Postoperative diffused alveolar hemorrhage complicated by pneumonia after lung cancer surgery in a patient with liver cirrhosis: a case report and review of literatures
Introduction
Liver cirrhosis is an aggravated stage of chronic inflammation in the liver when hepatocytes gradually progress to severe fibrosis with repeated destruction and proliferation. Despite the notable advances in surgery, studies showed that in patients undergoing abdominal surgery, liver cirrhosis is considered a critical threat carrying a morbidity and mortality boost (1,2). Generally, surgery in patients with severely compromised hepatic function might precipitate hepatic decompensation and is usually followed by a serious postoperative course (3). For another, lung cancer is featured with an additional death rate and a shorter life expectancy than liver cirrhosis even with hepatoma. Hence, with the management of perioperative cirrhosis-related complications, treatment for lung cancer might have a bearing on overall survival and would benefit the patients with both pulmonary carcinoma and cirrhosis. We report a case of a patient with severe cirrhosis who was successfully treated for lung cancer, followed by left superior segmentectomy and right superior wedge resection. This is the first case report to show a successfully staged perioperative strategy.
Case presentation
A 54-year-old man was admitted to a local hospital with discomfort in the hepatic region. He had been diagnosed with “cirrhosis, hypersplenism and portal hypertension” for many years. A preadmission CT scan showed multiple nodules at S4 and S5 in the liver that were considered to be hepatocellular carcinoma. Interventional surgery was conducted to manage the liver nodules. He was diagnosed with multiple early stage lung tumors and was referred to our hospital for surgery. The local hospital hematological laboratory data showed a significant decrease in platelets and fibrin. Thus, before admission to our hospital, he had been treated with thrombopoietin and cryoprecipitate. The patient’s hematological laboratory data after medical treatment at admission were as follows: white blood cell count, 6.07×109/L [normal range (NR) =3.5–9.5×109/L); hemoglobin, 124 g/L (NR =130–175 g/L); platelet count, 297×109/L (NR =125–350×109/L); total bilirubin, 29.3 µmol/L (NR =5.1–19.0 µmol/L); direct bilirubin, 11.8 µmol/L (NR =0.0–6.8 µmol/L); total serum protein, 59.5 g/L (NR =65.0–85.0 g/L); albumin, 32.7 g/L (NR =40.0–55.0 g/L); prothrombin time, 15.40 s (NR =8–14 s); partial thromboplastin activation time, 33.90 s (NR =25–31.3 s), carcinoembryonic antigen (CEA), 4.89 ng/mL (NR <4.7 ng/mL), carbohydrate antigen 199 (CA19-9), 269.10 U/mL (NR <39 U/mL), carbohydrate antigen 724 (CA72-4), 7.19 U/mL (NR <6.9 U/mL), cytokeratin 19 fragment (CYFRA21-1), 6.30 ng/mL (NR <3.30 ng/mL), and hepatitis B virus DNA <5.00E+02 IU/mL (NR <5.00E+02 IU/mL). Preoperative liver function was evaluated as Child-Pugh classification grade B, with a Child-Pugh score of 8 points.
Preadmission PET/CT showed hybrid ground glass density nodules in the posterior segment of the left upper lung that was 1.4×1.7 cm2 in size, and the anterior segment of the right upper lobe was 1.1×0.7 cm2 in size. There was no evidence of lymph node metastases or distant metastases. The preoperative diagnosis was lung cancer: clinical T1 N0 M0 stage IA on both sides, according to the TNM classification (4). We decided to perform surgery with the consultation of a hepatologist and infectious disease experts to exclude surgical contradictions. Left superior segmentectomy and right superior wedge resection were performed. A rapid operative pathological diagnosis indicated adenocarcinomas on both sides. The amount of bleeding was 200 mL, and the length of operation was 3 hours and 10 minutes. No blood transfusions were performed. Histopathological examination of the resected specimen revealed an invasive adenocarcinoma on the left and a well-differentiated adenocarcinoma on the right, both with negative margins and no evidence of metastasis in the lymph nodes (pT1bN0M0), including stations 2, 3, 4 and 7.
Six hours later, when the patient was transferred to the intensive care unit, the left thoracic duct effused 1,200 mL sanguineous drainage with an increased heart rate and hypotension. Plasma, red blood cells, platelets, and cryoprecipitate were repeatedly transfused into the patient to maintain vital signs. Atrial fibrillation and dyspnea occurred on POD1 after the surgery, which was diagnosed as acute respiratory distress syndrome (ARDS) and diffused alveolar hemorrhage (DAH). Stale blood clots were found in the phlegm after violent coughing.
The patient’s hematological laboratory data on POD2 postoperative were as follows: white blood cell count, 13.2×109/L (NR =3.5–9.5×109/L); hemoglobin, 66 g/L (NR =130–175 g/L); platelet count, 123×109/L (NR =125–350×109/L); prothrombin time, 15.80 s (NR =8–14 s); partial thromboplastin activation time, 35.40 s (NR =25–31.3 s), fibrinogen, 1.45 g/L (NR =2.00–4.00 g/L); total bilirubin, 33.7 µmol/L (NR =5.1–19.0 µmol/L); direct bilirubin, 15.3 µmol/L (NR =0.0–6.8 µmol/L); total serum protein, 50.1 g/L (NR =65.0–85.0 g/L); and albumin, 33.3 g/L (NR =40.0–55.0 g/L). The postoperative CT scan taken on POD4 showed that patchy infiltrates were scattered throughout both lungs.
Noninvasive ventilator-assisted ventilation was performed to improve oxygenation and enhance the partial pressure of oxygen when the patient’s pulse oxygen saturation dropped to 85% (Figure 1). Sanguineous drainage still effused from the left thoracic duct, and plasma and red blood cells were transfused. However, heart failure and pulmonary edema emerged overnight. Standard heart failure treatments and low-dose steroids that manipulated pulmonary edema were utilized.
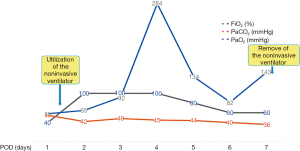
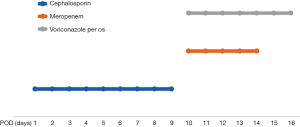
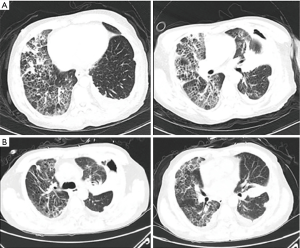
Left thoracic duct drainage gradually diminished on POD7. The patient’s oxygenation and partial pressure of oxygen improved daily with ventilation from a noninvasive ventilator. The results of sputum culture on POD10 showed Klebsiella pneumoniae and Candida albicans, for which meropenem and voriconazole were employed to control the infection (Figure 2). Chest CT reexamination on POD13 (Figure 3A,B) showed that fewer patchy infiltrates were scattered throughout both lungs than had been shown previously. Repeated sputum culture on POD14 showed that Candida albicans existed, and voriconazole per os was advised. The patient was discharged from the hospital on POD16. The patient’s postdischarge recovery was unremarkable, and at the 3-month follow-up, he had no evidence of recurrent disease.
Discussion
Perioperative mortality and morbidity remain high for patients with decompensated cirrhosis owing to the stress of anesthesia and surgical operation, notwithstanding intensive care has achieved significant advances (5). Three reasons have been reported for patients with liver cirrhosis adopted surgical procedures, who might have varied metabolic dysfunctions: diminished coagulation, inferior tolerance to invasive surgical interventions (6) and increased susceptibility to infection (7,8). Simultaneously, cirrhotic patients after surgery might sustain three major complications from these problems: liver failure could be exacerbated in patients with cirrhosis when the impaired liver function is deteriorated by surgical stress postoperatively, accompanied by hepatic encephalopathy, ascites and jaundice (3); operative field may acutely bleed (9); and malnutrition and metabolic anomaly may instigate postoperative severe infection due to the immune system disorder (10). Thus, liver cirrhosis-related events is a critical condition, which 50–90% of patients would die of, and the life expectancy of patients with cirrhosis is approximately 40% of those with the same age in the general population (11).
Thoracic surgery may be less invasive in liver cirrhosis than open abdominal surgery. Iwasaki et al. reported 4 liver failure deaths among 17 cirrhotic patients underwent pulmonary resection for cancer (11). Studies have shown that the preoperative serum total bilirubin level, not other factors like platelet count and prothrombin level, was the only predictive factor for postoperative liver failure, which in our case was slightly higher than normal range (12).
Pulmonary complications caused by cirrhosis in our case remained a challenge for perioperative recovery. DAH, one rare but life-threatening cirrhosis related emergency, has been reported to be associated with hematopoietic stem cell transplantation (1–5%) (13). DAH presents with acute alveolar infiltration, hypoxemia and progressive bloody alveolar lavage. For patients undergoing lung resection, DAH is usually supposed to be the consequence of lesion to the pulmonary microvasculature, coagulation disorders, infections even autoimmune diseases. Tachypnoea, hypoxemia, pyrexia and anemia are typical clinical manifestations of DAH. Imageology studies usually reveal a bilateral interstitial infiltration or ground glass opacification, which spares most of the apices and peripheral parts of the lungs (14). The diagnosis of DAH is made upon clinical, radiological and bronchoscopy findings when necessary, as also seen in our patient, which include acute respiratory failure, hemoglobin sudden decline, the presence of ground glass opacification indicating bilateral pulmonary infiltrates and blood clots in the phlegm after coughing.
The management of DAH with concurrent respiratory failure consists of three main ethics: supportive respiratory treatment, a combination of antimicrobial agents, and early rehabilitation practice. In our case, a noninvasive ventilator was utilized to support respiration. Due to severe lung tissue impairments after resection, microvascular vasodilatation and hyperemia lead to the exudation of gross erythrocytes and plasmocytes in the alveolus, which results in pulmonary regional hemodynamic disturbances and ventilation-perfusion mismatches, finally developing into fatal hypoxemia (15,16). Studies have demonstrated that noninvasive ventilators, especially PEEPs, can improve air exchange in DAH patients: noninvasive ventilation (I) accelerates the flow of interstitial fluid in the alveolus into the vascular wall through the augmentation of alveolar pressure and interstitial hydrostatic pressure (17,18); (II) dilates collapsed alveoli and eliminates the need for intrapulmonary shunt (18); (III) increases functional residual capacity and lung compliance (19); and (IV) generally reduces pulmonary blood flow, pulmonary hemorrhage and ventilation-perfusion imbalance (20). Although invasive positive pressure mechanical ventilation can achieve the same purpose, an artificial airway needs to be established, which will cause airway damage and increase bleeding tendency.
Two possible infections were cultured from sputum culture: Klebsiella pneumoniae and Candida albicans. Combinations of the broad-spectrum antibiotics meropenem and voriconazole were administered to address this problem. After several days, septicemia gradually resolved, and we only proceeded with long-term voriconazole per os for candidiasis.
The last, but not least important, strategy was early rehabilitation practice. From POD1, the patient was encouraged to exercise respiratory function via coughing and to promote lung recruitment, which could expel residual blood clots in the lungs and reduce the impact of DAH on pulmonary function.
Conclusions
In conclusion, patients with cirrhosis can both tolerate and benefit from surgical treatment for lung cancer. Strict perioperative analyses of patients’ liver function and systemic status and preparations such as blood and plasma transfusions are important for minimizing perioperative cirrhosis-related complications. Our distinct experience with this case suggests that cirrhosis-induced DAH should be considered in the differential diagnosis of early pulmonary complications after pulmonary surgery. Early diagnosis and proper but not aggressive treatment are crucial to achieving a favorable outcome (21).
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure from (available at http://dx.doi.org/10.21037/tcr.2020.01.65). The authors have no conflicts of interests to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Newman KL, Johnson KM, Cornia PB, et al. Perioperative Evaluation and Management of Patients with Cirrhosis: Risk Assessment, Surgical Outcomes, and Future Directions. Clin Gastroenterol Hepatol 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Reverter E, Cirera I, Albillos A, et al. The prognostic role of hepatic venous pressure gradient in cirrhotic patients undergoing elective extrahepatic surgery. J Hepatol 2019;71:942-50. [Crossref] [PubMed]
- Iwata T, Inoue K, Nishiyama N, et al. Long-term outcome of surgical treatment for non-small cell lung cancer with comorbid liver cirrhosis. Ann Thorac Surg 2007;84:1810-7. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Hickman L, Tanner L, Christein J, et al. Non-Hepatic Abdominal Surgery in Patients with Cirrhotic Liver Disease. J Gastrointest Surg 2019;23:634-42. [Crossref] [PubMed]
- Stravitz RT. Algorithms for managing coagulation disorders in liver disease. Hepatol Int 2018;12:390-401. [Crossref] [PubMed]
- Kujovich JL. Coagulopathy in liver disease: a balancing act. Hematology Am Soc Hematol Educ Program 2015;2015:243-9. [Crossref] [PubMed]
- Fernandez J, Tandon P, Mensa J, et al. Antibiotic prophylaxis in cirrhosis: Good and bad. Hepatology 2016;63:2019-31. [Crossref] [PubMed]
- Iwata T, Inoue K, Nishiyama N, et al. Factors predicting early postoperative liver cirrhosis-related complications after lung cancer surgery in patients with liver cirrhosis. Interact Cardiovasc Thorac Surg 2007;6:720-30. [Crossref] [PubMed]
- Lee JH, Yu CS, Lee JL, et al. Factors affecting the postoperative morbidity and survival of patients with liver cirrhosis following colorectal cancer surgery. Int J Colorectal Dis 2017;32:521-30. [Crossref] [PubMed]
- Iwasaki A, Shirakusa T, Okabayashi K, et al. Lung cancer surgery in patients with liver cirrhosis. Ann Thorac Surg 2006;82:1027-32. [Crossref] [PubMed]
- Toyoda H, Lai PB, O'Beirne J, et al. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: application of the ALBI grade. Br J Cancer 2016;114:744-50. [Crossref] [PubMed]
- Keklik F, Alrawi EB, Cao Q, et al. Diffuse alveolar hemorrhage is most often fatal and is affected by graft source, conditioning regimen toxicity, and engraftment kinetics. Haematologica 2018;103:2109-15. [Crossref] [PubMed]
- Kao KC, Hu HC, Chang CH, et al. Diffuse alveolar damage associated mortality in selected acute respiratory distress syndrome patients with open lung biopsy. Crit Care 2015;19:228. [Crossref] [PubMed]
- Feltracco P, Carollo C, Barbieri S, et al. Early respiratory complications after liver transplantation. World J Gastroenterol 2013;19:9271-81. [Crossref] [PubMed]
- Levesque E, Hoti E, Azoulay D, et al. Pulmonary complications after elective liver transplantation-incidence, risk factors, and outcome. Transplantation 2012;94:532-8. [Crossref] [PubMed]
- Britton D, Hoit JD, Benditt JO, et al. Swallowing with Noninvasive Positive-Pressure Ventilation (NPPV) in Individuals with Muscular Dystrophy: A Qualitative Analysis. Dysphagia 2019; [Epub ahead of print]. [PubMed]
- Seyfi S, Amri P, Mouodi S. New modalities for non-invasive positive pressure ventilation: A review article. Caspian J Intern Med 2019;10:1-6. [PubMed]
- Nam H, Cho JH, Choi EY, et al. Current Status of Noninvasive Ventilation Use in Korean Intensive Care Units: A Prospective Multicenter Observational Study. Tuberc Respir Dis (Seoul) 2019;82:242-50. [Crossref] [PubMed]
- Shioji N, Kanazawa T, Iwasaki T, et al. High-flow Nasal Cannula Versus Noninvasive ventilation for Postextubation Acute Respiratory Failure after Pediatric Cardiac Surgery. Acta Med Okayama 2019;73:15-20. [PubMed]
- Nugroho A, Lee KW, Kim H, et al. Challenging Alveolar Hemorrhage Complicating Pneumonia After Liver Transplantation: A Case Report. Transplant Proc 2018;50:4046-9. [Crossref] [PubMed]