
Initial experience of drug-eluting bead transarterial chemoembolization with CalliSpheres® microspheres in treating liver metastases patients
Introduction
Liver metastases, also called secondary liver cancer, commonly arise from blood metastasis of cancer cells from tumors at other parts of the body or through direct infiltration from adjacent organs (1). Unlike primary liver cancer, which is mainly attributed to chronic liver diseases, liver metastases are the consequence of progression and metastasis of other tumors, and are more prevalent than primary liver cancers (2). The most common primary tumors that would develop liver metastasis are gastrointestinal tumors. For instance, up to 60% of colorectal cancer patients develop liver metastasis during the course of disease (3). Although surgical resection is the first-line treatment for liver metastases which provides survival benefits, a large proportion of patients remain inoperable and response poorly to systemic chemotherapy. Besides, the indications for surgical resection need careful evaluation to avoid accelerating the progress of other metastatic lesions. Therefore, various interventional treatments including transarterial chemoembolization (TACE), radiofrequency or microwave ablation, cryotherapy, and internal radiation therapy are used as alternatives or as neoadjuvant and palliative treatments to surgical resection.
TACE acts by obstructing the blood supply and delivering chemotherapeutic drugs to the tumor through hepatic arteries periodically, and is recommended as preferred therapy for intermediated or advanced and inoperable liver cancer (4). Meanwhile, the application of lipiodol-based conventional TACE (cTACE) in liver metastasis of colorectal cancer, gastric cancer, breast cancer as well as renal cell carcinoma has been reported in the past few years (5-11). Although cTACE is previously shown to be effective in liver tumor with rich blood supply, the clinical response is usually not so satisfied in lesions lack blood supply, such as liver metastases (12). Compared to cTACE, the novel drug-eluting bead TACE (DEB-TACE) utilizes drug-eluting microspheres as embolization agent as well as drug-delivering agent, which has been shown to result in lower systemic concentration and higher tumor concentration of drugs, thereby reducing the systemic drug-related adverse events. Therefore, DEB-TACE is increasingly applied in tumors lacking blood supply and preceding the lipiodol-based cTACE in clinics (5,6,13). Despite the benefits of DEB-TACE in primary liver cancer, its clinical efficacy or safety in liver metastases are yet to be reported. Therefore, we retrospectively reviewed 39 liver metastases patients who received DEB-TACE and evaluated the treatment efficacy, survival, safety profiles as well as factors that affected the clinical outcomes of DEB-TACE in these patients.
Methods
Patients
Between 2015/2/28 and 2018/3/24, a total of 39 liver metastases patients underwent DEB-TACE treatments at our hospital were reviewed in this retrospective study. The screening criteria included: (I) confirmed diagnosis of liver metastases by clinical and imaging examinations; (II) underwent DEB-TACE treatment; (III) age more than 18 years; (IV) clinical records were accessible; (V) with 1-month response assessment post-DEB-TACE treatment. The study protocol was approved by the Institutional Review Board of our hospital (ethical approval number: 2019-432), and informed consents or verbal agreements with type recording were obtained from all enrolled patients or their guardians.
Data collection
Patients’ data were collected from the Electronic Medical Record, which included demographic information, tumor characteristics, history of treatment, examinations of blood routine, liver function, renal function, coagulation function and tumor markers, treatment procedures, response assessments, adverse events as well as follow-up records.
DEB-TACE treatment
The DEB-TACE procedure was performed according to previous studies (14-16). Briefly, percutaneous femoral artery puncture was carried out with the modified Seldinger technique, and hepatic angiography was performed to detect the tumor supplying vessels, then CalliSpheres® microspheres (CSM) (diameter: 100 to 300 µm, Jiangsu Hengrui Medicine Co., Ltd., Jiangsu Province, China) loading with chemotherapeutic reagents (such as pirarubicin, irinotecan, oxaliplatin and mitomycin) were injected into the tumor supplying vessel for chemoembolization precisely.
Treatment response assessment
Computed tomography (CT) or magnetic resonance imaging (MRI) was conducted within 1 week prior to the initial DEB-TACE procedure to evaluate the baseline tumor imaging, which was then performed at approximately 1 month (30±10 days) post-DEB-TACE to assess the treatment response. According to the modified Response Evaluation Criteria in Solid Tumors (17), treatment response was classified as (I) complete remission (CR): disappearance of any intratumoral arterial enhancement in all target nodules; (II) partial response (PR): at least 30% decrease in the sum of diameters of viable (enhancement in the arterial phase) target nodules, taking as reference the baseline sum of the diameters of target nodules; (III) stable disease (SD): any cases that do not meet the criteria of either PR or progressive disease (PD); (IV) PD: an increase of more than 20% in the sum of the diameters of viable (enhancing) target nodules, taking as reference the smallest sum of the diameters of viable (enhancing) target nodules recorded since the initiation of treatment. Besides, as for patients who underwent multiple cycles of DEB-TACE procedures, the last assessed treatment response was analyzed in the current study.
Survival assessment
Patients’ follow-up records were reviewed. The last follow-up date was 2019/03/24, with a median follow-up duration of 17.8 months (range, 3.0–63.4 months). Progression-free survival (PFS) and overall survival (OS) were calculated. PFS was defined as the time interval from the first DEB-TACE therapy to the disease progression, death or last follow-up; OS was defined as the time interval from the diagnosis of liver metastasis to death or last follow-up.
Laboratory indexes and adverse event assessment
Laboratory indexes were examined before DEB-TACE, within 1 week and at approximately 1 month (30±10 days) after DEB-TACE, which included (I) blood routine indexes: white blood cell (WBC), red blood cell (RBC), absolute neutrophil count (ANC), haemoglobin (Hb), and platelet (PLT); (II) liver function indexes: albumin (ALB), total protein (TP), total bilirubin (TBIL), total bile acid (TBA), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP); (III) renal function indexes: blood creatinine (BCr), blood urea nitrogen (BUN); (IV) coagulation function indexes: prothrombin time (PT) and prothrombin activity (PTA); (V) tumor markers: carcino-embryonic antigen (CEA), and carbohydrate antigen 199 (CA199). Besides, adverse events occurred after DEB-TACE treatment were reviewed, which consisted of pain, fever, nausea, vomiting and elevated liver function index.
Statistical analysis
Data were analyzed by SPSS 24.0 statistical software (SPSS Inc., Chicago, USA), and graphs were plotted by GraphPad Prism 7.02 (GraphPad Software Inc., San Diego, USA). The normality of continuous data was determined by the Shapiro-Wilk test. Continuous data were described as mean and standard deviation (SD) if normally distributed, and as median and interquartile range (IQR) if not normally distributed. Data were presented as count (percentage). The treatment response rate was calculated respectively based on the total treated cycles of DEB-TACE and the total treated nodules. Paired comparison (before and after treatment) of continuous data was determined by Wilcoxon signed-rank test. Kaplan-Meier curve was plotted to display survival profiles, and the log-rank test was applied to determine the difference of PFS and OS between groups. Univariable and multivariable Cox’s proportional hazard regression model analyses were performed to determine the factors affecting PFS. P value <0.05 was considered significant.
Results
Patients’ characteristics
Thirty-nine liver metastases patients aged 62.1±8.9 years on average were enrolled, of which 15 (38.5%) were females and 24 (61.5%) were males (Table 1). Regarding tumor characteristics, 30 (76.9%) patients had diffuse disease, 20 (51.3%) patients had extrahepatic lesions, and the mean tumor size was 6.0±3.6 cm. There were 12 (30.8%), 7 (17.9%), 8 (20.5%), 3 (7.7%) and 9 (23.1%) patients whose primary cancers were colon cancer, rectal cancer, pancreatic cancer, gastric cancer and others, respectively. In addition, 5 (12.8%) patients received liver resection before DEB-TACE. And 10 (25.6%), 16 (41.0%), 13 (33.3%) and 12 (30.8%) patients received combined therapy with cTACE, chemotherapy, targeted therapy and radiofrequency ablation respectively. The number of patients received 1, 2, 3 and 5 cycles of DEB-TACE was 12 (30.8%), 21 (53.8%), 5 (12.8%) and 1 (2.6%), respectively, and the mean duration of liver metastasis to first DEB-TACE was 9.4±9.0 months.
Table 1
| Characteristics | Patients (N=39) |
|---|---|
| Age (years), mean ± SD | 62.1±8.9 |
| Gender, No. (%) | |
| Female | 15 (38.5) |
| Male | 24 (61.5) |
| Diffuse disease, No. (%) | 30 (76.9) |
| Extrahepatic lesion, No. (%) | 20 (51.3) |
| Largest tumor size (cm), mean ± SD | 6.0±3.6 |
| Primary cancer, No. (%) | |
| Colon cancer | 12 (30.8) |
| Rectal cancer | 7 (17.9) |
| Pancreatic cancer | 8 (20.5) |
| Gastric cancer | 3 (7.7) |
| Others* | 9 (23.1) |
| Previous liver resection, No. (%) | 5 (12.8) |
| Combined treatments, No. (%) | |
| cTACE | 10 (25.6) |
| Chemotherapy | 16 (41.0) |
| Targeted therapy | 13 (33.3) |
| Radiofrequency ablation | 12 (30.8) |
| Cycles of DEB-TACE, No. (%) | |
| 1 cycle | 12 (30.8) |
| 2 cycles | 21 (53.8) |
| 3 cycles | 5 (12.8) |
| 5 cycles | 1 (2.6) |
| Duration of liver metastases to first DEB-TACE (months), mean ± SD | 9.4±9.0 |
*, others including breast cancer, duodenal papilla cancer, ovarian cancer, lung cancer, cervical cancer, leiomyosarcoma, nasopharyngeal cancer, gallbladder carcinoma and cecum cancer. DEB-TACE, drug-eluting bead transarterial chemoembolization; SD, standard deviation; cTACE, conventional transarterial chemoembolization.
Treatment response to DEB-TACE
Based on total treated cycles of DEB-TACE (n=64), the CR, PR, SD and PD were 1 (1.6%), 22 (34.4%), 35 (54.7%) and 6 (9.4%), respectively (Figure 1A). Based on the total treated nodules (n=161), the CR, PR, SD and PD were 8 (5.0%), 43 (26.7%), 93 (57.8%) and 17 (10.6%) respectively (Figure 1B). In addition, the median PFS was 15.3 months (95% CI: 9.7–20.8 months) and the median OS was 28.7 months (95% CI: 20.3–37.0 months).

Laboratory indexes of patients before and after DEB-TACE
The detailed laboratory indexes before (baseline), 1 week after and 1 month after DEB-TACE were listed in Table 2. For blood routine, WBC (P<0.001) and ANC (P<0.001) were increased, while RBC (P=0.019), Hb (P=0.010) and PLT (P=0.001) were deceased at 1 week after DEB-TACE compared to baseline. Whereas at 1 month after DEB-TACE, WBC was reduced (P=0.020) and Hb was lower (P=0.012) compared with baseline. Regarding liver function, ALB (P=0.001), TP (P=0.005) and ALP (P=0.010) were reduced, while TBIL (P<0.001), ALT (P<0.001) and AST (P<0.001) were promoted at 1 week after DEB-TACE compared with baseline. And at 1 month after DEB-TACE, ALB was lower (P=0.010) but ALP was higher (P<0.001) compared with baseline. As to coagulation function, PT was increased (P=0.009) and PTA was decreased (P=0.001) at 1 week after DEB-TACE, while both PT (P=0.808) and PTA (P=0.501) did not change at 1 month after DEB-TACE compared with baseline. Other biochemical indexes were unchanged after DEB-TACE compared with baseline (all P>0.05).
Table 2
| Items | Baseline | 1-week post-DEB-TACE | P value* | 1-month post-DEB-TACE | P value# |
|---|---|---|---|---|---|
| Blood routine | |||||
| WBC (×109 cell/L) | 6.1 (4.0–8.0) | 7.9 (5.2–10.4) | <0.001 | 5.8 (4.3–9.4) | 0.020 |
| RBC (×1012 cell/L) | 4.0 (3.6–4.7) | 4.0 (3.5–4.5) | 0.019 | 3.9 (3.4–4.3) | 0.164 |
| ANC (%) | 61.3 (55.7–70.6) | 79.1 (68.2–83.1) | <0.001 | 67.7 (54.4–72.3) | 0.622 |
| Hb (g/L) | 122.0 (108.0–136.5) | 116.0 (105.0–133.0) | 0.010 | 115.5 (100.0–125.8) | 0.012 |
| PLT (×109 cell/L) | 166.5 (129.8–230.3) | 161.0 (108.0–205.0) | 0.001 | 202.5 (109.3–287.5) | 0.248 |
| Liver function | |||||
| ALB (g/L) | 39.2 (37.2–42.1) | 36.4 (34.3–39.9) | 0.001 | 38.3 (35.7–39.8) | 0.010 |
| TP (g/L) | 67.1 (62.5–74.6) | 66.0 (62.0–68.2) | 0.005 | 70.3 (66.0–74.4) | 0.249 |
| TBIL (μmol/L) | 11.6 (9.8–15.3) | 18.2 (13.3–22.9) | <0.001 | 11.5 (9.3–15.6) | 0.589 |
| TBA (I/L) | 6.8 (4.4–13.4) | 6.7 (4.7–10.4) | 0.955 | 7.1 (5.3–10.9) | 0.284 |
| ALT (U/L) | 23.0 (16.8–32.0) | 65.0 (41.0–114.0) | <0.001 | 24.0 (16.0–33.0) | 0.845 |
| AST (U/L) | 29.0 (22.5–43.0) | 90.0 (52.0–150.0) | <0.001 | 34.0 (23.0–45.0) | 0.459 |
| ALP (U/L) | 129.0 (88.0–195.5) | 128.5 (96.3–235.3) | 0.010 | 154.0 (112.5–292.0) | <0.001 |
| Kidney function | |||||
| BCr (μmol/L) | 59.0 (48.5–68.5) | 57.0 (48.3–64.0) | 0.121 | 60.0 (48.0–75.0) | 0.909 |
| BUN (mmol/L) | 4.7 (3.6–5.9) | 4.4 (4.0–5.3) | 0.359 | 4.7 (3.5–5.4) | 0.642 |
| Coagulation function | |||||
| PT (s) | 13.0 (12.7–13.6) | 13.9 (13.2–14.3) | 0.009 | 13.2 (12.6–14.1) | 0.808 |
| PTA (%) | 101.5 (91.5–106.3) | 87.0 (83.0–98.5) | 0.001 | 97.5 (84.8–107.3) | 0.501 |
| Tumor markers | |||||
| CEA (μg/L) | 16.6 (3.4–281.8) | 72.3 (12.6–9,135.8) | 1.000 | 18.7 (4.7–139.8) | 0.077 |
| CA199 (μg/L) | 410.9 (13.8–4,679.6) | 528.7 (27.7–2,543.3) | 0.109 | 953.4 (12.8–3,655.8) | 0.131 |
Comparisons were determined by Wilcoxon signed-rank test. P value <0.05 was considered significant. *, 1-week post vs. baseline; #, 1-month post-DEB-TACE vs. baseline. DEB-TACE, drug-eluting bead transarterial chemoembolization; WBC, white blood cell; RBC, red blood cell; ANC, absolute neutrophil count; Hb, hemoglobin; PLT, platelet; ALB, albumin; TP, total protein; TBIL, total bilirubin; TBA, total bile acid; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; BCr, blood creatinine; BUN, blood urea nitrogen; PT, prothrombin time; PTA, prothrombin activity; CEA, carcino-embryonic antigen; CA199, carbohydrate antigen199.
Factors affecting PFS
Patients with previous liver resection (P=0.003) (Figure 2A), no targeted therapy (P=0.011) (Figure 2B) and non-disease control rate (non-DCR) after first DEB-TACE (P<0.001) (Figure 2C,D) were shown with worse PFS. Univariate Cox’s proportional hazard model regression revealed that previous liver resection [P=0.008, hazard ratio (HR) =4.268] was correlated with shorter PFS, while combined treatment with targeted therapy (P<0.001, HR =0.326), patients achieved DCR after first DEB-TACE (P<0.001, HR =0.084) and the primary nodule achieved DCR after first DEB-TACE (P<0.001, HR =0.122) was correlated with prolonged PFS (Table 3). Further multivariate Cox’s proportional hazard model regression showed that combined treatment with targeted therapy (P=0.031, HR =0.181) independently predicted longer PFS in liver metastases patients.
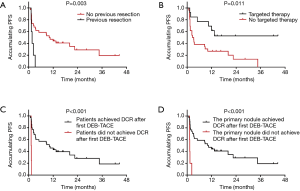
Table 3
| Items | Univariate Cox’s regression | Multivariate Cox’s regression | |||
|---|---|---|---|---|---|
| P value | HR (95% CI) | P value | HR (95% CI) | ||
| Age (>60 vs. ≤60 years) | 0.249 | 0.641 (0.300–1.366) | 0.816 | 1.138 (0.382–3.396) | |
| Gender (male vs. female) | 0.937 | 0.969 (0.442–2.125) | 0.985 | 0.989 (0.310–3.152) | |
| Diffuse disease (yes vs. no) | 0.351 | 0.676 (0.297–1.539) | 0.599 | 0.676 (0.157–2.916) | |
| Extrahepatic lesion (yes vs. no) | 0.415 | 0.734 (0.349–1.544) | 0.275 | 0.585 (0.223–1.532) | |
| Largest tumor size (>5 vs. ≤5 cm) | 0.329 | 0.684 (0.319–1.467) | 0.679 | 1.337 (0.338–5.296) | |
| Primary colorectal cancer vs. other cancers | 0.266 | 0.653 (0.308–1.383) | 0.662 | 0.730 (0.178–2.987) | |
| Previous liver resection (yes vs. no) | 0.008 | 4.268 (1.463–12.451) | 0.059 | 5.270 (0.940–29.542) | |
| Combined treatments | |||||
| cTACE (yes vs. no) | 0.996 | 0.998 (0.420–2.370) | 0.091 | 0.336 (0.095–1.188) | |
| Chemotherapy (yes vs. no) | 0.515 | 0.776 (0.362–1.665) | 0.456 | 1.491 (0.521–4.262) | |
| Targeted therapy (yes vs. no) | 0.016 | 0.326 (0.131–0.811) | 0.031 | 0.181 (0.039–0.853) | |
| Radiofrequency ablation (yes vs. no) | 0.790 | 0.897 (0.401–2.003) | 0.060 | 0.354 (0.119–1.047) | |
| Patients achieved ORR after first DEB-TACE (yes vs. no) | 0.656 | 0.843 (0.397–1.788) | 0.672 | 1.547 (0.206–11.636) | |
| Patients achieved DCR after first DEB-TACE (yes vs. no) | <0.001 | 0.084 (0.021–0.329) | 0.075 | 0.145 (0.017–1.217) | |
| The primary nodule achieved ORR after first DEB-TACE (yes vs. no) | 0.424 | 0.728 (0.334–1.584) | 0.105 | 0.206 (0.030–1.395) | |
| The primary nodule achieved DCR after first DEB-TACE (yes vs. no) | <0.001 | 0.122 (0.039–0.384) | 0.762 | 0.715 (0.082–6.240) | |
PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; cTACE, conventional transarterial chemo-embolization; DEB-TACE, drug-eluting bead transarterial chemoembolization; ORR, overall response rate; DCR, disease control rate.
Adverse events
Adverse events by DEB-TACE treatment were recorded. There were 20 (51.3%) patients presented with pain, 5 (12.8%) patients with fever, 7 (17.9%) patients with nausea and 5 (12.8%) patients with vomiting by DEB-TACE treatment.
Discussion
The prevalence of liver metastases is far more common than primary liver cancer, and a great part of liver metastases patients are not accessible for curative surgery (6,8). TACE is considered as an alternative to curative surgery in liver metastases, whose treatment efficacy has been reported in patients with liver metastasis from gastrointestinal tumors, breast cancer, etc. One previous study discloses that in colorectal cancer liver metastasis patients treated with cTACE followed by sustained hepatic arterial infusion chemotherapy, CR, PR, SD and overall response rate (ORR) rates are 0.05%, 29.0%, 45.7%, and 29.5% respectively (6). And another study including patients with ovarian cancer liver metastasis reveals that the local tumor control was PR in 16.9%, SD in 58.5% and PD in 24.6% of patients by cTACE using lipiodol and starch as embolization agent (11). Additionally, cTACE (lipiodol and starch embolized) is applied in patients with breast cancer liver metastasis, which leads to PR of 13%, SD of 50.5% and PD of 36.5% (10). These studies display a wide application of lipiodol-based cTACE in liver metastases patients with various primary cancers. However, it has been reported that compared with cTACE, the treatment efficacy of DEB-TACE has superior performance. For instance, the DEB-TACE treated melanoma liver metastasis presents tumor response rate of 55% and DCR of 80% (18). The existing evidence suggests that DEB-TACE is more effective in treating tumors lacking blood supply compared with cTACE (5,12,18-21). In our study, the CR, PR, SD and PD of total treated cycles were 1.6%, 34.4%, 54.7% and 9.4% respectively, and 5.0%, 26.7%, 57.8% and 10.6% of total treated nodules respectively, which showed a better prognostic value compared with that of cTACE in previous studies. The possible reason might be that, the microspheres used in DEB-TACE were shown to have better drug loading and releasing profiles as well as embolization effect than lipiodol used in cTACE, which might lead to higher drug concentration at tumor site and the blood supply blockage that induced tumor necrosis, thereby contributed to better treatment response (22). However, this conclusion needed further validation by comparing treatment response between cTACE and DEB-TACE in liver metastases patients.
Besides, the median PFS was 15.3 months and the median OS was 28.7 months, which were relatively longer compared with that in the previous studies. According to a previous related study, colorectal cancer liver metastasis patients treated with lipiodol-based cTACE and hepatic arterial infusion chemotherapy presents the median PFS and OS as 5.5 and 15.6 months respectively (6). As for patients with ovarian cancer liver metastasis treated with lipiodol-based cTACE, the median OS is 14 months (11). In addition, the median OS from the start of cTACE is 18.5 months in breast cancer liver metastasis patients treated with lipiodol and starch-based TACE (10). Although our study was lacking a control group, DEB-TACE treatment seemed to have a better outcome in disease control and patient survival.
Furthermore, the safety profiles of DEB-TACE in liver metastases were evaluated in this study. The safety profiles of cTACE in treating liver metastases have been documented in several studies, which present that the very common adverse events include abnormal liver function, abdominal pain, fever and nausea (5,23). In accordance with these studies, we also observed that the common adverse events manifested as pain, fever, nausea and vomiting to DEB-TACE in liver metastases patients, which could be effectively controlled after these complications occurred. At the same time, laboratory indexes including liver function, except ALP, were deteriorated 1 week after DEB-TACE, but recovered at 1 month after DEB-TACE. The slight increase of ALP might be induced by hepatocellular injury after DEB-TACE, which was still reflected in a safe range.
Predictive factors for treatment response to DEB-TACE, PFS in this study, were also evaluated. Concerns about whether OS is as significant as PFS in evaluation of prognosis on liver metastases patients still exists. Since many other factors such as the primary cancer sites, metastatic lesions at other sites, and general physical condition of the patient will greatly influence the OS. Therefore, PFS is chosen as a more suitable index to evaluate treatment efficacy in liver metastases patients in this study. Firstly, our study displayed that previous liver resection of metastatic lesions was correlated with worse PFS. This result seemed to be inconsistent with the previous evidence about the early resection efficiency in liver metastases. Although a successful resection of liver metastases may improve the prognosis of disease significantly, since a large part of liver metastases was found unresectable because of lesions locations and lymphatic metastasis, postoperative recurrence in our patients might indicate a failed treatment option. So, this could be explained by that tumor resection might facilitate the subsequent progression of lesions due to possible off-label resection, and it was vital to strictly screen the indications for surgical resection of liver metastases. Besides, combined with targeted therapy and DCR after the first DEB-TACE was correlated with prolonged PFS. These results inspire us that (I) the combined therapy with targeted drugs may prolong PFS in liver metastases, and bring a better treatment benefit compared with single DEB-TACE group in this study. Therefore, it would be practical to combine targeted therapy and DEB-TACE for effective control of disease progression on liver metastases in the future. (II) Patients who were evaluated as DCR after first DEB-TACE in whether patient level or primary nodules level, may suggest that they were more sensitive and responded better to DEB-TACE treatment, which might be a reliable feature for doctors to make an accurate prognostic evaluation after first DEB-TACE treatment.
Our study reported the treatment efficacy, safety profiles, and predictive factors of DEB-TACE in liver metastases in using detailed records like regular MRI reviews, while there remained several limitations. As a single-centered study, the selection of samples was subject to locoregional bias and the sample size was relatively insufficient, which may cause possible bias. Besides, although liver metastasis of distinct pathological types might respond differently to DEB-TACE, we did not investigate the distinction of liver metastasis transferred from different organs due to limited cases. In addition, the diameter of microspheres used in this study was 100–300 µm uniformly, while lesions with different pathological types might need different diameters.
In conclusion, DEB-TACE is an efficient and safe treatment choice for liver metastases, and strict screening of indications for resection as well as combined therapy with targeted therapy might improve the efficacy of DEB-TACE.
Table 4
| Items | Univariate Cox’s regression | Multivariate Cox’s regression | |||
|---|---|---|---|---|---|
| P value | HR (95% CI) | P value | HR (95% CI) | ||
| Age (>60 vs. ≤60 years) | 0.732 | 1.158 (0.500–2.681) | 0.486 | 1.542 (0.456–5.208) | |
| Gender (male vs. female) | 0.448 | 1.396 (0.590–3.304) | 0.698 | 1.253 (0.400–3.923) | |
| Diffuse disease (yes vs. no) | 0.120 | 2.388 (0.798–7.148) | 0.050 | 5.450 (1.000–29.710) | |
| Extrahepatic lesion (yes vs. no) | 0.873 | 1.070 (0.467–2.453) | 0.749 | 1.210 (0.377–3.884) | |
| Largest tumor size (>5 vs. ≤5 cm) | 0.907 | 1.050 (0.461–2.392) | 0.935 | 1.061 (0.252–4.477) | |
| Primary colorectal cancer vs. other cancers | 0.360 | 0.681 (0.299–1.552) | 0.469 | 0.544 (0.104–2.833) | |
| Previous liver resection (yes vs. no) | 0.413 | 1.580 (0.528–4.725) | 0.112 | 3.789 (0.732–19.621) | |
| Combined treatments | |||||
| cTACE (yes vs. no) | 0.337 | 0.615 (0.228–1.661) | 0.024 | 0.121 (0.019–0.752) | |
| Chemotherapy (yes vs. no) | 0.846 | 0.921 (0.401–2.114) | 0.909 | 1.071 (0.327–3.511) | |
| Targeted therapy (yes vs. no) | 0.368 | 0.650 (0.254–1.663) | 0.012 | 0.107 (0.019–0.617) | |
| Radiofrequency ablation (yes vs. no) | 0.473 | 0.717 (0.289–1.780) | 0.231 | 0.447 (0.120–1.669) | |
| Patients achieved ORR after first DEB-TACE (yes vs. no) | 0.704 | 0.850 (0.367–1.967) | 0.488 | 0.435 (0.041–4.572) | |
| Patients achieved DCR after first DEB-TACE (yes vs. no) | 0.775 | 1.237 (0.288–5.312) | 0.276 | 4.075 (0.326–50.894) | |
| The primary nodule achieved ORR after first DEB-TACE (yes vs. no) | 0.387 | 0.676 (0.278–1.644) | 0.517 | 0.476 (0.050–4.487) | |
| The primary nodule achieved DCR after first DEB-TACE (yes vs. no) | 0.327 | 0.581 (0.196–1.723) | 0.463 | 0.409 (0.037–4.461) | |
P value <0.05 was considered significant. OS, overall survival; HR, hazard ratio; CI, confidence interval; cTACE, conventional transarterial chemo-embolization; DEB-TACE, drug-eluting bead transarterial chemoembolization; ORR, overall response rate; DCR, disease control rate.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.01.61). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Institutional Review Board of our hospital (ethical approval number: 2019-432), and informed consents or verbal agreements with type recording were obtained from all enrolled patients or their guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sasson AR, Sigurdson ER. Surgical treatment of liver metastases. Semin Oncol 2002;29:107-18. [Crossref] [PubMed]
- Lee MH, Kim EJ, Lee H, et al. Liposomal Texaphyrin Theranostics for Metastatic Liver Cancer. J Am Chem Soc 2016;138:16380-7. [Crossref] [PubMed]
- Gruber-Rouh T, Marko C, Thalhammer A, et al. Current strategies in interventional oncology of colorectal liver metastases. Br J Radiol 2016;89:20151060. [Crossref] [PubMed]
- Jang JH, Lee JW, Hong JT, et al. Transarterial chemoembolization for hepatocellular carcinoma: an evidence-based review of its place in therapy. J Hepatocell Carcinoma 2015;2:123-9. [PubMed]
- Wei N, Zhang B, Wang Y, et al. Transarterial chemoembolization with raltitrexed-based or floxuridine-based chemotherapy for unresectable colorectal cancer liver metastasis. Clin Transl Oncol 2019;21:443-50. [Crossref] [PubMed]
- Zhang H, Guo J, Gao S, et al. Prognostic factors for transarterial chemoembolization combined with sustained oxaliplatin-based hepatic arterial infusion chemotherapy of colorectal cancer liver metastasis. Chin J Cancer Res 2017;29:36-44. [Crossref] [PubMed]
- Miura JT, Gamblin TC. Transarterial chemoembolization for primary liver malignancies and colorectal liver metastasis. Surg Oncol Clin N Am 2015;24:149-66. [Crossref] [PubMed]
- Matsuda H, Tamada S, Kato M, et al. Transarterial chemoembolization of liver metastasis from renal cell carcinoma. Urol Case Rep 2018;17:79-81. [Crossref] [PubMed]
- Nabil M, Gruber T, Yakoub D, et al. Repetitive transarterial chemoembolization (TACE) of liver metastases from renal cell carcinoma: local control and survival results. Eur Radiol 2008;18:1456-63. [Crossref] [PubMed]
- Vogl TJ, Naguib NN, Nour-Eldin NE, et al. Transarterial chemoembolization (TACE) with mitomycin C and gemcitabine for liver metastases in breast cancer. Eur Radiol 2010;20:173-80. [Crossref] [PubMed]
- Vogl TJ, Naguib NN, Lehnert T, et al. Initial experience with repetitive transarterial chemoembolization (TACE) as a third line treatment of ovarian cancer metastasis to the liver: indications, outcomes and role in patient's management. Gynecol Oncol 2012;124:225-9. [Crossref] [PubMed]
- Cao G, Zhu X, Li J, et al. A comparative study between Embosphere((R)) and conventional transcatheter arterial chemoembolization for treatment of unresectable liver metastasis from GIST. Chin J Cancer Res 2014;26:124-31. [PubMed]
- Ni JY, Xu LF, Wang WD, et al. Conventional transarterial chemoembolization vs microsphere embolization in hepatocellular carcinoma: a meta-analysis. World J Gastroenterol 2014;20:17206-17. [Crossref] [PubMed]
- Sun J, Zhou G, Zhang Y, et al. Comprehensive analysis of common safety profiles and their predictive factors in 520 records of liver cancer patients treated by drug-eluting beads transarterial chemoembolization. Medicine (Baltimore) 2018;97:e11131. [Crossref] [PubMed]
- Zhou GH, Han J, Sun JH, et al. Efficacy and safety profile of drug-eluting beads transarterial chemoembolization by CalliSpheres(R) beads in Chinese hepatocellular carcinoma patients. BMC Cancer 2018;18:644. [Crossref] [PubMed]
- Wu B, Zhou J, Ling G, et al. CalliSpheres drug-eluting beads versus lipiodol transarterial chemoembolization in the treatment of hepatocellular carcinoma: a short-term efficacy and safety study. World J Surg Oncol 2018;16:69. [Crossref] [PubMed]
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. [Crossref] [PubMed]
- Rostas J, Tam A, Sato T, et al. Image-Guided Transarterial Chemoembolization With Drug-Eluting Beads Loaded with Doxorubicin (DEBDOX) for Unresectable Hepatic Metastases from Melanoma: Technique and Outcomes. Cardiovasc Intervent Radiol 2017;40:1392-400. [Crossref] [PubMed]
- Yang Z, Chen G, Cui Y, et al. The safety and efficacy of TACE combined with apatinib on patients with advanced hepatocellular carcinoma: a retrospective study. Cancer Biol Ther 2019;20:321-7. [Crossref] [PubMed]
- Mizandari M, Paksashvili N, Kikodze N, et al. Long-term survival in a patient with low-level inflammatory markers and liver metastasis, converted resectable by TACE. Immunotherapy 2017;9:1067-9. [Crossref] [PubMed]
- Cornelis FH, Solomon SB. Treatment of Primary Liver Tumors and Liver Metastases, Part 2: Non-Nuclear Medicine Techniques. J Nucl Med 2018;59:1801-8. [Crossref] [PubMed]
- Zou JH, Zhang L, Ren ZG, et al. Efficacy and safety of cTACE versus DEB-TACE in patients with hepatocellular carcinoma: a meta-analysis. J Dig Dis 2016;17:510-7. [Crossref] [PubMed]
- Liu SF, Lu CR, Cheng HD, et al. Comparison of Therapeutic Efficacy between Gastrectomy with Transarterial Chemoembolization Plus Systemic Chemotherapy and Systemic Chemotherapy Alone in Gastric Cancer with Synchronous Liver Metastasis. Chin Med J (Engl) 2015;128:2194-201. [Crossref] [PubMed]


