
The role of lncRNA LSAT1 in the invasion and metastasis of non-small cell lung cancer under hypoxia
Introduction
The invasion and metastasis of non-small cell lung cancer (NSCLC) has become a key problem which affects the efficacy and long-term survival of patients. Previous studies about the invasion and metastasis of tumors are often confined to the change of internal factors of tumor cells, but ignore the interaction between tumor cells and tumor microenvironment. It is worth mentioning that as the important microenvironment for promoting the development of solid tumors, hypoxia has been verified to affect multiple malignant phenotypes, including invasion and metastasis (1-4). In recent years, long non-coding RNA (lncRNA) has gained more and more attention as a hot point in cancer research. lncRNA is a kind of non-coding RNA with the length of 200 nt. Compared with protein-coding genes, it has obvious specificity of advanced stage and tissue (5,6). The role and mechanism of lncRNA are various and one of the important points is that it can regulate the expression of protein-coding genes on multiple levels (7,8). Furthermore, a lot of researches have confirmed that lncRNA is of a vital importance in the invasion and metastasis of various tumors (9-11).
There are two transcripts of spermidine/spermine N1-acetyltransferase 1 (SAT1) gene, including SSAT1 (short SAT1) and LSAT1 (long SAT1). SSAT1 has six exons, which translate and produce arginine N-acetyltransferase, a protein that can promote tumor cell apoptosis. LSAT1 inserts a sequence between the third and fourth exons of SSAT1 transcripts, introduces a termination codon and forms a new reading frame (12). To clarify the role and potential mechanism of lncRNA in hypoxia-induced invasion and metastasis of non-small cell lung cancer (NSCLC), our study used high-throughput microarrays to compare and analyze the difference of lncRNA in three NSCLC cell lines induced by hypoxia and normoxia. And we further found that lncRNA LSAT1 participated in the invasion and metastasis of hypoxia-induced NSCLC through multiple screening strategies, clinical sample analysis and functional deficiency tests.
Methods
Clinical specimens and ethical approval
Twenty pairs of NSCLCs and paracancerous tissues were from the Laboratory Clinical Sample Bank. These tissues are all fresh samples and we kept it in liquid nitrogen tanks for a long time. In addition, we made a simple statistic on histological types and degree of differentiation of the 20 cases of NSCLC samples. As far as histological types are concerned, it included 4 cases of squamous cell carcinoma, 11 cases of adenocarcinoma (5 mucinous adenocarcinoma and 6 tubular adenocarcinoma), 3 cases of signet ring cell carcinoma and 2 cases of undifferentiated cancer. As far as degrees of differentiation are concerned, it included 12 cases of the highly differentiated, 5 cases of the moderately differentiated and 3 cases of the poorly differentiated.
All experimental protocols were approved by the Ethics Committee of Shanghai General Hospital, and all subjects involved in this study provided informed consent. Animal experiments were carried out according to the Shanghai General Hospital Animal Care and Use Guidelines, and the experimental.
Cell culture
Human NSCLC cell lines including NCI-H1299, NCI-H1650 and A549 were purchased from ATCC. Three NSCLC cell lines were cultured in RPMI1640 medium containing 10% fetal bovine serum and 1% penicillin and streptomycin. After the cell density was up to about 60–70%, they were cultured in conventional incubators (37 °C, 5% CO2) and hypoxia incubators (37 °C, 5% CO2, 94% N2, 1% O2).
RT-PCR
We uniformly extracted RNA from tissues or cell lines by RNA extraction kit from TaKaRa Company. According to the instructions of TaKaRa reverse transcription kit, reverse transcription reaction was carried out to obtain the required cDNA. The sequences of primers of LSAT1 and β-actin are as follows: LSAT1-F: 5'-GGCGGGGAGGTAACTAAAAG-3'; LSAT1-R: 5'-ACCATCCTCCCTGCTCTCCT-3'; β-actin-F: 5'-ATAGCACAGCCTGGATAGCAACGTAC-3'; β-actin-R: 5'-CACCTTCTACAATGAGCTGCGTGTG-3'.
Transfection of sinRNA and lentivirus
The siRNA sequence and lentivirus of LSAT1 were designed and provided from Jikai Company in Shanghai. The specific steps of transfection are referred to the transfection manual provided by Jikai Company. The siRNA sequence was as follows: LSAT1-shRNA1: 5'-GATCCACAGTCTCTAGCTTCGCCATGTACATCAAGAGTGTACATGGCGAAGCTAGAGACTGTTTTTTA-3'; LSAT1-shRNA2: 5'-GATCCCATGTACATGGCCCTTCCGTGTACATCAAGAGTGTACACGGAAGGGCCATGTACATGTTTTTA-3'.
Transwell migration and invasion experiments
Transwell migration experiment was mainly operated by Transwell chamber and 24-well plate. The cell solution suspended by 200 µL basic culture medium was added into the upper chamber, while the culture medium containing 20% fetal bovine serum was added outside the chamber (in the hole of 24-well plate). The incubator was incubated for a certain time, and then the chamber was taken out to enter it. Fixation, dyeing, cleaning and other treatments were performed, and finally photographs were taken for statistical analysis.
Animal studies
All nude mice were female in this experiment and were fed in Animal Laboratory Center of Shanghai General Hospital. Cultured cells in multiple culture flasks, when they grew to about 80–90% and were in good condition, digested suspended cells, counted cells with cell counter, and adjusted cell concentration to 1×107/mL; the tail vein of each nude mouse was injected with about 0.2 mL of cell suspension (containing about 2×106 cells), and each kind of cell was inoculated into 10 nude mice, and observed 2–3 times a week from the date of inoculation.
Statistical analysis
All data were processed using SPSS 20.0 statistical software. The experimental data were expressed as mean ± standard error (means ± S.E.M.). T-test was used for paired comparison. P<0.05 was considered statistically significant.
Results
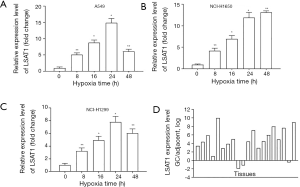
Expression of lncRNA LSAT1 in NSCLC cell and tissue induced by hypoxia
We utilized RT-PCR to verify the expression of lncRNA LSAT1 in NSCLC cells which were cultured under hypoxic condition. The result showed that lncRNA LSAT1 was significantly upregulated in NSCLC cells induced by hypoxia. It reached its peak value at 24 hours of hypoxia in A549 and NCI-H1299, 48 hours of hypoxia in NCI-H1650 respectively (Figure 1A,B,C). In addition, we tested the expression of 20 pairs of lncRNA LSAT1 in NSCLC tissue and its paracancerous tissues. The result showed that the expression of lncRNA LSAT1 was significantly higher than that of paracancerous tissues, whose difference was statistically significant (Figure 1D, P<0.05). Combining the above results, we speculated that lncRNA LSAT1 was like an oncogene gene which probably participated in the hypoxia-induced invasion and metastasis of NSCLC.
Screening of siRNA with the best interference effect
We constructed four interference sequences (si-1, si-2, si-3, si-4) and one contrast sequence (si-NC) for lncRNA LSAT1, and then transfected them into A549 and NCI-H1299 cells, respectively. After 24 hour-transfection, RNA was extracted from corresponding cells, and transfection efficiency was tested the by RT-PCR. The result showed that compared with control group, the expression of lncRNA LSAT1 in A549 and NCI-H1299 cells transfected by si-1 sequence was most significantly down-regulated (Figure 2A,B).
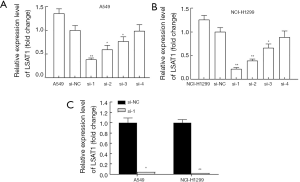
Construction of a cell line stably down-regulated lncRNA LSAT1
Through the verification of the above experiments, we found that after transfected by si-1, the transfection efficiency of lncRNA LSAT1 was the best. Therefore, we wrapped si-1 sequence in lentivirus and transfected NSCLC cells A549 and NCI-H1299. The result of RT-PCR showed that compared with control group, the expression of lncRNA LSAT1 in the cell line transfected by si-1 lentivirus was significantly down-regulated (Figure 2C). For convenience, we marked the steady cell lines we constructed as A549-si-LSAT1, NCI-H1299-si-LSAT1, A549-NC and NCI-H1299-NC.
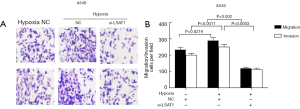
LSAT1 was involved in cell invasion and metastasis of NSCLC cells
In this study, we determined whether LSAT1 could affect normoxic NSCLC cell migration and invasion. Transwell assays with or without Matrigel indicated that A549 and NCI-H1299 cells with stable LSAT1 knockdown showed significantly decreased migration and invasion compared with control cells (Figure 3A,B). Conversely, upregulation of LSAT1 expression significantly enhanced cell migration and invasion (Figure 3C,D).

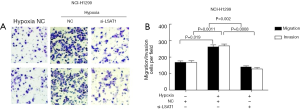
Influence of the down-regulation of lncRNA LSAT1 in the hypoxia-induced invasion and metastasis of NSCLC cells
The results of transwell migration and invasion experiments showed that the metastasis and invasion of A549 and NCI-H1299 cells cultured under hypoxic conditions were significantly enhanced compared with that cultured under normoxic conditions. However, under hypoxic conditions, the metastasis and invasion of A549-si-LSAT1 and NCI-H1299-si-LSAT1 cells were significantly reduced compared with that of A549-NC and NCI-H1299-NC (Figures 4,5). The above results indicated that hypoxia can significantly enhance the metastasis and invasion of NSCLC cells A549 and NCI-H1299. Of note, the hypoxia-induced metastasis and invasion of NSCLC cells were significantly reduced after successful down-regulation of the expression of lncRNA LSAT1.
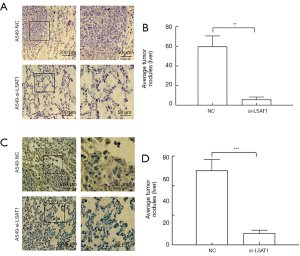
Effect of down-regulation of lncRNA LSAT1 on metastasis in nude mice after tail vein injection
The result of visceral metastasis in nude mice by tail vein injection showed that compared with control group, the number of metastatic nodules in liver and lung of nude mice injected with A549-si-LSAT1 cells decreased significantly (Figure 6).
Discussion
Invasion and metastasis of NSCLC is the cause of death in most patients with NSCLC. Its specific molecular mechanism has gained great attention from researchers. However, most researches are concentrated on the change of the internal molecule and signaling pathway of tumor cells, but ignore the microenvironment outside tumor cells. Hypoxic tumor microenvironment is a main feature of malignant solid tumor. And hypoxia in tumors plays an important role in tumorigenesis and development. Besides, it is closely related to tumor resistance to radiotherapy, chemotherapy, proliferation and invasion and metastasis (13-16).
LncRNA is a kind of non-coding RNA whose transcript length exceeds 200 nt. It cannot code proteins, but it regulates the expression of genes on multiple levels in the form of RNA. Studies in recent years indicated that lncRNA not only participated in the regulation of X chromosome silencing, genomic imprinting, chromatin modification, transcriptional activation, transcriptional interference, intranuclear transport, but also played an important role in the occurrence and development of various diseases, especially tumors. It would affect multiple malignant phenotypes of tumors including invasion and metastasis (6,7).
Our study firstly utilized high-throughput microarrays to screen 154 common differentially expressed lncRNA molecules in NSCLC cells induced by hypoxia, among which 84 were upregulated and 70 were down-regulated. Through further multiple screening strategies, we targeted lncRNA LSAT1. Then, we validated it at the cellular level and the result showed that the expression of lncRNA LSAT1 in hypoxia-induced NSCLC cells A549, NCI-H1650 and NCI-H1299 were significantly enhanced. Meanwhile, we also verified the expression of lncRNA LSAT1 in 20 pairs of NSCLC tissues and adjacent tissues, which showed that it was high expressed in NSCLC tissues. Finally, to find out whether lncRNA LSAT1 participated in the hypoxia-induced invasion and metastasis of NSCLC, A549 and NCI-H1299 cells stably down-regulated lncRNA LSAT1 were constructed through siRNA interference and lentivirus transfection. Further in vivo functional experiments confirmed that down-regulation of the expression of lncRNA LSAT1 could reduce the invasion and metastasis of NSCLC cells in vitro and in vivo. In summary, we found that lncRNA LSAT1 is a key molecule in the hypoxia-induced invasion and metastasis of NSCLC.
As we know, a lot of studies showed that lncRNA can regulate gene expression from multiple levels and perspectives, including epigenetic level, transcriptional level and post-transcriptional level. Besides, the mechanisms of lncRNA are various, among which the regulation of lncRNA in the expression of peripheral protein coding genes is one of the most important mechanisms (17-19).
LSAT1 is a transcript of SAT1 gene on X chromosome, which consists of six exons. Because of the presence of a termination codon on the fourth exon, bioinformatics methods predict that the transcript does not have the ability to encode proteins. In the past, most studies focused on the transcripts of SAT1 gene, but ignored the transcripts of LSAT1 which did not encode the protein (20). So far, there is no report about the specific function of LSAT1 in cells. In order to find transcription factors regulating LSAT1 expression under hypoxia, we found transcription factors binding to upstream genes on UCSC, and screened out hypoxia-related transcription factors by searching literature and we targeted at STAT3. Chip-seq data showed that STAT3 had strong signals upstream of SAT1 gene. In addition, through literature review, we found that STAT3 was closely related to hypoxia regulation, such that hypoxia-induced HIF promoted tumor metastasis, but HIF must be assisted by STAT3 to complete the regulation of its downstream genes, and STAT3 itself was also regulated by hypoxia (21,22). Therefore, we believed that STAT3 may be one of the target genes for lncRNA LSAT1 to play a regulatory role. In the follow-up study, we will discuss this part of the content in depth.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.12.101). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All experimental protocols were approved by the Ethics Committee of Shanghai General Hospital, and all subjects involved in this study provided informed consent. Animal experiments were carried out according to the Shanghai General Hospital Animal Care and Use Guidelines, and the experimental.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer 2014;14:430-9. [Crossref] [PubMed]
- DiGiacomo JW, Gilkes DM. Tumor Hypoxia As an Enhancer of Inflammation-Mediated Metastasis: Emerging Therapeutic Strategies. Target Oncol 2018;13:157-73. [Crossref] [PubMed]
- Jögi A, Ehinger A, Hartman L, et al. Expression of HIF-1α is related to a poor prognosis and tamoxifen resistance in contralateral breast cancer. PLoS One 2019;14:e0226150. [Crossref] [PubMed]
- Tang YA, Chen YF, Bao Y, et al. Hypoxic tumor microenvironment activates GLI2 via HIF-1α and TGF-β2 to promote chemoresistance in colorectal cancer. Proc Natl Acad Sci U S A 2018;115:E5990-9. [Crossref] [PubMed]
- Fico A, Fiorenzano A, Pascale E, et al. Long non-coding RNA in stem cell pluripotency and lineage commitment: functions and evolutionary conservation. Cell Mol Life Sci 2019;76:1459-71. [Crossref] [PubMed]
- Ulitsky I, Shkumatava A, Jan CH, et al. Conserved function of lincRNAs in vertebrate embryonic development despite rapid sequence evolution. Cell 2011;147:1537-50. [Crossref] [PubMed]
- Zhang Y, He XY, Qin S, et al. Upregulation of PUM1 Expression in Preeclampsia Impairs Trophoblast Invasion by Negatively Regulating the Expression of the lncRNA HOTAIR. Mol Ther 2019; [Epub ahead of print]. [PubMed]
- Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature 2012;482:339-46. [Crossref] [PubMed]
- Ke S, Li RC, Meng FK, et al. NKILA inhibits NF-κB signaling and suppresses tumor metastasis. Aging (Albany NY) 2018;10:56-71. [Crossref] [PubMed]
- Cao W, Peng T, Zhou Y. Long noncoding RNA activated by transforming growth factor-β promotes cancer development and is a prognostic marker in cervical cancer. J Cancer Res Ther 2017;13:801-6. [Crossref] [PubMed]
- DiStefano JK. Long noncoding RNAs in the initiation, progression, and metastasis of hepatocellular carcinoma. Noncoding RNA Res 2017;2:129-36. [Crossref] [PubMed]
- Yang Q, Deng Y, Xu Y, et al. Knockdown of SSATX, an alternative splicing variant of the SAT1 gene, promotes melanoma progression. Gene 2019;716:144010. [Crossref] [PubMed]
- Chen TI, Chiu HW, Pan YC, et al. Intermittent hypoxia-induced protein phosphatase 2A activation reduces PC12 cell proliferation and differentiation. J Biomed Sci 2014;21:46. [Crossref] [PubMed]
- Nabavi N, Bennewith KL, Churg A, et al. Switching off malignant mesothelioma: exploiting the hypoxic microenvironment. Genes Cancer 2016;7:340-54. [PubMed]
- Galzio R, Cristiano L, Fidoamore A, et al. Hypoxia modulation of peroxisome proliferator-activated receptors (PPARs) in human glioblastoma stem cells. Implications for therapy. J Cell Biochem 2012;113:3342-52. [Crossref] [PubMed]
- Seyfi D, Behzad SB, Nabiuni M, et al. Verbascoside Attenuates Rac-1 and HIF-1α Signaling Cascade in Colorectal Cancer Cells. Anticancer Agents Med Chem 2018;18:2149-55. [Crossref] [PubMed]
- Wang X, Arai S, Song X, et al. Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature 2008;454:126-30. [Crossref] [PubMed]
- Song H, Xu Y, Shi L, et al. LncRNA THOR increases the stemness of gastric cancer cells via enhancing SOX9 mRNA stability. Biomed Pharmacother 2018;108:338-46. [Crossref] [PubMed]
- Barbagallo C, Brex D, Caponnetto A, et al. LncRNA UCA1, Upregulated in CRC Biopsies and Downregulated in Serum Exosomes, Controls mRNA Expression by RNA-RNA Interactions. Mol Ther Nucleic Acids 2018;12:229-41. [Crossref] [PubMed]
- Thakur VS, Aguila B, Brett-Morris A, et al. Spermidine/spermine N1-acetyltransferase 1 is a gene-specific transcriptional regulator that drives brain tumor aggressiveness. Oncogene 2019;38:6794-800. [Crossref] [PubMed]
- Jung JE, Lee HG, Cho IH, et al. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J 2005;19:1296-8. [Crossref] [PubMed]
- Xu Q, Briggs J, Park S, et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene 2005;24:5552-60. [Crossref] [PubMed]


