
Expression and correlation of Bmi-1, AEG-1 and FHIT in bladder transitional cell carcinoma
Introduction
As one of the most common tumors in the urogenital system, bladder carcinoma (BC) is the second leading cause of death (1). To calculate mortality, the number of patients with BC is estimated to be more than 160,000 every year. For the past 20 years, the incidence of BC has been rising, which ranks the 7th in male and the 17th in female worldwide (2). Bladder transitional cell carcinoma (BTCC) is the most common malignancy in the bladder and accounts for 90% of all malignant bladder tumors (3,4). At present, although the mechanism of occurrence and progression in BC have been intensively studied, it remains unclear how to prevent the tumorigenesis of BTCC. There is a high recurrence rate of up to 50–70% after resection (5). Despite surgical removal of cancer tissue, with cisplatin-based adjuvant chemotherapy after surgery, approximately half of the patients die within 4–5 years after surgery (6). The tumorigenesis, progression and invasion of BTCC were complex processes which may involve a variety of genes’ interactive effects.
The B-cell-specific Moloney murine leukemia virus insertion site 1 (Bmi-1) gene was firstly separated as an oncogene in murine models and it could collaborate with cellular-myelocytomatosis (c-Myc) to generate lymphadenomas (7,8). It was reported that Bmi-1 played key roles in cell multiplication and tumor progression, including in lung cancer, laryngeal squamous cell carcinoma, colorectal cancer and gallbladder carcinoma (9-12). The astrocyte elevated gene-1 (AEG-1) was initially distinguished in the human fetal astrocytes, where it could be induced by human immunodeficiency virus type 1 (HIV-1) infection (13). The AEG-1 participated in genesis and development of diverse cancers, including colorectal carcinoma, glioblastoma and lung carcinoma (14-17), which is related to tumor multiplication, metastasis, and invasion (18,19). As an important member of histidine triad gene family, the fragile histidine triad (FHIT) is a tumor suppressor gene, of which abnormal expression is related to various malignant tumors. Studies have shown that FHIT can regulate the process of cell proliferation and apoptosis (20,21).
The tumorigenesis, progression and invasion of BTCC may involve in a series of genetic factors. In the current study, our purpose was to explore the expression levels and correlation of Bmi-1, AEG-1 and FHIT in BTCC. We aimed to provide mechanism insights into Bmi-1, AEG-1 and FHIT function and support the research of therapeutic strategy that targets at these genes (or their downstream mediators of transformation and angiogenesis) by a genetic [antisense or small interfering ribonucleic acid (siRNA)] or pharmacological (small-molecule) approach, thus to develop an effective rational strategy for the therapy of BTCC.
Methods
Human clinical specimens
Forty-six cases of BC tissue samples were obtained from patients (ranged 40–80 years) diagnosed with primary BC during the first radical cystectomy between June 2010 and January 2014 in the Characteristic Medical Center of the Chinese People’s Armed Police Force Hospital. All patients were not treated with chemoradiotherapy. Meanwhile, 10 non-cancer bladder mucous membrane tissue samples in the same period were obtained from patients with no symptoms and signs of bladder cancer who had undergone the surgical treatment for benign prostatic hyperplasia. All the tissue samples were collected during surgery and further confirmed by histopathological, which were trimmed to pieces of 0.5 cm size after washed by physiological saline repeatedly. Some samples were stored in −80 °C deep freezer for quantitative real time polymerase chain reaction (qRT-PCR) and Western blot. Some samples were unbuffered formalin fixed and paraffin embedded, which were send to the specimen library for immunohistochemistry. Some samples were used to pathological diagnosis.
Of all the BC tissue samples, 16 samples were lymphatic metastasis. Histological cell type of the tumor was assigned according to the World Health Organization (WHO) classification: 24 were classified as low grade papillary urothelial carcinoma (LGPUC), 22 as high grade papillary urothelial carcinoma (HGPUC). Staging was reviewed based on the International Union Against Cancer (UICC)-tumour node metastases (TNM) staging system: 27 were stage Tis-T1, 19 were stage T2-T4.
qRT-PCR
The TRIzol reagent (Tianjin Biotechnology, Tianjin, China) was used to extract total RNA from samples, according to the manufacturer’s protocol. Then the extracted total RNA was reversely transcripted into cDNA (Complementary deoxyribonucleic acid) with PrimeScript RT-PCR kit (Takara Biotechnology, Dalian, China). The qRT-PCR reaction conditions were as follows: pre-denaturation at 95 °C for 5 s, denaturation at 95 °C for 5 s, annealing at 60 °C for 30 s, 40 cycles. β-actin was used as an internal control. The 2-ΔΔCt method was used to evaluate expression fold changes. The Bmi-1 sense primer was 5'-AATCTAAGGAGGAGGTGA-3' and the anti-sense primer was 5'-CAAACAAGAAGAGGTGGA-3'; the AEG-1 sense primer was 5'-AAATAGCCAGCCTATCAAGACTC-3', and the anti-sense primer was 5'-TTCAGACTTGGTCTGTGAAGGAG-3'; the FHIT sense primer was 5'-GCTCTTGTGAATAGGAAACC-3', and the anti-sense primer was 5'- TCACTGGTTGAAGAATACAGG-3'. For the β-actin gene, the sense primer was 5'-CGCTGCGCTGGTCGTCGACA-3', and the anti-sense primer was 5'-GTCACGCACGATTTCCCGCT-3'.
Western blot
In short, the total cellular protein was extracted with lysis buffer, followed by ice bath for 30 min and centrifuge at 12,000 ×g for 15 min at 4 °C, then the protein was quantified by bicinchoninic acid (BCA) method. Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) was used to separate 40 µg proteins, which was then transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, Massachusetts, USA). Blocking the membranes with 5% BSA (bovine serum albumin, ServiceBio, Beijing, China) for 1 hour, and then probing the membranes with antibodies against Bmi-1 (1:1,000, Sigama, Saint Louis, USA), AEG-1 (1:1,000, Sigama), FHIT (1:1,000, Sigama) and β-actin (1:1,000, Sigama) at 4 °C overnight. Washing the membranes three times with TBST and incubating the membranes with horse-radish peroxidase-conjugated second antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 hour. The protein bands were visualized by enhanced chemiluminescence (ECL) reagents (Pierce, Rockford, IL, USA). The membranes were stripped and re-probed with β-actin antibodies as the loading controls. Image J software (National Institutes of Health, Bethesda, USA) was utilized for densitometric quantification of the Western blot bands.
Immunohistochemistry
Briefly, xylene (ChuanghuaChem, Nanjing, China) was used for deparaffinization. Four mL tissue sections which were formalin-fixed and paraffin-embedded, then rehydrated in serial dilution of ethanol, and boiled (microwave) for 15 min in ethylene diamine tetraacetic acid (EDTA) buffer (pH 8.0, SolarBio, Beijing, China) for antigen retrieval of Bmi-1, AEG-1 and FHIT; 3% hydrogen peroxide (ChuanghuaChem, Nanjing, China) was used to incubate these tissue sections for 10 min to quench the activity of endogenous peroxidase, followed by incubating with goat serum to block nonspecific binding. Washed slides with PBS (phosphate buffered solution, SolarBio, Beijing, China) and incubated with polyclonal antibodies (Weiao Biotechnology, Shanghai, China) at room temperature for 1 h. Then they were reacted with biotinylated secondary antibodies (Weiao Biotechnology) for 10 min and followed by incubating with the streptavidin-horseradish-peroxidase complex. DAB (Diaminobenzidine, Weiao Biotechnology) was used on the slides as substrates after further washing. These sections were counterstained with Mayer’s hematoxylin, dehydrated with ascending concentrations of alcohol, and mounted by crystal mount. Taking place of the primary antibodies with non-immune IgG (Weiao Biotechnology) of the same isotype to be negative controls.
Statistical analysis
Statistical analysis was conducted using SPSS 20.0 (SPSS Inc, Chicago, Illinois, USA). Continuous variables were expressed as the mean ± SD. Differences between groups were evaluated by the Student’s t-test for two groups or one-way analysis of variance (ANOVA) followed by Tukey post hoc test for three groups. Categorical variables were represented as percentages and were tested by chi-square test. Relationships between Bmi-1, AEG-1 and FHIT were determined by using linear correlation coefficient. For all the tests, P<0.05 was considered as statistical significance.
Results
The messenger RNA (mRNA) expression of Bmi-1, AEG-1 and FHIT in normal tissues and BTCC tissues evaluating by qRT-PCR
The mRNA expression of Bmi-1 and AEG-1 in BTCC tissues were significantly higher than those in normal tissues (0.60±0.03 vs. 0.16±0.03, 0.58±0.03 vs. 0.14±0.03, P<0.01). Conversely the mRNA expression of FHIT was much higher in normal tissues than BTCC tissues (0.83±0.03 vs. 0.42±0.02, P<0.01). There were also significant differences in the mRNA expression of Bmi-1, AEG-1 and FHIT in different histological cell type, the differences between groups were about 1.7–1.9 times (P<0.01). The mRNA expressions of Bmi-1, AEG-1 and FHIT in BTCC patients with lymph node metastasis were significantly different compared with non-lymph node metastasis patients (P<0.01), as well. Detailed data was showed in Figure 1.
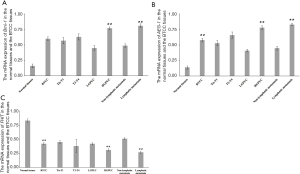
The linear correlation analysis showed that the mRNA expression of Bmi-1 was positively related to the mRNA expression of AEG-1 (r=0.90, P<0.01). The mRNA expression of Bmi-1 and AEG-1 had negative relationships with the mRNA expression of FHIT (r=−0.84, P=0.03; r=−0.89, P<0.01). Detailed data were showed in Table 1.
Table 1
| The mRNA expression | Linear correlation coefficient | ||
|---|---|---|---|
| Bmi-1 | AEG-1 | FHIT | |
| Bmi-1 | – | 0.90* | −0.84* |
| AEG-1 | 0.90* | – | −0.89* |
| FHIT | −0.84* | −0.89* | – |
*, P<0.05.
The protein expression of Bmi-1, AEG-1 and FHIT in normal tissues and BTCC tissues evaluating by Western blot
The expression levels of Bmi-1, AEG-1 and FHIT protein in BTCC tissues evaluating by Western blot were showed in Figure 2. Compared with normal tissues, the protein expression of Bmi-1 and AEG-1 in BTCC tissues were significantly higher (P<0.05). On the contrary, the protein expression of FHIT was higher in normal tissues than BTCC tissues (P<0.05). There were significant differences in the protein expression of Bmi-1, AEG-1 and FHIT in difference histological cell type (P<0.05). In BTCC patients with lymph node metastasis, the protein expression of Bmi-1, AEG-1 and FHIT were significantly different compared with non-lymph node metastasis patients (P<0.05).
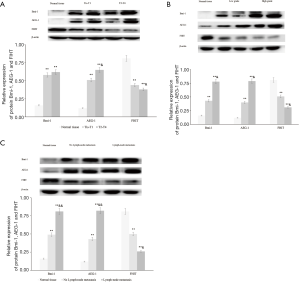
The linear correlation analysis showed that the protein expression of Bmi-1 was positively related to the protein expression of AEG-1 (r=0.94, P<0.01). The protein expression of Bmi-1 and AEG-1 had negative relationships with the protein expression of FHIT (r=−0.84, P<0.05; r=−0.97, P<0.01). Detailed data were showed in Table 2.
Table 2
| The mRNA expression | Linear correlation coefficient | ||
|---|---|---|---|
| Bmi-1 | AEG-1 | FHIT | |
| Bmi-1 | – | 0.94 | −0.84* |
| AEG-1 | 0.94* | – | −0.97* |
| FHIT | −0.84* | −0.97* | – |
*, P<0.05.
The protein expression of Bmi-1, AEG-1 and FHIT in normal tissues and BTCC tissues evaluating by immunohistochemistry
As shown in Figure 3, the Bmi-1 expression was located mainly in the nuclei of tumor cells, and the AEG-1 expression was localized within nuclear membranes or cytoplasm; the FHIT expression was closely distributed in the cytoplasm of normal cells.
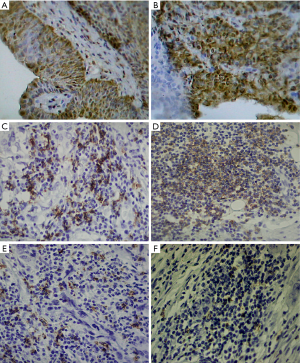
There were more samples in BTCC tissues, of which Bmi-1 and AEG-1 expressed positively, than normal tissues (P<0.01). In patients with BTCC, more proportions were positive expression of Bmi-1 and AEG-1 in HGPUC compared with LGPUC (P<0.05). The rates of positive expression of Bmi-1 and AEG-1 were higher in the patients with lymph node metastasis than the patients with non-lymph node metastasis (P<0.05). The expression of FHIT was opposite to Bmi-1 and AEG-1. Detailed data was showed in Tables 3-5.
Table 3
| Group | N | Protein expression of Bmi-1 | χ2 | P value | |
|---|---|---|---|---|---|
| Positive (%) | Negative (%) | ||||
| Pathological diagnosis | 8.51 | <0.01 | |||
| Normal tissues | 10 | 1 (10.00) | 9 (90.00) | ||
| BTCC | 46 | 28 (60.87) | 18 (39.13) | ||
| Clinical pathological stage | 0.78 | 0.28 | |||
| Tis-T1 | 27 | 15 (55.56) | 12 (44.44) | ||
| T2-T4 | 19 | 13 (68.42) | 6 (31.58) | ||
| Histological cell type | 11.51 | <0.01 | |||
| LGPUC | 24 | 9 (37.50) | 15 (62.50) | ||
| HGPUC | 22 | 19 (86.36) | 3 (13.64) | ||
| Lymphatic metastasis | 11.14 | <0.01 | |||
| No | 30 | 13 (43.33) | 17 (56.67) | ||
| Yes | 16 | 15 (93.75) | 1 (6.25) | ||
BTCC, bladder transitional cell carcinoma; LGPUC, low grade papillary urothelial carcinoma; HGPUC, high grade papillary urothelial carcinoma.
Table 4
| Group | N | Protein expression of AEG-1 | χ2 | P value | |
|---|---|---|---|---|---|
| Positive (%) | Negative (%) | ||||
| Pathological diagnosis | 10.40 | <0.01 | |||
| Normal tissues | 10 | 2 (20.00) | 8 (80.00) | ||
| BTCC | 46 | 34 (73.91) | 12 (26.09) | ||
| Clinical pathological stage | 1.78 | 0.16 | |||
| Tis-T1 | 27 | 18 (66.67) | 9 (33.33) | ||
| T2-T4 | 19 | 16 (84.21) | 3 (15.79) | ||
| Histological cell type | 6.32 | 0.01 | |||
| LGPUC | 24 | 14 (58.33) | 10 (41.67) | ||
| HGPUC | 22 | 20 (90.91) | 2 (9.09) | ||
| Lymphatic metastasis | 8.66 | <0.01 | |||
| No | 30 | 18 (60.00) | 12 (40.00) | ||
| Yes | 16 | 16 (100.00) | 0 (0.00) | ||
BTCC, bladder transitional cell carcinoma; LGPUC, low grade papillary urothelial carcinoma; HGPUC, high grade papillary urothelial carcinoma.
Table 5
| Group | N | Protein expression of FHIT | χ2 | P value | |
|---|---|---|---|---|---|
| Positive (%) | Negative (%) | ||||
| Pathological diagnosis | 7.12 | < 0.01 | |||
| Normal tissues | 10 | 9 (90.00) | 1 (10.00) | ||
| BTCC | 46 | 20 (43.48) | 26 (56.52) | ||
| Clinical pathological stage | 0.58 | 0.32 | |||
| Tis-T1 | 27 | 13 (48.15) | 14 (51.85) | ||
| T2-T4 | 19 | 7 (36.84) | 12 (63.16) | ||
| Histological cell type | 4.51 | 0.03 | |||
| LGPUC | 24 | 14 (58.33) | 10 (41.67) | ||
| HGPUC | 22 | 6 (27.27) | 16 (72.73) | ||
| Lymphatic metastasis | 6.11 | 0.01 | |||
| No | 30 | 17 (56.67) | 13 (43.33) | ||
| Yes | 16 | 3 (18.75) | 13 (81.25) | ||
BTCC, bladder transitional cell carcinoma; LGPUC, low grade papillary urothelial carcinoma; HGPUC, high grade papillary urothelial carcinoma.
Discussion
Although some genes, such as the epidermal growth factor receptor (EGFR) and human epithelial growth factor receptor 2 (HER-2) have been reported to associate with BC in previous study (22), it still remains unclear about the molecular mechanisms of the occurrence and development in BC. There are a series of genetic factors involving the above processes, including the inactivation of tumor suppressor genes and the activation of oncogenes (22-24). The current study demonstrated that the expression of Bmi-1 and AEG-1 were up-regulated and the down-regulated expression of FHIT in BTCC tissues. The interaction of Bmi-1, AEG-1 and FHIT may associate with tumorigenesis and progression of BTCC.
There is often an up-regulated of polycomb group (PcG) protein in many human cancers. As a member of the PcG family, the over-expression of Bmi-1 could repress the p19Arf targets and p16Ink4a/CDKN2A (cyclin-dependent kinase Inhibitor 2A) (25,26). Because the lack of p16Ink4a, the retinoblastoma product (pRB) can be phosphorylated by the cyclin D/Cdk4/6 complex, which allows the transcription factor (E2F)-dependent transcription that leads to the progression of cell cycle and synthesis of DNA. Ma et al. (27) found that Bmi-1 may play an important role in the progression of liver cancer by the independent pathway of p16Ink4a to regulate the cell multiplication. Furthermore, murine double minute 2 (MDM2)-mediated p53 degradation causes low p53 levels in the absence of p19Arf, thus could prevent cell cycle arrest and apoptosis. It has been proved that MDM2 and p53 can repress the transactivation of nuclear factor-κB (NF-κB) (28). Compared with normal tissues, the expression of Bmi-1 in BTCC tissues was significantly higher in the current study, which was in consistent with Qin’s study (29). There were significant differences in the expression levels of Bmi-1 in various histological cell types. Compared with non-lymph node metastasis patients, BTCC patients with lymph node metastasis showed the significantly higher expression levels of Bmi-1. Invasion and metastasis of tumor cells depend on the hydrolysis of extracellular protein matrix mediated by matrix metallopeptidase 9 (MMP-9). Yu’s study demonstrated that Bmi-1 could induce the expression and the activity of MMP-9 through the NF-κB activation in glioma, whereas blocking the activity of NF-κB drastically reduced the pro-invasive effects of Bmi-1 and prevented upregulation of MMP-9 (30). Thus, we believed that the Bmi-1 probably played a key role in the cell multiplication, tumor progression and metastasis in BTCC by the NF-κB/MMP-9 pathway.
As the Ha-Ras’s downstream target, AEG-1 has an important role in regulating the occurrence, invading, migration and angiogenesis of tumors. To be consistent with previous study, an over-expression of AEG-1 in BTCC tissues was observed in the current study (31). The over-expression of AEG-1 was also related to histological cell type. The invasive ability of the HeLa cells could be enhanced by the over-expression of AEG-1 (32). In sharp contrast, the knockdown of AEG-1 could reduce cell activity and promote apoptosis in prostatic carcinoma cells. It was reported that cervical cancer cell migration could be depressed by the down-regulation of AEG-1 (33). A study on glioma demonstrated that the AEG-1 could induced the degradation of inhibitor of NF-κB (IκB) to accelerate the activation of NF-κB, and that tumor cells proliferation was promoted eventually (34). On the other hand, the metastasis and invading of glioma cells could be enhanced by the up-regulated of AEG-1, which was promoted by MMP-9. When the AEG-1 was knockdown, their metastasis and invading would be inhibited. In summary, AEG-1 was related to the initiation, development and invasion in BTCC via the NF-κB/MMP-9 pathway.
The FHIT is located on human chromosome 3p14.2, of which protein product is 16.8 kDa in size (35). The protein can involve in the process of cell cycle and DNA repair. Previous study showed that the growth of human colon cancer cell line can be significantly inhibited by FHIT protein and finally promoted apoptosis (36). Our study demonstrated the down-regulated expression of FHIT in BTCC tissues. And the down-regulation of FHIT had relationship with histological cell types and lymphatic metastasis. When FHIT was transfected into lung carcinoma cells which was in absent of FHIT gene, the lung carcinoma cell cycle at G0/G1 phase was blocked and the cells’ apoptosis was promoted (37). In colon cancer cell lines, FHIT protein inhibited cell growth by attenuating the signaling mediated by NF-κB (38). It was demonstrated that FHIT silencing in bronchial cells could regulate cell invasion though inducing the overexpression of MMP-9 (39). We cherish the belief that FHIT also take part in the regulating the process of cell cycle and cell apoptosis in BTCC through the NF-κB/MMP-9 pathway.
In addition, we also analyzed the correlation among Bmi-1, AEG-1 and FHIT. A positive relationship between the expression of Bmi-1 and AEG-1 was observed. The aggressiveness of glioma could be promoted by Bmi-1 activating the NF-κB/MMP-9 signaling pathway (40). AEG-1 was also involved in this pathway (41). Therefore, Bmi-1 and AEG-1 may promote the tumorigenesis, progression and invasion of BTCC synergistically. FHIT could also cause cell apoptosis and metastasis through NF-κB/MMP-9 pathway, which may explain why the expression of FHIT was negatively related to the expression of Bmi-1 and AEG-1 in our study.
Conclusions
The tumorigenesis, progression and invasion of BTCC were complex processes which may involve a variety of genes’ interactive effects. This study demonstrated that the expression of Bmi-1 and AEG-1 were up-regulated and the expression of FHIT was down-regulated in BTCC. The expression of Bmi-1 was positively related to the expression AEG-1, and the expression of FHIT had a negative correlation with the expression of Bmi-1 and AEG-1 in BTCC. It is believed that there may be common pathways and regulatory factors in the three genes, which may provide a new idea for gene therapy of BTCC. The present study provides mechanism insights into Bmi-1, AEG-1 and FHIT function and supports the research of therapeutic strategy which targeted at these genes (or their downstream mediators of transformation and angiogenesis) by a genetic (antisense or siRNA) or pharmacological (small-molecule) approach to develop an effective rational strategy for the therapy of BTCC.
Acknowledgments
Funding: This study was funded by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.01.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the Characteristic Medical Center of the Chinese People’s Armed Police Force Hospital (No. wjtsmc089) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stojnev S, Ristic-Petrovic A, Velickovic LJ, et al. Prognostic significance of mucin expression in urothelial bladder cancer. Int J Clin Exp Pathol 2014;7:4945-58. [PubMed]
- Antoni S, Ferlay J, Soerjomataram I, et al. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol 2017;71:96-108. [Crossref] [PubMed]
- Md Noh MS, Abdul Aziz AF, Mohd Ghani KA, et al. Giant Intradiverticular Bladder Tumor. Am J Case Rep 2017;18:212-6. [Crossref] [PubMed]
- Chou R, Gore JL, Buckley D, et al. Urinary Biomarkers for Diagnosis of Bladder Cancer: A Systematic Review and Meta-analysis. Ann Intern Med 2015;163:922-31. [Crossref] [PubMed]
- Sverrisson EF, Espiritu PN, Spiess PE. New therapeutic targets in the management of urothelial carcinoma of the bladder. Res Rep Urol 2013;5:53-65. [PubMed]
- Seiler R, Oo HZ, Tortora D, et al. An Oncofetal Glycosaminoglycan Modification Provides Therapeutic Access to Cisplatin-resistant Bladder Cancer. Eur Urol 2017;72:142-50. [Crossref] [PubMed]
- Haupt Y, Alexander WS, Barri G, et al. Novel zinc finger gene implicated as myc collaborator by retrovirally accelerated lymphomagenesis in E mu-myc transgenic mice. Cell 1991;65:753-63. [Crossref] [PubMed]
- van Lohuizen M, Verbeek S, Scheijen B, et al. Identification of cooperating oncogenes in E mu-myc transgenic mice by provirus tagging. Cell 1991;65:737-52. [Crossref] [PubMed]
- Hu C, Zhang Q, Tang Q, et al. CBX4 promotes the proliferation and metastasis via regulating BMI-1 in lung cancer. J Cell Mol Med 2020;24:618-31. [Crossref] [PubMed]
- Wang LQ, Wang Y, Jin H, et al. Expressions of SALL4, Bmi-1 and p27 and their correlation in laryngeal squamous cell carcinoma. J Biol Regul Homeost Agents 2019;33:1533-8. [PubMed]
- Wang J, Xing Y, Wang Y, et al. A novel BMI-1 inhibitor QW24 for the treatment of stem-like colorectal cancer. J Exp Clin Cancer Res 2019;38:422. [Crossref] [PubMed]
- Jiao K, Jiang W, Zhao C, et al. Bmi-1 in gallbladder carcinoma: Clinicopathology and mechanism of regulation of human gallbladder carcinoma proliferation. Oncol Lett 2019;18:1365-71. [PubMed]
- Emdad L, Sarkar D, Su ZZ, et al. Activation of the nuclear factor kappaB pathway by astrocyte elevated gene-1: implications for tumor progression and metastasis. Cancer Res 2006;66:1509-16. [Crossref] [PubMed]
- Zhang Y, Li ZY, Hou XX, et al. Clinical significance and effect of AEG-1 on the proliferation, invasion, and migration of NSCLC: a study based on immunohistochemistry, TCGA, bioinformatics, in vitro and in vivo verification. Oncotarget 2017;8:16531-52. [PubMed]
- Gnosa S, Capodanno A, Murthy RV, et al. AEG-1 knockdown in colon cancer cell lines inhibits radiation-enhanced migration and invasion in vitro and in a novel in vivo zebrafish model. Oncotarget 2016;7:81634-44. [Crossref] [PubMed]
- Park SY, Choi M, Park D, et al. AEG-1 promotes mesenchymal transition through the activation of Rho GTPases in human glioblastoma cells. Oncol Rep 2016;36:2641-6. [Crossref] [PubMed]
- Ren F, Ding H, Huang S, et al. Expression and clinicopathological significance of miR-193a-3p and its potential target astrocyte elevated gene-1 in non-small lung cancer tissues. Cancer Cell Int 2015;15:80. [Crossref] [PubMed]
- Liang X, Li H, Fu D, et al. MicroRNA-1297 inhibits prostate cancer cell proliferation and invasion by targeting the AEG-1/Wnt signaling pathway. Biochem Biophys Res Commun 2016;480:208-14. [Crossref] [PubMed]
- Wang Y, Zhang W, Zhu X, et al. Upregulation of AEG-1 Involves in Schwann Cell Proliferation and Migration After Sciatic Nerve Crush. J Mol Neurosci 2016;60:248-57. [Crossref] [PubMed]
- Fu Y, Shan X, Song W, et al. Correlations of breast cancer FHIT gene with the incidence and prognosis of breast cancer. J buon 2019;24:40-7. [PubMed]
- Kiss DL, Baez W, Huebner K, et al. Impact of FHIT loss on the translation of cancer-associated mRNAs. Mol Cancer 2017;16:179. [Crossref] [PubMed]
- Li W, Wang Y, Tan S, et al. Overexpression of Epidermal Growth Factor Receptor (EGFR) and HER-2 in Bladder Carcinoma and Its Association with Patients' Clinical Features. Med Sci Monit 2018;24:7178-85. [Crossref] [PubMed]
- Ye F, Wang L, Castillo-Martin M, et al. Biomarkers for bladder cancer management: present and future. Am J Clin Exp Urol 2014;2:1-14. [PubMed]
- Zhang L, Yang G, Chen H, et al. Depletion of astrocyte elevated gene-1 suppresses tumorigenesis through inhibition of Akt activity in bladder cancer cells. Am J Transl Res 2017;9:5422-31. [PubMed]
- Wang X, Wang C, Zhang X, et al. Bmi-1 regulates stem cell-like properties of gastric cancer cells via modulating miRNAs. J Hematol Oncol 2016;9:90. [Crossref] [PubMed]
- Zhang X, Tian T, Sun W, et al. Bmi-1 overexpression as an efficient prognostic marker in patients with nonsmall cell lung cancer. Medicine (Baltimore) 2017;96:e7346. [Crossref] [PubMed]
- Ma DQ, Zhang YH, Ding DP, et al. Effect of Bmi-1-mediated NF-kappaB signaling pathway on the stem-like properties of CD133+ human liver cancer cells. Cancer Biomark 2018;22:575-85. [Crossref] [PubMed]
- Hientz K, Mohr A, Bhakta-Guha D, et al. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget 2017;8:8921-46. [Crossref] [PubMed]
- Qin ZK, Yang JA, Ye YL, et al. Expression of Bmi-1 is a prognostic marker in bladder cancer. BMC Cancer 2009;9:61. [Crossref] [PubMed]
- Yu GH, Li AM, Li X, et al. Bispecific antibody suppresses osteosarcoma aggressiveness through regulation of NF-kappaB signaling pathway. Tumour Biol 2017;39:1010428317705572. [Crossref] [PubMed]
- Yang G, Zhang L, Lin S, et al. AEG-1 is associated with tumor progression in nonmuscle-invasive bladder cancer. Med Oncol 2014;31:986. [Crossref] [PubMed]
- Zhang X, Cai D, Meng L, et al. MicroRNA-124 inhibits proliferation, invasion, migration and epithelial-mesenchymal transition of cervical carcinoma cells by targeting astrocyte-elevated gene-1. Oncol Rep 2016;36:2321-8. [Crossref] [PubMed]
- Liu X, Wang D, Liu H, et al. Knockdown of astrocyte elevated gene-1 (AEG-1) in cervical cancer cells decreases their invasiveness, epithelial to mesenchymal transition, and chemoresistance. Cell Cycle 2014;13:1702-7. [Crossref] [PubMed]
- Emdad L, Sarkar D, Lee SG, et al. Astrocyte elevated gene-1: a novel target for human glioma therapy. Mol Cancer Ther 2010;9:79-88. [Crossref] [PubMed]
- Xu Z, Wu J, Cai P, et al. Effects of FHIT gene on proliferation and apoptosis of osteosarcoma cells. Oncol Lett 2019;17:877-82. [PubMed]
- Morikawa H, Nakagawa Y, Hashimoto K, et al. Frequent altered expression of fragile histidine triad protein in human colorectal adenomas. Biochem Biophys Res Commun 2000;278:205-10. [Crossref] [PubMed]
- Hu B, Ying X, Wang J, et al. Identification of a tumor-suppressive human-specific microRNA within the FHIT tumor-suppressor gene. Cancer Res 2014;74:2283-94. [Crossref] [PubMed]
- Nakagawa Y, Akao Y. Fhit protein inhibits cell growth by attenuating the signaling mediated by nuclear factor-kappaB in colon cancer cell lines. Exp Cell Res 2006;312:2433-42. [Crossref] [PubMed]
- Joannes A, Grelet S, Duca L, et al. Fhit regulates EMT targets through an EGFR/Src/ERK/Slug signaling axis in human bronchial cells. Mol Cancer Res 2014;12:775-83. [Crossref] [PubMed]
- Jiang L, Wu J, Yang Y, et al. Bmi-1 promotes the aggressiveness of glioma via activating the NF-kappaB/MMP-9 signaling pathway. BMC Cancer 2012;12:406. [Crossref] [PubMed]
- Huang LL, Wang Z, Cao CJ, et al. AEG-1 associates with metastasis in papillary thyroid cancer through upregulation of MMP2/9. Int J Oncol 2017;51:812-22. [Crossref] [PubMed]


