
Effects of knockout of long-chain non-coding RNA LSINCT5 on proliferation, apoptosis, epithelial-mesenchymal transition, and p38MAPK pathway of pancreatic cancer PANC-1 cells
Introduction
Pancreatic cancer is a highly malignant gastrointestinal tumor with a 5-year survival rate of only around 6%, rendering it one of the world’s most lethal malignant tumors (1). While the pathogenesis of pancreatic cancer remains unclear, studies have shown that smoking (2), diabetes (3), obesity (4), alcohol (5), dietary habits (6), and genetic factors (7) are correlated with pathogenesis of pancreatic cancer.
Furthermore, the diagnosis and treatment of pancreatic cancer are highly challenging, and most patients do not have the chance to receive surgery for this highly occult malignancy. The cure rate is even lower, along with the invasion and metastasis of pancreatic cancer cells. Long non-coding RNAs (lncRNAs) are RNAs in the human genome that can be transcribed into RNA but do not express proteins. Their length exceeds 200 bp. Many studies have proved that lncRNAs play a key role in the occurrence and progression of cancer. LncRNA LSINCT5 may promote the development of a variety of malignant tumors such as human gliomas (8), esophageal cancer (9), ovarian cancer (10), and osteosarcoma (11). Therefore, in our current study, we constructed sh-LSINCT5 to knock out lncRNA LSINCT5, to explore the effect of lncRNA LSINCT5 on pancreatic cancer and p38MAPK pathway and thus provide a theoretical basis for future studies on the pathogenic mechanisms and therapeutic targets of pancreatic cancer.
Methods
Materials
Tumor tissues were collected from 21 patients with pancreatic cancer who were treated in our center from 2014 to 2016. Informed consent was obtained from the patients and their families, and the ethics committee had approved the study of our center. Human pancreatic cancer PANC-1 cell lines were purchased from ATCC, USA; RPMI-1640, trypsin, and fetal bovine serum (FBS) were purchased from Gibco, USA; Trizol and Lipofectamine 2000 were purchased from Invitrogen, USA; reverse transcription kits were purchased from TaKaRa, Japan; Cell Counting Kit-8 (CCK-8) was purchased from DOJINDO, Japan; RIPA lysate was purchased from Solarbio (Beijing, China); Annexin V-EGFP Apoptosis Staining/Detection Kit was purchased from BestBio, Shanghai, China; E-cadherin, N-cadherin, Vimentin, Caspase-3, Caspase-9, PCNA, SOX2, OCT4, and GAPDH antibody were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA); Ki67 was purchased from Abcam; HRP-labeled goat anti-mouse and HRP-labeled goat anti-rabbit secondary antibodies were purchased from Bioss, Beijing, China. GenePharma, Shanghai, China synthesized the primers. The cell incubators were bought from Thermo, USA, the vertical electrophoresis system was bought from Bio-Rad, USA, and the flow cytometers were purchased from BD, USA.
Cell culture
The human pancreatic cancer PANC-1 cell line was inoculated in RPMI 1640 medium holding 10% FBS and 1% penicillin-streptomycin and cultured in a cell incubator containing 5% CO2 at 37 °C. The cells were passaged when the cells were 90% confluent, and the culture medium was changed every two days. The cells were used for later experiments after their status became stable.
Vector construction and transfection
The small hairpin RNA (shRNA) vector targeting LSINCT5 (sh-LSINCT5) and the negative control vector (shRNA-NC) were designed and synthesized by Invitrogen (Beijing, China). The interference sequence of sh-LSINCT5 was as follows: CGAAAGCACGTAATCGCCGGTGTAACGAATTACACCGGCGATTAC. The PANC-1 cells were cultured in a 6-well plate at a density of 5×104 cells per well till they were 50% confluent and then used for later transfection. The cells were randomly divided into a control group, the shRNA-NC group, and the sh-LSINCT5 group, with five replicates in each group. After the cells became adherent, the shRNA-NC group was transfected with the shRNA-NC vector, the sh-LSINCT5 group was transfected with sh-LSINCT5 vector, and the control group was added with an equal amount of PBS.
Reverse transcription polymerase chain reaction (RT-PCR)
RNA was extracted with TRIzol, and cDNA was synthesized according to the kit instructions. The LSINCT5 primer was as follows: forward: 5'-CCAGCUACAAACCUCUGAATT-3'; reverse: 5'-UUCAGAGGUUUGUAGCUG GTT-3'. The amplification conditions were as follows: pre-denaturation at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 10 s, annealing at 60 °C for 20 s, and extension at 72 °C for 10 s. The control group sample was set as standard 1, and the relative expressions of the target genes were analyzed and compared.
CCK-8 cell proliferation assay
After the density of PANC-1 cells was adjusted to 5×104 cells/mL, the cells were seeded in 96-well plates, and 100 µL of cell suspension was added to each well. The cells were grouped as described above, and the medium was changed every two days. The proliferation of the cells in each group was detected 1, 2, 3, and 4 days after transfection, and three replicates were set in different cell groups at each time point. After 10 µL of CCK-8 solution was added to each well, the cells were incubated at room temperature in the dark for 4 h. Absorbance was measured spectrophotometrically at 450 nm, which was repeated three times to calculate the average.
Apoptosis assayed by flow cytometry
After transfection, 1×105 cells were taken from each group and then added with 500 µL of Binding Buffer suspension cells. Subsequently, 100 µL of cell suspension was added to the Falcon tube. Then, 5 µL of Annexin V-EGFP was added and mixed well, followed by the adding of 5 µL of propidium iodide. After the mixture was cultured in the dark for 10 min, the apoptosis of cells was detected by flow cytometry.
Spherogenesis assay
In each group, 100 cells were obtained and then seeded into a 24-well ultra-low attachment culture plate. Five replicates were set for each group. The cells were cultured in a serum-free medium for 1 week, and the spherogenesis was observed under an inverted microscope daily.
Western blotting
After the transfection, the PANC-1 cells were cultured in 6-well plates at a density of 1×105 cells/well for 24 h at 37 °C in a humidified 5% CO2 atmosphere. The total protein was extracted from the lysed cells. A proper amount of proteins was loaded and then transferred to a PVDF membrane after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After the filters were blocked for 2 h at room temperature with 5% skim milk, the products were incubated with the primary and secondary antibodies. After rinsing the wells with PBST, BCL was added for exposing. The protein band was visualized and analyzed for gray-level analysis using ImageJ software (Bio-Rad, California, USA).
Statistical analysis
Statistical analyses were implemented by using SPSS 19.0 software package. The experimental results are presented as mean ± standard deviation (). Normality and homogeneity of the data were confirmed before statistical analysis. Pairwise comparisons were performed using independent t-tests. Differences between groups were evaluated using a one-way analysis of variance (ANOVA). Survival analysis was based on the Kaplan-Meier test, and survival curves were drawn. A P value of <0.05 was considered significantly different.
Results
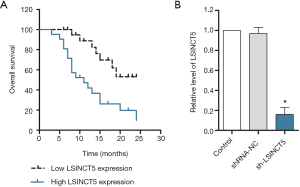
The survival rate was higher in patients with low expression of lncRNA LSINCT5
Twenty-one patients with pancreatic cancer were clinically collected, and Kaplan-Meyer curves were drawn to compare the survival rates of patients with low or high expression of lncRNA LSINCT5. As shown in Figure 1A, the survival rate of patients with low expression of lncRNA LSINCT5 was higher than that of patients with high expression of lncRNA LSINCT5. The shRNA-NC and sh-LSINCT5 were transfected into PANC-1 cells, respectively, and RT-PCR detected the LINCT5 expression. As shown in Figure 1B, compared with the control group, the sh-MIAT group had significantly lower expression of lncRNA LSINCT5 (P<0.05), showing successful interference.
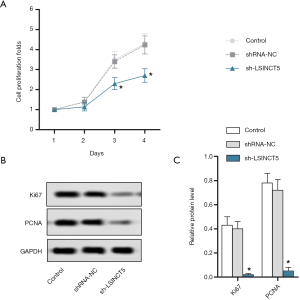
lncRNA SINCT5 knockout inhibited the proliferation of PANC-1 cells
CCK-8 detected the proliferation of PANC-1 cells. As shown in Figure 2A, compared with the control group, the sh-LSINCT5 group had a significantly lower cell proliferation ratio (P<0.05). Western blotting was used to detect Ki67 and PANC expressions. As shown in Figure 2B,C, compared with the control group, the sh-LSINCT5 group had significantly lower expressions of Ki67 and PANC (both P<0.05). Thus, the knockout of the LSCINC5 gene inhibited the proliferation of pancreatic cancer PANC-1 cells.
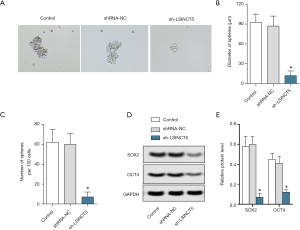
lncRNA SINCT5 knockout inhibited spherogenesis of PANC-1 cells
Spherogenesis assay was applied to detect the proliferation ability of PANC-1 cells. As shown in Figure 3A,B,C, compared with the control group, the sh-LSINCT5 group had a significantly smaller sphere diameter and fewer spheres (both P<0.05). Western blotting was used to detect the expressions of SOX2 and OCT4. As shown in Figure 3D,E, compared with the control group, the sh-LSINCT5 group had significantly lower expressions of SOX2 and OCT4 (both P<0.05). Thus, the knockout of the LSCINC5 gene inhibited the stemness and proliferation ability of pancreatic cancer PANC-1 cells.
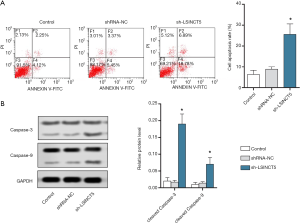
Knockout of lncRNA SINCT5 promoted apoptosis of PANC-1 cells
The apoptosis of pancreatic cancer PANC-1 cells was detected by flow cytometry. As shown in Figure 4A, compared with the control group, the sh-LSINCT5 group had significantly more apoptotic cells (P<0.05). Western blotting was used to detect the expressions of cleaved Caspase-3 and cleaved Caspase-9. As shown in Figure 4B, compared with the control group, the sh-LSINCT5 group had significantly higher expressions of cleaved Caspase-3 and cleaved Caspase-9 (both P<0.05). Thus, knockout of lncRNA SINCT5 promoted the apoptosis of PANC-1 cells.
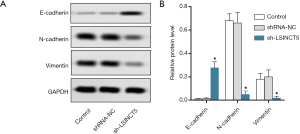
lncRNA SINCT5 knockout inhibited EMT of PANC-1 cells
The expressions of E-cadherin, N-cadherin, and Vimentin were detected by Western blotting. As shown in Figure 5, compared with the control group, the sh-LSINCT5 group had significantly decreased expressions of N-cadherin and Vimentin (both P<0.05) and significantly increased E-cadherin (P<0.05). Thus, lncRNA SINCT5 knockout inhibited EMT of PANC-1 cells.
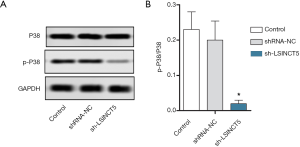
Knockout of lncRNA SINCT5 inhibited p38MAPK pathway
The activation of p38 was detected by Western blotting. As shown in Figure 6, compared with the control group, the sh-LSINCT5 group had a significantly lower p-P38/P38 ratio (P<0.05). Thus, LSNCT5 knockout significantly suppressed p38 activation.
Discussion
Pancreatic cancer in the digestive system is a highly occult malignancy, so patients often miss the opportunity for surgical treatment. Particularly challenging is the diagnosis and treatment of pancreatic cancer; pancreatic cancer in particular responds poorly to chemoradiotherapy (12,13). More in-depth work on the role of oncogenesis and therapeutic targets for pancreatic cancer are therefore urgently needed.
Many studies have reported that lncRNA LSINCT5 plays a pro-carcinogenic role in a variety of malignant tumors. The expression of lncRNA LSINCT5 was significantly up-regulated in hepatocellular carcinoma tissues and cell lines, and patients with low lncRNA LSINCT5 expression in cancer tissues had a higher survival rate than those with high lncRNA LSINCT5 expression. Meanwhile, lncRNA LSINCT5 could promote the invasion of hepatocellular carcinoma cells and stimulate the forming of tumor spheres, showing an apparent pro-carcinogenic effect (14). In addition, Long et al. found that the expression of lncRNA LSINCT5 was higher in ovarian cancer tissues than in normal ovarian tissues and was also positively correlated with the lymphatic metastasis of cancer cells. Knockout of LINCT5 significantly decreased the expression of chemokine receptor 4 (CXCR4) and suppressed the proliferation, migration, and invasion of SKOV3 cells (15). As shown in our current study, patients with high lncRNA LSINCT5 expression had a lower survival rate, and knockout of lncRNA LSINCT5 significantly reduced the expressions of Ki67, PANC, SOX2, and OCT4 in PANC-1 cells and decreased the proliferation and spherogenesis of PANC-1 cells. Ki67 is expressed only in the nuclei of proliferating cells, while the proliferating cell nuclear antigen (PCNA) plays a crucial role in DNA replication and replication-related processes. Both are closely related to tumor cell proliferation and growth. In addition, OCT4 and SOX2 are highly expressed in a variety of tumor cells and serve as the cellular markers of tumor stem cells. Therefore, knockout of lncRNA LSINCT5 can inhibit the proliferation and spherogenesis of PANC-1 cells, thus suppressing the development and progression of pancreatic cancer.
The uncontrolled proliferation of cells is the main feature of a malignant tumor, while apoptosis is an important mechanism to regulate the development of tumors. Cysteinyl aspartate specific proteinase (caspase) can selectively cleave certain proteins to regulate apoptosis (16). Studies have shown that lncRNA LSINCT5 was highly expressed in glioma tissues, and knockout of lncRNA LSINCT5 could inhibit the growth and metastasis of glioma GL15 cells and promote the apoptosis of GL15 cells (8). Another study revealed that knockout of lncRNA LSINCT5 significantly increased the sensitivity of ovarian cancer cells to cisplatin; meanwhile, it up-regulated the expression of Caspase-3 and promoted the apoptosis of ovarian cancer cells (17). In our current study, knockout of lncRNA LSINCT5 significantly increased the amount of apoptotic PANC-1 cells and up-regulated the expression of cleaved Caspase-3 and cleaved Caspase-9. Thus, knockout of lncRNA LSINCT5 may inhibit the proliferation of pancreatic cancer cells and the development of pancreatic cancer by promoting the apoptosis of pancreatic cancer cells.
The invasion and metastasis of tumor cells are one of the significant causes of cancer mortality in patients with pancreatic cancer. Epithelial-mesenchymal transition (EMT) is a process by which epithelial cells become a mesenchymal cell phenotype, which is an essential process for the invasion and metastasis of cancer cells (18). The downregulation of cell adhesion factor E-cadherin and the up-regulation of N-cadherin and vimentin represent EMT activation. Studies have shown that knockout of lncRNA LSINCT5 could up-regulate the expression of E-cadherin and down-regulate the expression of N-cadherin, thereby inhibiting the migration and invasion of gastric cancer cells (19). In addition, Jing et al. found that the expression of lncRNA LSINCT5 was significantly up-regulated in esophageal squamous cell carcinoma (ESCC) cells, and knockout of lncRNA LSINCT5 significantly inhibited the proliferation, migration, invasion, and EMT of ESCC cells (9). In our current study, we found that the knockout of the LSCINC5 gene significantly up-regulated the expression of E-cadherin and decreased the expressions of N-cadherin and vimentin in PANC-1 cells. Therefore, knockout of LSCINCT may inhibit the invasion and metastasis of pancreatic cancer cells.
Mitogen-activated protein kinase (MAPK) is a vital substance that transmits cell surface signals to the nucleus; notably, p38 mitogen-activated protein kinases (p38MAPK) pathway is an essential member of the MAPK family and participates in the regulation of cell proliferation and differentiation. Research has shown that inhibiting p38 activation could effectively suppress the progression of pancreatic cancer (20). It was therefore hypothesized that knockout of lncRNA LSINCT5 could affect p38 activity and thus affect the development of pancreatic cancer. The results of our current study showed that knockout of lncRNA LSINCT5 significantly inhibits the phosphorylation level of p38 protein. Therefore, the knockout of lncRNA SINCT5 can affect the development of pancreatic cancer by inhibiting the p38MAPK pathway.
To sum up, the survival rate of patients with high lncRNA LSINCT5 expression was significantly lower than that of patients with low lncRNA LSINCT5 expression, and the knockout of lncRNA LSINCT5 could significantly inhibit the proliferation, spherogenesis, and EMT of pancreatic cancer PANC-1 cells and meanwhile suppress the activity of the p38MAPK pathway. Therefore, lncRNA LSINCT5 may be a promising therapeutic target for pancreatic cancer. In our future studies, we will investigate the effect of knocking out lncRNA LSINCT5 on the oncogenesis of pancreatic cancer and the relationship between the p38MAPK pathway and pancreatic cancer progression in mouse models of slow-growing pancreatic cancer.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.01.50). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from the patients and their families, and the ethics committee had approved the study of our center.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016;22:9694-705. [Crossref] [PubMed]
- Korc M, Jeon CY, Edderkaoui M, et al. Tobacco and alcohol as risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol 2017;31:529-36. [Crossref] [PubMed]
- Rahn S, Zimmermann V, Viol F, et al. Diabetes as risk factor for pancreatic cancer: Hyperglycemia promotes epithelial-mesenchymal-transition and stem cell properties in pancreatic ductal epithelial cells. Cancer Lett 2018;415:129-50. [Crossref] [PubMed]
- Incio J, Liu H, Suboj P, et al. Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy. Cancer Discov 2016;6:852-69. [Crossref] [PubMed]
- Jayasekara H, English DR, Hodge AM, et al. Lifetime alcohol intake and pancreatic cancer incidence and survival: findings from the Melbourne Collaborative Cohort Study. Cancer Causes Control 2019;30:323-31. [Crossref] [PubMed]
- Chang HH, Moro A, Takakura K, et al. Incidence of pancreatic cancer is dramatically increased by a high fat, high calorie diet in KrasG12D mice. PLoS One 2017;12:e0184455. [Crossref] [PubMed]
- Cao SS, Su AP, Du XJ, et al. The expressions and correlation of bcl-2 and Beclin-1 in pancreatic cancer. Sichuan Da Xue Xue Bao Yi Xue Ban 2012;43:156-60. [PubMed]
- Liu B, Cao W, Ma H. Knockdown of lncRNA LSINCT5 suppresses growth and metastasis of human glioma cells via up-regulating miR-451. Artif Cells Nanomed Biotechnol 2019;47:2507-15. [Crossref] [PubMed]
- Jing L, Lin J, Zhao Y, et al. Long noncoding RNA LSINCT5 is upregulated and promotes the progression of esophageal squamous cell carcinoma. Eur Rev Med Pharmacol Sci 2019;23:5195-205. [PubMed]
- Ren C, Li X, Wang T, et al. Functions and mechanisms of long noncoding rnas in ovarian cancer. Int J Gynecol Cancer 2015;25:566-9. [Crossref] [PubMed]
- Cao YY, Yang GZ, Gong YJ, et al. Effect of Huntingtin-associated Protein 1 Gene on Proliferation of Mouse Fibroblasts. Sichuan Da Xue Xue Bao Yi Xue Ban 2019;50:298-304. [PubMed]
- Ercan G, Karlitepe A, Ozpolat B. Pancreatic Cancer Stem Cells and Therapeutic Approaches. Anticancer Res 2017;37:2761-75. [PubMed]
- Rossi ML, Rehman AA, Gondi CS. Therapeutic options for the management of pancreatic cancer. World J Gastroenterol 2014;20:11142-59. [Crossref] [PubMed]
- Li O, Li Z, Tang Q, et al. Long stress induced non-coding transcripts 5 (LSINCT5) promotes hepatocellular carcinoma progression through interaction with high-mobility group at-hook 2 and mir-4516. Med Sci Monit 2018;24:8510-23. [Crossref] [PubMed]
- Long X, Li L, Zhou Q, et al. Long non-coding RNA LSINCT5 promotes ovarian cancer cell proliferation, migration and invasion by disrupting the CXCL12/CXCR4 signalling axis. Oncol Lett 2018;15:7200-6. [PubMed]
- Miller DK. The role of the Caspase family of cysteine proteases in apoptosis. Semin Immunol 1997;9:35-49. [Crossref] [PubMed]
- Wang L, Wang HY, Huang Q, et al. Effect of siRNA LSINCT5 combined with cisplatin on the proliferation and apoptosis of ovarian cancer SKOV3 cells. Progress in Obstetrics and Gynecology 2016;25:349-53.
- Huang R, Zong X. Aberrant cancer metabolism in epithelial-mesenchymal transition and cancer metastasis: Mechanisms in cancer progression. Crit Rev Oncol Hematol 2017;115:13-22. [Crossref] [PubMed]
- Qi P, Lin WR, Zhang M, et al. E2F1 induces LSINCT5 transcriptional activity and promotes gastric cancer progression by affecting the epithelial-mesenchymal transition. Cancer Manag Res 2018;10:2563-71. [Crossref] [PubMed]
- Alam MS, Gaida MM, Bergmann F, et al. Selective inhibition of the p38 alternative activation pathway in infiltrating T cells inhibits pancreatic cancer progression. Nat Med 2015;21:1337-43. [Crossref] [PubMed]


