crossref] [PubMed]
- Terai A, Ito N, Yoshimura K, et al. Laparoscopic partial nephrectomy using microwave tissue coagulator for small renal tumors: usefulness and complications. Eur Urol 2004;45:744-8. [Crossref] [PubMed]
- Mir MC, Ercole C, Takagi T, et al. Decline in renal function after partial nephrectomy: etiology and prevention. J Urol 2015;193:1889-98. [Crossref] [PubMed]
- Bertolo R, Fiori C, Piramide F, et al. Assessment of the relationship between renal volume and renal function after minimally-invasive partial nephrectomy: the role of computed tomography and nuclear renal scan. Minerva Urol Nefrol 2018;70:509-17. [Crossref] [PubMed]
- Zhu L, Wu G, Huang J, et al. Comparing renal function preservation after laparoscopic radio frequency ablation assisted tumor enucleation and laparoscopic partial nephrectomy for clinical T1a renal tumor: using a 3D parenchyma measurement system. J Cancer Res Clin Oncol 2017;143:905-12. [Crossref] [PubMed]