
Cerebral metastases of parathyroid carcinoma: a case report and literature review
Background
As a rare endocrine tumor, parathyroid carcinoma (PC) accounts for 1% of parathyroid gland tumors and less than 1% of primary hyperparathyroidism (PHPT) (1). The typical PC histology shows that tumor cells are arranged in nests and separated by thin fibrous bands with multiple thin-walled capillaries (2). PC clinical manifestations are dominated by an excessive secretion of parathormone [human parathyroid hormone (PTH)] with symptoms ranging from none to severe hypercalcemia, or para thyrotoxicosis, usually including bone pain, pathological fractures, urinary stones, hematuria, or peptic ulcers (3).
Among metastatic PC cases, more than 25% were reported to involve lungs, but occasionally also involve the bones, liver, pleura, or pancreas (4). PCs intracranial metastases are extremely rare. For those patients presented in the emergency department, for brain occupying symptoms and without a cancer history, the diagnosis remains a challenge for the clinicians due to the limited clinical information. In this study, we report one of these rare cases.
Case presentation
On May 23, 2018, a 60-year-old woman, with no cancer history, presented at the emergency department with headache, nausea and vomiting. Using computed tomography (CT) scan analysis, at the local hospital, we identified an evident mass in the right frontal lobe of the brain. Since PC was not initially suspected, only limited laboratory tests were performed. The patient calcium blood level, analyzed by gas analysis, was 1.75 mmol/L (our reference interval: 1.15–1.29 mmol/L). The patient chest X-ray showed that the soft tissue density, at the upper end of the esophagus, was approximatively 31 mm × 21 mm at maximum dimension. A contrast-enhanced CT scan and a magnetic resonance imaging (MRI) of the head confirmed the lesion at the right frontal lobe and showed the presence of surrounding oedema, a mass effect and a solid mixed cyst, measuring 53 mm × 40 mm at the maximum dimension (Figure 1). The patient underwent tumor resection on May 28 and discharged after one week. On May 2019, the subject went to their local hospital and resected the primary lesion. The serum calcium and PTH levels were 2.70 mmol/L (our reference interval: 2.15–2.57 mmol/L) and 458.3 pg/mL (our reference interval: 12–88 pg/mL), respectively. The postoperative pathology analysis confirmed the diagnosis of PC. Indeed, the patient’s serum PTH and calcium levels returned towards baselines after tumor resection.
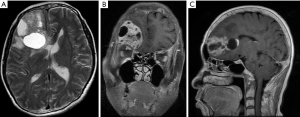
Investigation
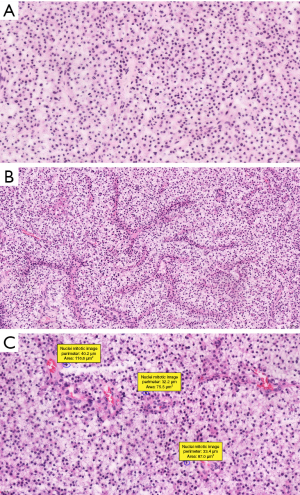
Morpho-pathological analysis
The cross-examination of the brain lesion demonstrated the presence of a grayish solid mass, measuring 6.0×3.5×2.5 cm3. Both frozen and unfrozen resected specimens were processed in 10% neutral-buffered formalin fixation, paraffin embedded, sectioned and stained with hematoxylin and eosin (HE). A microscopic examination demonstrated that the tumor was composed of relatively consistent cells, which were organized in nests and separated by thin fibrous bands and abundant blood vessels. Most tumor cells had ample and clear, or faint eosinophilic cytoplasm with a round to ovoid nuclei, containing dense chromatin and inconspicuous nucleoli. We registered 5 mitoses per ten high power fields, and no capsular or probable vascular space invasions, were detected (Figure 2). In the second surgical specimen, the tumors demonstrated irregular cell nests of vacuolated and clear neuroendocrine cells. These tumor cells had abundant and clear, or eosinophilic cytoplasm, with rounded nuclei and low mitotic activity. The morpho-pathological analysis reveals similarities between the two specimens of the two resections.
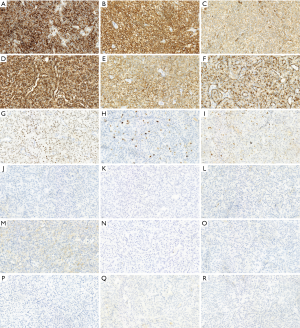
Immunohistochemistry
The EnVision method was used on the 5 µm paraffin-embedded tissue sections. In brief, the sections were preprocessed in 10 mM citrate buffer solution (pH 6.0), treated with 0.3% hydrogen peroxide in methanol for 30 min, and subsequently washed in phosphate-buffered saline. Then, the sections were incubated overnight at 4 °C with the following antibodies: PTH [working solution (WS), ZM-0268, Roche], oligodendrocyte transcription factor 2 (OLIG2; WS, ZA-0561, Roche), antigen Ki67 (KI67; WS, ZA-0187, Roche), glial fibrillary acidic protein (GFAP; WS, ZA-0529, Roche), paired-box gene 8 (PAX8; WS, ZM-0468, Roche), carbonic anhydrase 9 (CA9; WS, ZM-0161, Roche), endothelial transcription factor 3 (GATA3; WS, ZA-0661, Roche), alpha-methylacyl coenzyme A racemase (AMACR; WS, ZM-0227, Roche), synaptophysin (SYN; WS, ZM-0246, Roche), chromogranin-A (CHGA; WS, ZM-076, Roche), S-100 proteins (S-100; WS, ZA-0225, Roche), vimentin (Vimentin; WS, ZA-0511, Roche), spectrum of cellular keratin (CK-PAN; WS, ZM-0069, Roche), common acute lymphoblastic leukemia antigen (CALLA; WS, ZA-0526, Roche), epithelial membrane antigen (EMA; WS, Kit-0011, Roche), Caudal-related Homeobox Transcription Factor 2 (CDX-2; WS, RMA-0631, Roche), somatostatin receptor 2 (SSTR2; WS, ZA-0578, Roche), thyroid transcription factor-1 (TTF-1; WS, ZM-0270, Roche). After washing excess antibodies, hybridizations with corresponding secondary antibodies (Roche, Germany) were performed for 20 min at 37 °C and hematoxylin or DAB (WS, Kit-0014) staining was applied. Immunohistochemistry demonstrated that the tumor cells were positive for GATA3, PTH, CK-PAN, Vimentin, SYN, PAX-8, CA9, CHGA and AMACR, but negative for CALLA, OLIG2, SSTR2, S100, GFAP, EMA, TTF-1 and CDX-2. The proliferation index using Ki 67 showed an approximatively 10% positivity in the tumor cells, p53 shows about 20% (Figure 3). The results of immunohistochemistry of the second surgical specimen showed similar results.
Discussion
PCs are rarely non-functional and their main clinical manifestations include hyperparathyroidism, or hypercalcemia. PCs grow slowly, and at early stages, lack typical symptoms. The physical examination exhibits a palpable neck mass in 30 to 75% of PC patients. Approximately 50% of patients relapse within three years after diagnosis. In this report, we describe a case of a 60-year-old woman presented with brain metastasis originated from a PC, who solely presented symptoms of a space-occupying lesion of the brain. Although one-third of PC patients have metastases, intracranial metastases rarely occur, and only 10 cases were previously reported (Table 1) (1,2,4-12). In those cases, the mean age at diagnosis, was 55 years (range, 27–61 years) regardless of gender. Only one case displayed a single metastasis to the brain; whereas, the others showed multi-organ metastases, encompassing lungs, and/or lymph nodes. In the reported case, the primary lesion was impalpable, and the brain occupying symptoms were the main manifestation.
Table 1
| No. | Age, sex | Serum calcium at presentation (mg/dL) | PTH at presentation (pg/mL) | Sites of metastases | Location in the brain | Survival after diagnosis of HPT (months) | Survival-after diagnosis-of intracranial metastasis (months) | Author, year |
|---|---|---|---|---|---|---|---|---|
| 1 | 27, M | 9.2-9.8 | NR | Lymph nodes, lung, brain | NR | 27 | 58 | Aldinger et al., 1982 |
| 2 | 45, M | 12.9 | NR | Lymph nodes, lung, brain | Right-occipital lobe | 117 (alive) | 5 (alive) | Yamamoto et al., 1996 |
| 3 | 44, M | 15.2 | 467 | Brain | Left parasagittal front parietal | 61 | 13 | Tyler et al., 2001 |
| 4 | 45, F | NR | NR | Lung, brain | The sellar region | 45 | 31 | Eurelings et al., 2002 |
| 5 | 35, F | 17.8 | NR | Lung, brain | Multiple | 38 | 0 | Kar et al., 2003 |
| 6 | 54, F | 10.76 | 271 | Lymph nodes, lung, brain | Right frontal lobe | 29 | 8 | Kern et al., 2004 |
| 7 | 61, F | 13.2 | 730 | Lung, brain | Right frontal lobe | 19 | 6 | Yoshida et al., 2006 |
| 8 | 49, M | 15.2 | 475 | Lymph nodes, lung, brain, bone | Left cerebellar | 1 | 0 | Barker et al., 2014 |
| 9 | 62, F | 11.1 | 37 | Lymph nodes, lung, brain, right orbit | multiple in both hemispheres | 18 | 2 | Studentova et al., 2015 |
| 10 | 49, M | 22.3 | 3,560.7 | Thyroid, lung, brain, surrounding soft tissue | Multiple in left occipital lobe | 4 | 4 | Sadacharan et al., 2017 |
| 11 | 80, F | 7 | NR | Brain, bone | Right frontal lobe | 12 (alive) | 12 (alive) | Current report |
Note: the patient’s survival time was as reported in the article. NR, not reported.
Most PCs display abundant band-forming fibrosis, with multiple thin-walled capillaries, tumor cells with dense chromatin, and mitosis that can be found in 50% of malignant tumors, which usually contain ≥5 mitoses per 10 high power fields. In this case, some tumor cells were arranged in nests, mitoses were frequent, but fibrous cords were not seen. The diagnosis of malignancy should be restricted to tumors, with evidence of invasive growth involving adjacent structures, such as capsular and/or extracapsular blood vessels, or perineural spaces and/or those with documented metastases (13).
Morphologically, PCs could resemble other endocrine tumors, such as pituitary carcinoma, metastases of well-differentiated neuroendocrine tumors of the gastrointestinal tract or lung, and metastases of the renal cell carcinoma. Immunohistochemistry contributes in distinguishing these tumors, since the positivity to CHGA, SYP and CD56, and most importantly to PTH and GATA3, are characteristic of PCs. Recently, Yu et al. showed that PTH and CHGA are useful markers for the diagnosis of parathyroid neoplasms; while, SYP and INSM1 lack sensitivity (14). A parathyroid tumor is usually positive for PAX8, GATA3, and OLIG2 (14,15). In the absence of PHPT symptoms, PC is difficult to diagnose by radiology. In fact, in the present case, the diagnostic options initially included glioma, metastasis of unknown origin, and meningioma.
The etiology of PC is largely unknown; however, it has been reported in Hyperparathyroidism-jaw tumors (HPT-JT), a rare autosomal syndrome in which the incidence of PC is around 15%. Mutations of the CDC73 gene are strongly associated with the HPT-JT syndrome, HPT-JT-related PC, and with 70% of sporadic PCs (16). CDC73 gene locates at the chromosome locus 1q31, and encodes a protein involved in transcriptional and post-transcriptional regulations (17). PHPT is the first and most common manifestation of the HPT-JT syndrome and frequently involves a single parathyroid gland. The high incidence of PC is a characteristic of familial HPT-JT. This tumor has a larger size, white color, adherence to adjacent tissues, and firm consistency (18).
The gold standard therapy of PC is the en bloc resection of the primary tumor by performing a parathyroidectomy together with ipsilateral thyroid lobectomy. The whole tumor resection significantly improves long-term survival of PC patients. The postoperative management of the patients includes the monitoring of the serum calcium level. For multiple metastatic lesions, the long-term survival is compromised; hence, the reduced sensibility to chemotherapy and radiotherapy of the PC metastasis (19). In the present case, after the resection of the brain metastasis, the primary lesion surgery was performed in the absence of other metastases.
To conclude, this article reported a case of an intracranial metastasis originated from a PC in a 60-year-old female who displayed symptoms of space-occupying brain lesion, without the classic PHPT signs, derived from PC. Therefore, patients in whom brain masses are observed, PC should be considered in the differential diagnosis.
Acknowledgments
We thank the Ethics Committee of the Affiliated Hospital of Qingdao University and the patient’s family for sharing case data and EditSprings for the provided expert linguistic services.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.01.66). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. A written informed consent and any accompanying images were obtained from the patient for publication of this Case report. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Salcuni AS, Cetani F, Guarnieri V, et al. Parathyroid carcinoma. Best Pract Res Clin Endocrinol Metab 2018;32:877-89. [Crossref] [PubMed]
- Barker HS, Podoll MB, Parker JR, et al. Parathyroid carcinoma with intracranial metastasis at diagnosis in a patient with uncontrolled hypercalcemia. Ann Clin Lab Sci 2014;44:484-8. [PubMed]
- Bondeson L, Grimelius L, DeLellis RA, et al. Parathyroid carcinoma. In: DeLellis RA, Lloyd RV, Heitz PU, et al. editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Endocrine Organs. IARC Press, Lyon 2004:124-27.
- Studentova H, Melichar B, Cincibuch J, et al. Brain metastases of parathyroid carcinoma: Review of the literature and a case report. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2015;159:360-5. [Crossref] [PubMed]
- Aldinger KA, Hickey RC, Ibanez ML, et al. Parathyroid carcinoma: a clinical study of seven cases of functioning and two cases of nonfunctioning parathyroid cancer. Cancer 1982;49:388-97. [Crossref] [PubMed]
- Yamamoto T, Matsumura A, Fujita K, et al. Cerebral metastasis of parathyroid carcinoma. Neurol Med Chir (Tokyo) 1996;36:96-8. [Crossref] [PubMed]
- Tyler D, Mandybur G, Dhillon G, et al. Intracranial metastatic parathyroid carcinoma: case report. Neurosurgery 2001;48:937-9; discussion 939-40. [PubMed]
- Eurelings M, Frijns CJ, Jeurissen FJ. Painful ophthalmoplegia from metastatic nonproducing parathyroid carcinoma: case study and review of the literature. Neuro Oncol 2002;4:44-8. [Crossref] [PubMed]
- Kar DK, Mishra A, Mishra SK. Brain metastasis from parathyroid carcinoma. J Assoc Physicians India 2003;51:68-70. [PubMed]
- Kern M, Lee G, Robbins P, et al. Intracranial metastatic parathyroid carcinoma. Case report and review of the literature. J Neurosurg 2004;101:1065-9. [Crossref] [PubMed]
- Yoshida S. Intracranial metastatic parathyroid carcinoma: case report. Surg Neurol 2006;65:81-3. [Crossref] [PubMed]
- Sadacharan D, Mahadevan S, Ferdinant J, et al. Hypercalcaemic encephalopathy due to metastatic parathyroid carcinoma. BMJ Case Rep 2017; [Crossref] [PubMed]
- DeLellis RA, Arnold A, Bilezikian JP, et al. Parathyroid carcinoma 2016.The 2016 World Health Classification of Tumors of the Central Nervous System. 2016:147-52.
- Yu Q, Hardin H, Chu YH, et al. Parathyroid Neoplasms: Immunohistochemical Characterization and Long Noncoding RNA (lncRNA) Expression. Endocr Pathol 2019;30:96-105. [Crossref] [PubMed]
- Higgins SE, Barletta JA. Applications of Immunohistochemistry to Endocrine Pathology. Adv Anat Pathol 2018;25:413-29. [Crossref] [PubMed]
- Cetani F, Pardi E, Marcocci C. Parathyroid Carcinoma. Front Horm Res 2019;51:63-76. [Crossref] [PubMed]
- Cetani F, Pardi E, Marcocci C. Parathyroid carcinoma: a clinical and genetic perspective. Minerva Endocrinol 2018;43:144-55. [PubMed]
- Quinn CE, Healy J, Lebastchi AH, et al. Modern experience with aggressive parathyroid tumors in a high-volume New England referral center. J Am Coll Surg 2015;220:1054-62. [Crossref] [PubMed]
- Enomoto K, Uchino S, Ito A, et al. The surgical strategy and the molecular analysis of patients with parathyroid cancer. World J Surg 2010;34:2604-10. [Crossref] [PubMed]


