
A promising treatment option for refractory male primary choriocarcinoma: report of two cases
Introduction
Choriocarcinoma is a rare trophoblastic tumor that can secrete human chorionic gonadotropin (HCG). It can be categorized as non-gestational choriocarcinoma (primary choriocarcinoma) and gestational choriocarcinoma. Primary choriocarcinoma is highly invasive and extremely rare in men. It can be further subdivided into gonadal choriocarcinoma and extragonadal choriocarcinoma based on origin and primary site (1,2). The commonly used chemotherapy regimens are EMA/CO (etoposide, methotrexate, actinomycin D, cyclophosphamide and vincristine) and TP (paclitaxel and cisplatin), but advanced male primary choriocarcinoma is insensitive to chemotherapy and has a poor prognosis, with a median overall survival of only about half a year (3,4). Herein we retrospectively analyzed two patients with advanced male primary choriocarcinoma, one who received chemotherapy alone and died of progressive disease, the other who received pembrolizumab combined with chemotherapy and achieved complete response, and no recurrence was observed during 36 months of follow-up. To date, no successful treatment of male primary choriocarcinoma with pembrolizumab has been reported. We aim to highlight the limited response of male primary choriocarcinoma to chemotherapy, as well as favorable response to pembrolizumab in patients with high expression of programmed death ligand 1 (PD-L1) on tumor cells. We present the following case in accordance with the Care Guideline (5).
Case presentation
Patient 1
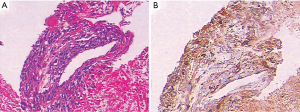
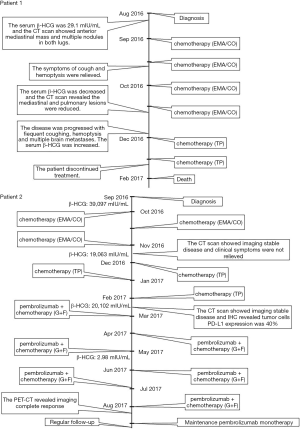
A 26-year-old man was admitted to the Second Xiangya Hospital of Central South University in August 2016 due to cough and hemoptysis for more than 20 days. He had no special past medical, familial, or psychosocial history. Physical examination showed mild bilateral breast swelling and atrophy of the left testis. Serum tumor marker detection showed that β-HCG was 29.1 mIU/mL (normal <3.0 mIU/mL). Chest contrast-enhanced computed tomography (CT) revealed an anterior superior mediastinal mass and multiple nodules in both lungs. Serum sex hormone examination showed low follicle-stimulating hormone (0.05 mIU/mL, normal 0.95–11.95 mIU/mL) and luteinizing hormone (0.25 mIU/mL, normal 1.14–8.75 mIU/mL) levels and high prolactin (63.74 ng/mL, normal 3.46–19.40 ng/mL), estradiol (378.48 pg/mL, normal 11.00–44.00 pg/mL), and progesterone (1.79 ng/mL, normal 0.10–0.20 ng/mL) levels. He was diagnosed with primary mediastinal choriocarcinoma after CT-guided percutaneous transthoracic aspiration biopsy of the anterior mediastinal mass (Figure 1A,B). After 4 cycles of first-line chemotherapy with EMA/CO (day 1, actinomycin D 0.5 mg, etoposide 100 mg/m2 and methotrexate 100 mg/m2 then 200 mg/m2 over 12 h; day 2, actinomycin D 0.5 mg, etoposide 100 mg/m2 and leucovorin 15 mg quarterly (4 doses, 24 h after the first administration of methotrexate); day 8, vincristine 1 mg/m2 and cyclophosphamide 600 mg/m2, every 2 weeks), the symptoms of cough and hemoptysis were relieved, the Eastern Cooperative Oncology Group score was decreased to 1, and the prolactin, estradiol, and progesterone levels returned to normal. During chemotherapy, the patient developed grade 3 myelosuppression and severe vomiting, which improved after treatment with recombinant human granulocyte-stimulating factor and aprepitant. However, the disease soon progressed with frequent coughing, hemoptysis, increased serum β-HCG and multiple brain metastases. He did not respond to 2 cycles of TP regimen (day 1, paclitaxel 175 mg/m2 and cisplatin 75 mg/m2, every 3 weeks). His serum β-HCG continued to rise, and symptoms of intracranial hypertension such as headache, dizziness, and vomiting were present. The patient subsequently discontinued treatment and died in February 2017 with an overall survival of 6.5 months.
Patient 2
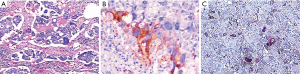
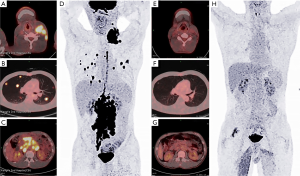
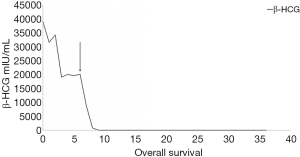
A 40-year-old man was admitted to the Second Xiangya Hospital of Central South University in September 2016 for a left neck mass and abdominal pain for 1 month. The patient was known to have hepatitis C and right inguinal hernia, and the rest of medical, family or psychosocial histories were unremarkable. One month ago, he suffered from repeated abdominal pain, which was relieved by oxycodone/acetaminophen (5 mg/325 mg, four times a day). Physical examination indicated abdominal tenderness and a painless lump in the neck. He was diagnosed with primary neck choriocarcinoma through pathologic examination of the left neck mass biopsy (Figure 2A,B,C). Serum tumor marker examination showed that his β-HCG level was 39,097 mIU/mL, and 18F-fluorodeoxyglucose (FDG)-positron emission tomography-CT (PET-CT) (Figure 3) showed enlarged lymph nodes with increased FDG uptake all over the body and multiple nodules with increased FDG uptake in both lungs (Figure 3A,B,C,D). After receiving 3 cycles of EMA/CO chemotherapy (day 1, actinomycin D 0.5 mg, etoposide 100 mg/m2 and methotrexate 100 mg/m2 then 200 mg/m2 over 12 h; day 2, actinomycin D 0.5 mg, etoposide 100 mg/m2 and leucovorin 15 mg quarterly (4 doses, 24 h after the first administration of methotrexate); day 8, vincristine 1 mg/m2 and cyclophosphamide 600 mg/m2, every 2 weeks), the patient’s serum β-HCG had decreased to 19,063 mIU/mL, but enhanced CT showed no significant changes in the multiple enlarged lymph nodes all over the body and multiple nodules in both lungs. Further, he was still experiencing abdominal pain. The patient then received second-line TP (day 1, paclitaxel 175 mg/m2 and cisplatin 75 mg/m2, every 3 weeks) for 3 cycles. CT revealed stable disease, and his serum β-HCG had increased to 20,102 mIU/mL. His abdominal pain had still not improved. Immunohistochemistry showed that the expression level of PD-L1 on the tumor cells was 40%. Therefore, third-line chemotherapy (day 1, gemcitabine 2,000 mg, fluorouracil 600 mg and leucovorin 600 mg, fluorouracil 3,500 mg continuous intravenous infusion over 46 h; day 8, gemcitabine 2,000 mg, every 3 weeks) combined with pembrolizumab (day 9, 200 mg, every 3 weeks) was administered for 3 cycles. After this treatment, the patient’s serum β-HCG decreased to within the normal range and his abdominal pain improved significantly. He then received another 3 cycles, and PET-CT showed that the multiple enlarged lymph nodes all over the body disappeared and multiple pulmonary nodules were significantly reduced with no FDG uptake (Figure 3E,F,G,H). The combination therapy was well tolerated, the patient developed grade 2 myelosuppression and mild nausea, and there was no evidence of pembrolizumab-related adverse events such as thyroid dysfunction, myositis and pneumonitis. The patient was then administered maintenance pembrolizumab monotherapy (200 mg, every 3 weeks). Serum β-HCG was monitored and enhanced CT was performed periodically during follow-up, and no recurrence has been observed for 36 months (Figure 4). The clear timeline of two patients including treatment process and outcomes were shown in Figure 5.
Discussion
Primary choriocarcinoma is a rare and aggressive malignancy with poor prognosis. It usually occurs in the midline of the body, including the retroperitoneum, mediastinum, and pineal region, and often coexists with other malignant tumor components, such as teratoma, dysgerminoma, or spermatocytoma (6,7). Patients often have significantly increased serum β-HCG level, and male patients often have other specific signs, including feminization of the breast, testicular atrophy, and loss of libido (8). Hematogenous metastasis usually occurs early, and the lung is the most common site of metastasis (9). Male primary choriocarcinoma progresses rapidly, with a median overall survival of only 7.7 months and a 1-month mortality rate of 23.8% (10). Serum β-HCG level can be used as a good indicator of diagnosis, prognosis, and therapeutic effect. Female choriocarcinoma is sensitive to chemotherapy, and the EMA/CO regimen is the first choice (3). Cisplatin, etoposide, and bleomycin can be used in patients with germ-cell tumor components (11). Paclitaxel, isophosphamide, phosphoadenamine, platinum agents, and epirubicin can be selected for second-line therapy (12,13). There is currently no standard chemotherapy regimen for male primary choriocarcinoma, and the high-intensity chemotherapy regimens common for female choriocarcinoma are generally used. However, as observed in these two cases, male primary choriocarcinoma is insensitive to chemotherapy alone. Since no targetable driver gene has yet been identified in male primary choriocarcinoma, clinicians should explore other methods on the basis of traditional chemotherapy to improve efficiency and prognosis, such as chemotherapy combined with bevacizumab or PD-1/PD-L1 monoclonal antibodies.
Pembrolizumab is a potent, highly selective, fully human IgG4 anti-PD-1 immune checkpoint inhibitor that can activate T cells to kill tumor cells by blocking the binding of the PD-1 receptor with PD-L1/2. Studies have confirmed its efficacy in several advanced malignancies and the expression of PD-L1 is considered an important biomarker to predict the efficacy (14,15). Studies have shown that PD-L1 expression is significantly higher in choriocarcinoma than in embryonal carcinoma, spermatocytoma, and other types of extragonadal germ cell tumors, and its increased expression is associated with poor prognosis (16). There was a previous report of a female choriocarcinoma treated with pembrolizumab in which the patient achieved biochemical complete response (17). Additionally, preclinical studies have demonstrated that chemotherapy enhances the anti-tumor immune response by upregulating tumor antigens, inducing dendritic cell maturation, and inhibiting regulatory T cells (18), suggesting that pembrolizumab combined with chemotherapy may be a promising option. However, the morbidity of male primary choriocarcinoma is extremely low, making it difficult to perform large-scale clinical trials to select the optimal population for pembrolizumab treatment. In other malignancies, the expression of PD-L1 on tumor cells, tumor mutation burden (TMB) and high microsatellite instability (MSI-H) have been shown to be biomarkers for predicting the efficacy of pembrolizumab (19). It was regrettable that we did not detect TMB and MSI status in our second patient, so it was unknown whether these two indicators had predictive efficacy in male primary choriocarcinoma. For this rare disease, we can only refer to other malignancies when selecting the optimal population, and the clinicians can try to detect TMB or MSI status when the patient had negative PD-L1 expression on tumor cells. It is also important to note that the dramatic efficacies of the pembrolizumab commonly accompanied by side effects. Even though the tolerance and safety of our second patient were generally good, it has been widely reported that pembrolizumab can cause severe or fatal immune-related adverse events such as cardiotoxicity, pneumonitis and neurological toxicities (20), and early recognition and management is crucial to improve patient outcomes. The PD-L1 expression in our second patient was 40%, which prompted the administration of pembrolizumab. After 6 cycles of chemotherapy combined with pembrolizumab, the patient achieved imaging and biochemical complete response. Pembrolizumab was subsequently used for maintenance therapy and no recurrence has been found. Therefore, pembrolizumab has further demonstrated its potential as a new treatment option for refractory male primary choriocarcinoma.
Conclusions
There is currently no standard treatment for male primary choriocarcinoma. We successfully treated refractory male primary choriocarcinoma with pembrolizumab combined with chemotherapy. This may be a new treatment option for this disease and suggests that the expression of PD-L1 is important. However, further investigations are warranted to provide evidence for pembrolizumab in the treatment of this rare disease.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Seckl MJ, Fisher RA, Salerno G, et al. Choriocarcinoma and partial hydatidiform moles. Lancet 2000;356:36-9. [Crossref] [PubMed]
- Jiang F, Xiang Y, Feng FZ, et al. Clinical analysis of 13 males with primary choriocarcinoma and review of the literature. Onco Targets Ther 2014;7:1135-41. [Crossref] [PubMed]
- Lu WG, Ye F, Shen YM, et al. EMA-CO chemotherapy for high-risk gestational trophoblastic neoplasia: a clinical analysis of 54 patients. Int J Gynecol Cancer 2008;18:357-62. [Crossref] [PubMed]
- Amikura T, Aoki Y, Banzai C, et al. Metastatic choriocarcinoma successfully treated with paclitaxel and carboplatin after interstitial lung disease induced by EMA-CO. Gynecol Oncol 2006;102:573-5. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE 2013 Explanations and Elaborations: Reporting Guidelines for Case Reports. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Jiang F, Yang X, Feng FZ, et al. Clinical analysis of 13 males with primary choriocarcinoma and review of the literature. Onco Targets Ther 2014;7:1135-41. [Crossref] [PubMed]
- Gaude GS, Patil P, Malur PR, et al. Primary mediastinal choriocarcinoma. South Asian J Cancer 2013;2:79. [Crossref] [PubMed]
- Vegh GL, Szigetvari I, Soltesz I, et al. Primary pulmonary choriocarcinoma: a case report. J Reprod Med 2008;53:369-72. [PubMed]
- Snoj Z. Kocijiancic, Skof E. Primary pulmonary choriocarcinoma. Radiol Oncol 2016;51:1-7. [Crossref] [PubMed]
- Yokoi K, Tanaka N, Furukawa K, et al. Male choriocarcinoma with metastasis to the jejunum: a case report and review of the literature. J Nippon Med Sch 2008;75:116-21. [Crossref] [PubMed]
- Bokemeyer C, Kollmannsberger C, Meisner C, et al. First-line high-dose chemotherapy compared with standard-dose PEB/VIP chemotherapy in patients with advanced germ cell tumors: A multivariate and matched-pair analysis. J Clin Oncol 1999;17:3450-6. [Crossref] [PubMed]
- Kondagunta GV, Bacik J, Donadio A, et al. Combination of paclitaxel, ifosfamide, and cisplatin is an effective second-line therapy for patients with relapsed testicular germ cell tumors. J Clin Oncol 2005;23:6549-55. [Crossref] [PubMed]
- Bedano PM, Brames MJ, Williams SD, et al. Phase II study of cisplatin plus epirubicin salvage chemotherapy in refractory germ cell tumors. J Clin Oncol 2006;24:5403-7. [Crossref] [PubMed]
- Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015;372:2521-32. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Cierna Z, Mego M, Miskovska V, et al. Prognostic value of programmed-death-1 receptor (PD-1) and its ligand 1 (PD-L1) in testicular germ cell tumors. Ann Oncol 2016;27:300-5. [Crossref] [PubMed]
- Huang M, Pinto A, Castillo RP, et al. Complete Serologic Response to Pembrolizumab in a Woman With Chemoresistant Metastatic Choriocarcinoma. J Clin Oncol 2017;35:3172-4. [Crossref] [PubMed]
- Santabarbara G, Maione P, Rossi A, et al. Novel immunotherapy in the treatment of advanced non-small cell lung cancer. Expert Rev Clin Pharmacol 2016;9:1571-81. [Crossref] [PubMed]
- Gelsomino F, Lamberti G, Parisi C, et al. The evolving landscape of immunotherapy in small-cell lung cancer: A focus on predictive biomarkers. Cancer Treat Rev 2019;79:101887. [Crossref] [PubMed]
- Wang DY, Salem JE, Cohen JV, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:1721-8. [Crossref] [PubMed]


