
Evaluation of serum midkine and carcinoembryonic antigen as diagnostic biomarkers for rectal cancer and synchronous metastasis
Introduction
Rectal cancer concerned about cancer of the large intestine and rectum is one of the most common malignancies that affect the digestive system. As with most other neoplasms, the incidence rate for rectal cancer rises dramatically with age. In the United States, an estimated 135,430 cases of rectal cancer are diagnosed in 2017, resulting in approximately 50,260 deaths (1). The key to decreasing rectal cancer mortality is early detection, diagnosis and treatment. However, most rectal cancer patients miss the optimal treatment strategy and have poor prognosis owing to no early warning signs and symptoms. At present, the early diagnosis and prognosis of rectal cancer mainly depend on colonoscopy, imaging and other examinations with relatively high expenses. Patients exhibit very low levels of adherence on subsequent return to the hospital. In the diagnosis and therapy of rectal cancer, molecular tumor markers have been playing an increasingly vital role. However, conventional biomarkers like carcinoembryonic antigen (CEA) are unable to satisfy the demand of patients in clinical practice. For over 40 years, American Society of Clinical Oncologists has recommended performing routine measurement of CEA to monitor local recurrence or metastatic relapse in rectal cancer patients, however, CEA increase only occurs for 38.7% of rectal cancer (2). In addition to rising in most rectal cancer, pancreatic cancer, stomach cancer, lung cancer, and breast cancer, the CEA is also seemed to be slightly elevated in some benign diseases. Therefore, it is of great significance to identify effective serum biomarkers with both satisfactory sensitivity and specificity to detect rectal cancer before surgery and to predict the prognosis.
Serum midkine (S-MK), firstly reported as the product of a retinoic acid-responsive gene, is a heparin-binding growth factor (3). It specifically advances in neuronal survival and differentiation and also has a critical role in cell growth, survival, migration, angiogenesis, and carcinogenesis (4). The expression of S-MK is up-regulated in neuroblastoma, gastrointestinal cancers, bladder cancer (5-10). However, little is known about S-MK levels in the multistep process of oncogenesis and development of rectal cancer. In 2013, Krzystek-Korpacka et al. (11) published a paper describing that S-MK was higher in rectal cancer than in inflammatory bowel disease (IBD), adenoma or controls. Nonetheless, the diagnostic and prognostic values of serum S-MK have not yet been comprehensively elucidated in patients with rectal cancer.
Therefore, the footing of this study was to determine the diagnostic significance and the prediction ability for synchronous metastasis of serum S-MK for rectal cancer before the radical resection. To achieve this, the study among S-MK, CEA and combination of S-MK and CEA were conducted respectively. Sensitivity, specificity, and diagnostic accuracy, positive predictive value (PPV) and negative predictive value (NPV) were evaluated by statistically analyzing and compiling the measured parameters for differential diagnosis.
Methods
Patients
A group of 106 subjects were investigated in this study. There were 76 individuals with rectal cancer, 30 subjects with benign rectal polyps. Enrolled patients were treated between October 2015 and October 2017 at the surgical department of Tianjin Union Medical Center (China). Blood samples were collected at the admission or a close time-point preceding treatments.
Sera from blood donors were considered healthy according to routine blood tests. The control group enrolled 30 individuals from the cohorts in the health management department of Tianjin Union Medical Center.
Patient recruitment and sample collection were performed within the guidelines of protocols approved by the institutional review boards and informed consent was obtained from all subjects. The ID/number of ethical approval is 2019C03 approved by Tianjin Union Medical Center.
Serum parameter evaluation
CEA (reference 0–5.00 ng/mL), carbohydrate antigen 19-9 (CA19-9) (reference 0–37.00 U/mL) and carbohydrate antigen 72-4 (CA72-4) (reference 0–69.00 U/mL) assays were performed on a fully automated ADVIA Centaur analyzer (Siemens Healthcare Diagnostics, New York, USA). All these assays were conducted with respect to the chemiluminescent reaction principle.
Serum S-MK measurement
Blood samples (5 mL) were obtained by venipuncture and immediately centrifuged at 3,000 ×g for 10 min. The serum was frozen at −80 °C until the assay was performed. We avoided repeating thawing and freezing of samples. Enzyme-linked immunosorbent assay (ELISA) (DuoSet ELISA, R&D Systems Inc., USA) was performed to measure sample S-MK level. The assay was conducted according to the manufacturer's instructions and values were reported as pg/mL. Briefly, the microplate was pre-coated with goat anti-human S-MK antibody (R&D Systems UK) and then blocked by 1% bovine serum albumin (BSA, St. Louis, USA). One hundred µL of each serum sample (1:10 dilution) or standard was incubated in a 96-well microplate (Corning UK) for 2 hours at room temperature. Firstly, biotinylated goat anti-human S-MK antibody was added and incubated with captured S-MK for 2 hours at room temperature, after washing three times. Conducting another three washes, we added 100 µL aliquots of streptavidin-conjugated horseradish-peroxidase (San Francisco, USA) which allowing reacting for 30 minutes in a dark place. After plate washing, substrate solutions (1:1 mixture of H2O2 and tetramethylbenzidine) were added to the wells (100 µL per well) for a 20-minute reaction. Finally, 1 mol/L H2SO4 (stop solution) was added (50 µL per well), and the optical densities of the wells were measured at 450 nm with a Multiskan MS plate Reader (Labsystems, Helsinki, Finland). After creating a standard curve by a four-parameter logistic curve-fit method, concentrations of the samples were determined. All specimens were tested blinded and in duplicate.
Statistical analysis
Quantitative data with normal distribution were presented as mean ± standard deviation, and other data was characterized by median (min–max). Comparisons between paired groups were made using the paired t-test. Either Pearson’s (normally distributed data) or Spearman’s rank (skewed or categorical data) correlation test was used for correlation analysis. The influence of age was determined with the Chi-square test. All calculated P values were two-sided and P<0.05 was generally considered significant.
Receiver operating characteristic (ROC) curves were drawn to evaluate the discriminative power of a given parameter. Once areas under ROC curves (AUC) were determined, optimal cut-off values were selected, and sensitivity, specificity, diagnostic accuracy, PPV and NPV for differential diagnosis were assessed, respectively.
All statistical analyses were carried out using SPSS 23.0 software (SPSS Incorporated, Chicago, Illinois, USA).
Results
Both S-MK and CEA had good prognostic values to differentiate rectal cancer, benign rectal polyps and normal controls
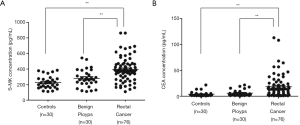
The pre-surgical S-MK levels and pre-surgical CEA in patients with malignant rectal cancer (380.95 pg/mL, 309.55–463.52 pg/mL) were remarkably higher than that with benign rectal polyps (261.85 pg/mL, 213.41–353.98 pg/mL) or healthy controls (219.3 pg/mL, 165.01–275.78 pg/mL) (Table 1, P<0.001). Similarity, the pre-surgical CEA level in the malignant rectal cancer group (13.64 ng/mL, 3.15–22.31 ng/mL) was significantly greater than that in the benign rectal polyps group (3.5 ng/mL, 2.17–8.13 ng/mL) and the control group (2.43 ng/mL, 1.43–4.43 ng/mL) (Table 1, P<0.001). We also measured CA19-9, showing a similar significant difference between the malignant and benign groups, malignant and normal groups but no obvious difference between benign groups and control groups. Other parameters like CA7-24 and age showed no significant differences among the groups. Scatter graphs of S-MK and CEA were plotted to demonstrate the intergroup differences as well (Figure 1).
Table 1
| Group* [case number] | Age (years old) | S-MK (pg/mL) | CEA (ng/mL) | CA19-9 (U/mL) | CA72-4 (U/mL) |
|---|---|---|---|---|---|
| 1 [30] | 64.63±11.14 | 219.3 (165.01–275.78) | 2.43 (1.43–4.43) | 6.1 (4.83–13.35) | 2.49 (1.78–4.32) |
| 2 [30] | 66.50±11.43 | 261.85 (213.41–353.98) | 3.5 (2.17–8.13) | 3.25 (1.88–4.7) | 2.34 (1.54–3.93) |
| 3 [76] | 62.88±9.95 | 380.95 (309.55–463.52) | 13.64 (3.15–22.31) | 6.25 (3.2–17.75) | 2.03 (1.27–4.92) |
| T value(1):(2)# | t=−0.641 | z=−2.087 | z=−1.528 | z=0.978 | z=0.449 |
| P value(1):(2)# | 0.524 | 0.041 | 0.032 | 0.549 | 0.801 |
| T value(1):(3)# | t=0.789 | z=−7.33 | z=−5.514 | z=−2.391 | z=−0.166 |
| P value(1):(3)# | 0.432 | <0.001 | <0.001 | <0.001 | 0.868 |
| T value(2):(3)# | t=1.616 | z=−3.734 | z=−4.586 | z=−2.555 | z=−0.258 |
| P value(2):(3)# | 0.109 | <0.001 | <0.001 | 0.013 | 0.797 |
*: group 1 = normal control group, group 2 = rectal polyps group, group 3 = rectal cancer group; #, analyzed by independent samples t-test. S-MK, serum midkine; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; CA72-4, carbohydrate antigen 72-4.
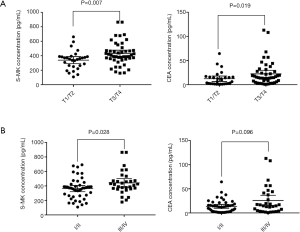
Both levels of S-MK and CEA in T3/T4 phases were higher than those in T1/T2 phases, more significant in S-MK (P=0.007) than CEA (P=0.019). No marked correlation was observed between S-MK, CEA and nodal involvement, or distant metastasis (Table 2). Both levels of S-MK and CEA gradually increased along with the stage. S-MK was higher in stage III/IV comparing to stage I/II [426.55 (360.72–471.12) vs. 298.33 (184.62–340.93) pg/mL, P=0.028]. CEA was also higher in stage III/IV than in stage I/II but with no significantly difference [17.03 (3.15–30.48) vs. 10.32 (1.03–25.59) ng/mL, P>0.05 (Table 2). In order to investigate the relationship between S-MK, CEA and clinicopathological variables, the scatter graphs were plotted (Figure 2).
Table 2
| Variable | S-MK (pg/mL) | P value | CEA | P value |
|---|---|---|---|---|
| Tumor depth | 0.007 | 0.019 | ||
| T1/T2 | 298.33 (184.62–340.93) | 6.71 (1.77–13.01) | ||
| T3/T4 | 398.84 (305.68–453.9) | 12.65 (5.39–21.83) | ||
| N factor | ||||
| N0 | 298.33 (184.62–340.93) | 0.323 (N0 vs. N1) | 11.25 (5.29–18.79) | 0.156 (N0 vs. N1) |
| N1 | 405.37 (254.63–447.21) | 1.000 (N1 vs. N2) | 10.16 (2.18–24.28) | 0.428 (N1 vs. N2) |
| N2 | 398.03 (350.91–512.99) | 0.146 (N0 vs. N2) | 11.65 (2.85–21.54) | 0.838 (N0 vs. N2) |
| M factor | 0.221 | 0.111 | ||
| M0 | 298.33 (184.62–340.93) | 14.47 (6.27–23.24) | ||
| M1 | 405.37 (236.42–727.15) | 18.63 (2.85–59.39) | ||
| Stage | 0.028 | 0.096 | ||
| I/II | 298.33 (184.62–340.93) | 10.32 (1.03–25.59) | ||
| III/IV | 426.55 (360.72–471.12) | 17.03 (3.15–30.84) |
We compared the diagnostic efficacy of pre-surgical S-MK and CEA, individually and in combination, analyzing in two separate assays. In the first analysis, differentiation between rectal cancer and rectal polyps was carried out and the ROC curve was drawn in Figure 3A. The area under the curve of S-MK was higher than that of CEA but smaller than integrating the two (Table 3). Furthermore, when the optimal cut-off values were determined (328.32 pg/mL for S-MK, 5.91 ng/mL for CEA and 449.23 for S-MK + CEA), the sensitivity, specificity, diagnostic accuracy, PPV, and NPV of S-MK + CEA were all higher than corresponding values of isolated S-MK or CEA. Subsequently, a differentiation analysis between rectal cancer and normal control participants was conducted. Table 4 demonstrated a larger area under the ROC curve of S-MK compared to the CEA under-curve area, but lower than the combination of S-MK and CEA (Figure 3B). Quantitative values of optimal cut-off values, the sensitivity, specificity, diagnostic accuracy, PPV, and NPV of S-MK, CEA and S-MK + CEA were also shown in Table 4.
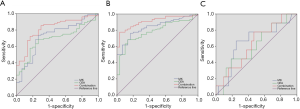
Table 3
| Variable | S-MK (pg/mL) | CEA (ng/mL) | S-MK + CEA |
|---|---|---|---|
| Area under the curve | 0.727 | 0.694 | 0.821 |
| Optimal cut-off value | 328.32 | 5.91 | 449.23 |
| Sensitivity (%) | 73.68 | 68.42 | 71.05 |
| Specificity (%) | 73.33 | 80 | 83.33 |
| Accuracy (%) | 73.58 | 71.7 | 74.53 |
| PPV (%) | 87.5 | 89.66 | 91.53 |
| NPV (%) | 52.38 | 50 | 53.19 |
S-MK, serum midkine; CEA, carcinoembryonic antigen; PPV, positive predictive value; NPV, negative predictive value.
Table 4
| Variable | S-MK (pg/mL) | CEA (ng/mL) | S-MK + CEA |
|---|---|---|---|
| Area under the curve | 0.839 | 0.787 | 0.91 |
| Optimal cut-off value | 299.14 | 5.69 | 420.591 |
| Sensitivity (%) | 76.32 | 68.42 | 77.63 |
| Specificity (%) | 83.33 | 86.67 | 96.67 |
| Accuracy (%) | 78.3 | 73.58 | 83.02 |
| PPV (%) | 92.06 | 92.86 | 98.33 |
| NPV (%) | 58.14 | 52 | 63.04 |
S-MK, serum midkine; CEA, carcinoembryonic antigen; PPV, positive predictive value; NPV, negative predictive value.
Both S-MK and CEA had good predictive values for metastatic existence
Totally 41 of the 75 rectal cancer patients with TxN0M0 were continuously followed up for 12 months to appraise the completeness of metastasis existed, once every month. Based on the 12-month follow-up information, the 41 patients were further allocated into two groups according to whether with metastasis (lymph node, lung, liver or skeletal metastasis). As shown in Table 5, 9 patients had metastasis with 7 lymph node metastasis, 1 liver metastasis and 1 skeletal metastasis. Comparisons of these two groups were performed. S-MK, CEA, and CA19-9 in metastasis group were higher than those in no metastasis group but with no statistical significance (P>0.05).
Table 5
| Group* [case number] | Age (years old) | S-MK (pg/mL) | CEA (ng/mL) | CA19-9 (U/mL) | CA72-4 (U/mL) |
|---|---|---|---|---|---|
| 1 [32] | 64±9.98 | 365.61 (269.91–392.09) | 10.99 (2.21–15.93) | 5.4 (3.12–8.5) | 1.9 (1.22–3.75) |
| 2 [9] | 66 (63.5–79) | 409.98 (322.84–489.11) | 13.78 (5.81–25.8) | 6.3 (2.9–18.1) | 1.4 (1.21–3.34) |
| T value(1):(2)# | t=−1.411 | z=−0.934 | z=−0.86 | z=−0.62 | z=0.26 |
| P value(1):(2)# | 0.230 | 0.164 | 0.359 | 0.676 | 0.793 |
*: group 1 = no metastases group, group 2 = metastases group; #, analyzed by independent samples t-test. S-MK, serum midkine; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; CA72-4, carbohydrate antigen 72-4.
The predictive efficacies of those parameters for the synchronous metastasis were tested by the ROC curve (Figure 3C) and it demonstrated good predictive capabilities of S-MK + CEA, with diagnostic accuracy around 80%, higher than that of S-MK and CEA individually (Table 6).
Table 6
| Variable | S-MK (pg/mL) | CEA (ng/mL) | S-MK + CEA |
|---|---|---|---|
| Area under the curve | 0.655 | 0.604 | 0.628 |
| Optimal cut-off value | 389.11 | 17.34 | 516.2 |
| Sensitivity (%) | 66.67 | 44.44 | 77.78 |
| Specificity (%) | 75 | 81.25 | 77.27 |
| Accuracy (%) | 73.17 | 73.17 | 77.42 |
| PPV (%) | 42.86 | 40 | 58.33 |
| NPV (%) | 88.89 | 83.87 | 89.47 |
S-MK, serum midkine; CEA, carcinoembryonic antigen; PPV, positive predictive value; NPV, negative predictive value.
Discussion
S-MK, also known as neurite growth-promoting factor 2 (NEGF2), is a heparin-binding growth factor that was initially discovered during the retinoic acid-induced differentiation of embryonic tumor cells. Since the first report in 1988 (3), several pieces of literature relating to S-MK expression have been published. Jones et al. reported that the average level of S-MK was around 253 pg/mL in healthy human subjects (12). Consistently, our control healthy participants had a mean level of 255.01 pg/mL for the S-MK expression.
It is reported that S-MK has five biological functions: (I) improve angiogenesis and mitosis; (II) increase the prothrombinase activity of aortic endothelial cells; (III) promote neuronal survival and induce neurite outgrowth (13); (IV) rescues Wilms’ tumor cells from apoptosis (14); and (V) exhibit chemotaxis to neutrophils (15). Specifically, the latter two may contribute to the infiltration and survival of tumor cells. In normal human tissue, the expression of S-MK is highly restricted, and low levels of S-MK can be detected in the kidneys, lungs, stomach, ovary and thyroid. However, high levels of S-MK are observed in at least 20 malignant tumors, such as breast cancer, lung cancer, esophageal cancer, and glial cell tumors. Expression conformity of S-MK in plasma is in accordance with that in tissue.
In recent years, investigators have evaluated S-MK to differentiate malignancy from benignancy in various pathological conditions. Kishida et al. reviewed the involvement of S-MK in neuroblastoma tumorigenesis, and they concluded that S-MK was expressed in chemoresistant cells and was involved in anticancer drug-resistance. In contrast, neuroblastoma cells were associated with a high level of S-MK expression and that an S-MK targeted therapy could exert a vital role in these cells (16). Ren et al. found that S-MK was overexpressed in the cytoplasm of esophageal squamous cell carcinoma (ESCC). Noticeably, the high intensity of S-MK was found abundant in vessels and the invading border of the tumors. Moreover, it was concluded that S-MK expression was correlated with tumor cell differentiation with more intensely expressed in well-differentiated tumors (76.9%) than in moderately and poorly differentiated tumors (43.1% and 41.2%, respectively) (17). As a tumor biomarker, S-MK showed an obviously higher sensitivity compared with alpha-fetoprotein (AFP) in the investigation of hepatocellular carcinoma (HCC). In another wide-ranging study, Zhu et al. showed that the sensitivity of serum S-MK was much higher than that of AFP (86.9% vs. 51.9%) with similar specificities (83.9% vs. 86.3%). Even in very early stages, S-MK held a two times higher sensitivity than AFP (80% vs. 40%) (18). Sun et al. further explained that S-MK promotes HCC metastasis by elevating anoikis resistance of circulating tumor cells. Activation of PI3K/Akt/NF-κB/TrkB signaling by midkine-activated anaplastic lymphoma kinase (ALK) is responsible for the anoikis resistance (19).
For rectal cancer, S-MK expression in colorectal tumors (low-grade dysplasia, adenomas with high-grade dysplasia (HGD) and invasive adenocarcinomas) was significantly higher than in the normal mucosa. Ulcerative colitis (UC)-associated lesions (regenerative mucosa of UC, UC-associated dysplasia and UC-associated adenocarcinoma) had similar variations, with higher S-MK expression than in the normal mucosa (20). Ye et al. had suggested that S-MK expression level was significantly elevated in carcinomas than that in normal tissues by Northern blotting. S-MK immunostaining was positive in the adenomas with moderate- and severe-grade dysplasia and in the carcinomas, but not in mild-grade dysplasia or in normal tissues (21). Krzystek-Korpacka et al. reported that the average multiple of S-MK expression in cancerous versus noncancerous tissue was 30.1. Higher S-MK levels express in stages III/IV vs. stages I/II of rectal cancer, while conversely in the colon cancer. Tissue expression of S-MK was positively correlated with its serum levels (22).
In this study, both S-MK and CEA were significantly higher in rectal cancer patients than other individuals (Table 1). Their blood levels increased significantly along with tumor depth and stage, but the S-MK level increased more significantly than CEA (Table 2). An optimal cut-off value of 328.32 pg/mL for S-MK was set to differentiate malignant from rectal polyps, which produced the sensitivity and diagnostic accuracy of 73.68% and 73.58%, respectively. These data were better than the results from CEA (Table 3). In the analysis to differentiate rectal cancer from normal participants without any rectal disease, a cut-off value of 299.14 pg/mL for S-MK was determined. The sensitivity and diagnostic accuracy determined by S-MK were higher than the parameters calculated from CEA (Table 4). The diagnostic sensitivity, specificity and accuracy of combined detection were higher than that of the single assay (Table 4). These results proved the efficacy of S-MK to be used as a promising early diagnostic biomarker for rectal cancer screening. Furthermore, the joint detection entails better pre-surgical diagnosis.
S-MK could serve as a practical marker for cancer screening, but also helpful in the evaluation of prognosis. The application value of S-MK as a prognostic biomarker was listed in a number of studies by the Kaplan-Meier curve research. According to Ota et al., oral squamous cell carcinoma patients with high S-MK concentrations showed a lower 5-year survival rate than patients in low S-MK groups. Even in early-stage tumor, the increased S-MK expression was strongly associated with poor survival (23). Maeda et al. first reported the association between S-MK and pancreatic head carcinoma. Patients with positive S-MK (53.3%) had a significantly lower 5-year survival rate than those with a negative one. The authors also revealed that S-MK expression was an independent prognostic factor (24). In the study by Rawnaq et al., S-MK concentrations were significantly higher in the gastrointestinal stromal tumor with recurrence compared with those without. For 400 pg/mL as a threshold value, patients with S-MK levels higher than the threshold showed a significantly worse 5-year recurrence-free survival (25). According to Ikematsu et al., there was a striking correlation between high plasma S-MK and poor prognosis for human neuroblastoma with a threshold value of 900 pg/mL (26). For rectal cancer, Krzystek-Korpacka et al. showed that higher S-MK levels express in stage N1 vs. stages N0 of rectal cancer, while conversely in the colon cancer (22).
The above studies indicate that S-MK and CEA in blood could be good biomarkers for the diagnosis of rectal cancer and prediction of synchronous metastasis. We found that significantly elevated expressions of S-MK and CEA in rectal cancer. Results showed good diagnostic accuracies of S-MK, CEA, S-MK + CEA for the purpose of differential diagnosis by ROC (Figure 3A,B, Tables 3,4). Furthermore, we divided the rectal cancer patients into two subgroups according to the status of metastases. ROC analyses also demonstrated good diagnostic abilities of the combination of S-MK and CEA to distinguish whether metastases existed (Figure 3C, Table 6).
There are two limitations in this study, which warrant further researches. Firstly, we recruited a relatively small number of cases and conducted a retrospective study. Therefore, a direct cause-effect link between activations of MK and CEA activities cannot clearly be shown through our analysis. Studies on the transcriptional regulation of these factors are needed to further investigate. Secondly, since rectal cancer has a relatively good survival rate, a longer time follow-up study is needed in the following studies.
Conclusions
Pre-surgical S-MK level as the rectal cancer indicator was superior to CEA, it may play a significant role in rectal cancer carcinogenesis and progression. Though this study did not confirm the role of S-MK for the prognosis, it did partially substantiate the correlation between high S-MK and tumor metastasis. The results also proved the potential usefulness of combined detection of S-MK and CEA for the diagnosis of rectal cancer, which might improve the diagnosis of rectal cancer.
Acknowledgments
Funding: This investigation was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.43). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Patient recruitment and sample collection were performed within the guidelines of protocols approved by the institutional review boards and informed consent was obtained from all subjects. The ID/number of ethical approval is 2019C03 approved by Tianjin Union Medical Center. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177-93. [Crossref] [PubMed]
- Shibutani M, Maeda K, Nagahara H, et al. Significance of CEA and CA19-9 combination as a prognostic indicator and for recurrence monitoring in patients with stage II colorectal cancer. Anticancer Res 2014;34:3753-8. [PubMed]
- Kadomatsu K, Hagihara M, Akhter S, et al. Midkine induces the transformation of NIH3T3 cells. Br J Cancer 1997;75:354. [Crossref] [PubMed]
- Shindo E, Nanki T, Kusunoki N, et al. The growth factor midkine may play a pathophysiological role in rheumatoid arthritis. Mod Rheumatol 2017;27:54-9. [Crossref] [PubMed]
- Ma J, Kong Y, Nan H, et al. Pleiotrophin as a potential biomarker in breast cancer patients. Clin Chim Acta 2017;466:6-12. [Crossref] [PubMed]
- EI-Edel RH. Evaluation of serum Midkine as a novel marker in hepatocellular carcinomas. Menoufia Med J 2018;31:1094.
- Shiratori F, Ito M, Yajima S, et al. The effectiveness of serum midkine in detecting esophageal squamous cell carcinoma. Esophagus 2019;16:246-51. [Crossref] [PubMed]
- Filippou PS, Karagiannis GS, Constantinidou A. Midkine (MDK) growth factor: a key player in cancer progression and a promising therapeutic target. Oncogene 2020;39:2040-54. [PubMed]
- Kalamatianos T, Denekou D, Stranjalis G, et al. Anaplastic lymphoma kinase in glioblastoma: detection/diagnostic methods and therapeutic options. Recent Pat Anticancer Drug Discov 2018;13:209-23. [Crossref] [PubMed]
- Jeiroodi N, Bagherpour M, Zare R, et al. Evaluation of midkine expression in dentigerous cysts, odontogenic keratocysts and different types of ameloblastoma. Turk J Pathol 2018;1:001-7.
- Krzystek-Korpacka M, Diakowska D, Neubauer K, et al. Circulating midkine in malignant and non-malignant colorectal diseases. Cytokine 2013;64:158-64. [Crossref] [PubMed]
- Jones D. Measuring midkine: the utility of midkine as a biomarker in cancer and other diseases. Br J Pharmacol 2014;171:2925-39. [Crossref] [PubMed]
- Kaneda N, Talukder AH, Nishiyama H, et al. Midkine, a heparin-binding growth/differentiation factor, exhibits nerve cell adhesion and guidance activity for neurite outgrowth in vitro. J Biochem 1996;119:1150-6. [Crossref] [PubMed]
- Qi M, Ikematsu S, Icbihara-Tanaka K, et al. Midkine rescues Wilms, tumor cells from cisplatin-induced apoptosis: regulation of Bcl-2 expression by Midkine. J Biochem 2000;127:269-77. [Crossref] [PubMed]
- Takada T, Toriyama K, Muramatsu H, et al. Midkine, a retinoic acid-inducible heparin-binding cytokine in inflammatory responses: chemotactic activity to neutrophils and association with inflammatory synovitis. J Biochem 1997;122:453-8. [Crossref] [PubMed]
- Kishida S, Kadomatsu K. Involvement of midkine in neuroblastoma tumourigenesis. Br J Pharmacol 2014;171:896-904. [Crossref] [PubMed]
- Ren YJ, Zhang QY. Expression of midkine and its clinical significance in esophageal squamous cell carcinoma. World J Gastroenterol 2006;12:2006-10. [Crossref] [PubMed]
- Zhu WW, Guo JJ, Guo L, et al. Evaluation of midkine as a diagnostic serum biomarker in hepatocellular carcinoma. Clin Cancer Res 2013;19:3944-54. [Crossref] [PubMed]
- Sun B, Hu C, Yang Z, et al. Midkine promotes hepatocellular carcinoma metastasis by elevating anoikis resistance of circulating tumor cells. Oncotarget 2017;8:32523. [PubMed]
- Tokuyama W, Mikami T, Fujiwara M, et al. Midkine expression in colorectal tumors: Correlation with Kiith Kition with Ki radic, but not ulcerative colitis‐associated ones. Pathol Int 2007;57:260-7. [Crossref] [PubMed]
- Ye C, Qi M, Fan Q, et al. Expression of midkine in the early stage of carcinogenesis in human colorectal cancer. Br J Cancer 1999;79:179. [Crossref] [PubMed]
- Krzystek-Korpacka M, Diakowska D, Grabowski K, et al. Tumor location determines midkine level and its association with the disease progression in colorectal cancer patients: a pilot study. Int J Colorectal Dis 2012;27:1319-24. [Crossref] [PubMed]
- Ota K, Fujimori H, Ueda M, et al. Midkine as a prognostic biomarker in oral squamous cell carcinoma. Br J Cancer 2008;99:655. [Crossref] [PubMed]
- Maeda S, Shinchi H, Kurahara H, et al. Clinical significance of midkine expression in pancreatic head carcinoma. Br J Cancer 2007;97:405. [Crossref] [PubMed]
- Rawnaq T, Kunkel M, Bachmann K, et al. Serum midkine correlates with tumor progression and imatinib response in gastrointestinal stromal tumors. Ann Surg Oncol 2011;18:559-65. [Crossref] [PubMed]
- Ikematsu S, Nakagawara A, Nakamura Y, et al. Plasma midkine level is a prognostic factor for human neuroblastoma. Cancer Sci 2008;99:2070-4. [Crossref] [PubMed]


