
Outcome evaluation of neoadjuvant chemotherapy in patients with stage IB2 or IIA cervical cancer: a retrospective comparative study
Introduction
Cervical cancer is common among women worldwide, with most cases occurring in developing countries (1). Along with the development of socioeconomics associated with urbanization, as well as the aging population, the incidence and mortality of cervical cancer keep increasing in China. It was estimated to have about 132,000 new cases and 30,000 deaths each year in China (2). Early-stage cervical cancer is mainly managed by surgical resection, with a 5-year survival rate over 90%. Radiation therapy is recommended to patients with locally advanced cervical cancer, but the prognosis is relatively poor with a 5-year survival rate around 50–60% (3). Recently, neoadjuvant chemotherapy (NACT) was proposed before the surgical treatment to improve the outcomes of surgical resection.
NACT, also known as pre-chemotherapy, refers to the chemotherapy performed before the main treatment methods (radical surgical resection or radiotherapy), which is different from the traditional adjuvant chemotherapy that is usually given after the completion of the main treatment (4). In patients with locally advanced cervical cancer or patients with stage IIB cervical cancer who wish to receive surgery after chemotherapy, NACT has been reported to shrink the tumor mass and control the subclinical lesions, and thus might lower the clinical stages of locally advanced cancer (5). At the same time, the NACT might improve prognosis and reduce the chance of local metastasis by inhibiting tumor activities, reducing the difficulty of surgical resection, and increasing the tumor resection rate (6). However, there was no consensus on the beneficial effects of NACT so far. The study reported by Cai et al. found that NACT could improve the 5-year disease-free survival rate compared to surgical treatment alone (84.6% versus 75.9%, P<0.05) (7). However, this improvement was only observed in patients with a large cervical tumor, but not in patients with a tumor diameter <4 cm. Another meta-analysis showed that NACT could reduce the high-risk postoperative pathological results without affecting the long-term survival rate (8). Some researchers even believed that NACT could mask the postoperative pathological examination. A recent phase-II study by Benson et al. has reported that weekly NACT followed by gefitinib maintenance is associated with a good response rate in locally advanced cervical cancer (9). All of these suggested the efficacy of NACT requires further investigation.
In the current study, we performed a retrospective investigation and analyzed the outcomes of patients who received NACT, followed by surgery and versus the patients underwent only primary surgery with stage I B2 or IIA cervical cancer.
Methods
Study design and participants
A retrospective study was performed in an urban hospital in Jilin, China. The study protocol was approved by the Second Hospital of Jilin University ethics committee (Ethic approval ID: 2018-227). The inclusion criteria for study participants selection were: (I), stage IB2 or stage IIA (including IIA1 and IIA 2) cervical cancer based on FIGO staging (10) and reviewed by two senior gynecologists (with consultations to additional gynecologist if there was any discrepancy in the staging); (II), 18≤ age ≤75 years old; (III), received treatment in our hospital between February 2014 and December 2016. Patients with post-operative reports that showed positive periuterine or surgical margins, or positive para-aortic lymph nodes, were excluded.
Treatment protocol
NACT was given with TP paclitaxel (175 mg/m2 D1), + cisplatin (75 mg/m2 D1) or nedaplatin (80 mg/m2 D1) or lobaplatin (30 mg/m2 D1) for 3 weeks. The total period for chemotherapy was 157 cycles. Surgery operation was performed 3–4 weeks after the completion of NACT.
All patients enrolled in the current study received C-type radical resection and pelvic lymphadenectomy for their cervical cancers. The lymph node dissection included bilateral pelvic lymphadenectomy, which included the removal of internal iliac, external iliac, iliac, obturator, and presacral lymph nodes. Postoperative pathological examination was performed by two senior pathologists, with consultations to additional attending pathologists if there was any discrepancy. Tumor differentiation, vascular tumor thrombus, the size and depth of invasion of the tumor (>50%), and the number of metastatic lymph nodes were recorded.
Postoperative adjuvant chemoradiotherapy (CRT) was based on the postoperative pathology reports, and the decision of the patients and their families. Patients with positive lymph nodes and tumor size ≥4 cm were recommended to receive combined postoperative radiotherapy with chemotherapy. Patients with a poorly differentiated tumor, lymphatic vessel infiltration, or depth of invasion >50% were recommended on the Sedlis criteria based on the guideline from 2017 National Comprehensive Cancer Network (NCCN). The final treatment strategy was determined based on the decision of the patients and their families.
Postoperative radiotherapy included intensity-modulated radiation therapy or rotary volume-enhanced radiation therapy. The dose of external irradiation was 45–50.4 Gy/1.8–2.0 Gy/25–28 F, with the synchronous sensitization chemotherapy with weekly cisplatin (40 mg/m2). The radiotherapy was performed for 5 weeks.
Postoperative chemotherapy was given as TP paclitaxel (175 mg/m2 D1) + cisplatin (75 mg/m2 D1), or nedaplatin (80 mg/m2 D1), or lobaplatin (30 mg/m2 D1) for 3 weeks. Postoperative chemotherapy was commonly completed within 3–6 months after the surgery, with total of 576 cycles.
Outcome measurements
All the patients were followed up through either telephone interview or clinical visits until July 31st, 2018. We considered death as the primary outcome, and deterioration of the clinical conditions as the secondary outcome. The duration of survival was calculated from the completion of the surgery until the occurrence of death.
Statistical analysis
Continuous data were presented as mean ± standard deviation and were compared by student t-test or non-parametric rank-sum test, when appropriate. Categorical data were presented as percentage (%) and were compared by Chi-square analysis or Fisher exact test. Odds ratios (OR) for death and deterioration were calculated by univariate or multivariate logistic regression analysis. Two-sided tests were performed with statistical software SAS9.3 (SAS, North Carolina, USA). P<0.05 was considered statistically significant.
Results
Baseline characteristics between patients with or without NACT
Among the 363 identified patients, 114 patients received NACT in addition to surgical resection and postoperative CRT, and 249 patients only received surgical resection and postoperative CRT. The median follow-up period was 36.4 months, ranging from 28 to 55 months. Among all the 114 patients who received preoperative NACT, 7 of them received 3 cycles of treatment, 29 received 2 cycles of treatment, and 78 received only one cycle of treatment. Among those 279 patients who received postoperative radiotherapy, 72 only received radiotherapy, and 207 received both radiation and chemotherapy. In 211 patients who received postoperative chemotherapy, 158 received 1–3 cycles and 52 received 4–6 cycles of chemotherapy treatment. Baseline characteristics of patients with or without NACT are listed in Table 1. There were statistically significant differences in the tumor differentiation, clinical stages, vascular tumor thrombus, and postoperative radiotherapy between these two groups. Thus, further multivariate logistic analysis was performed to compare the mortality and deterioration rates between these two groups after adjustment of these four variables.
Table 1
| Characteristics | Patients with neoadjuvant chemotherapy (N=114) | Patients without neoadjuvant chemotherapy (N=249) | P |
|---|---|---|---|
| Age, year, mean ± standard deviation | 53.1±8.1 | 54.1±10.3 | 0.99 |
| Tumor differentiation, N (%) | <0.01 | ||
| Poorly differentiated | 5 (4.4) | 31 (12.4) | |
| Moderately differentiated | 90 (78.9) | 209 (83.9) | |
| Well differentiated | 1 (0.9) | 3 (1.2) | |
| Undifferentiated | 18 (15.8) | 6 (2.4) | |
| Clinical stage, N (%) | <0.01 | ||
| IB2 | 25 (21.9) | 63 (25.3) | |
| IIA1 | 54 (47.4) | 165 (66.3) | |
| IIA2 | 35 (30.7) | 21 (8.4) | |
| Infiltration depth, N (%) | 0.20 | ||
| More than half the depth | 90 (78.9) | 181 (72.7) | |
| Not more than half the depth | 24 (21.1) | 68 (27.3) | |
| Vascular tumor thrombus, N (%) | 0.01 | ||
| Yes | 44 (38.6) | 135 (54.2) | |
| No | 70 (61.4) | 114 (45.8) | |
| Lymph node metastasis, N (%) | 0.70 | ||
| Yes | 31 (27.2) | 63 (25.3) | |
| No | 83 (72.8) | 186 (74.7) | |
| Postoperative radiation therapy, N (%) | 0.03 | ||
| Yes | 96 (84.2) | 183 (73.5) | |
| No | 18 (15.8) | 66 (26.5) | |
| Postoperative chemotherapy, N (%) | 0.81 | ||
| Yes | 67 (58.8) | 143 (57.4) | |
| No | 47 (41.2) | 106 (42.6) |
Mortality comparisons between patients with or without NACT
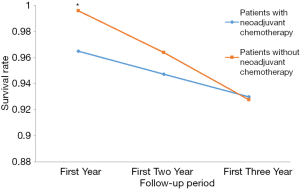
After adjusted by the tumor differentiation, clinical stages, vascular tumor thrombus, and postoperative radiotherapy, the mortality rate was statistically significantly higher in the patients who received NACT as compared to the patients who did not receive this therapy within one year after the surgery. When the observation period was extended to two or three years, this statistically significant difference disappeared, as shown in Tables 2,3 and Figure 1.
Table 2
| Variables | First year | First two years* | First three years* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Death, N (%) | Adjusted OR (95% CI)† | Adjusted P† | Death, N (%) | Adjusted OR (95% CI)† | Adjusted P† | Death, N (%) | Adjusted OR (95% CI)† | Adjusted P † |
|||
| Neoadjuvant | 0.02 | 0.16 | 0.44 | ||||||||
| Yes (N=114) | 4 (3.5) | 24.9 (1.8, 347.3) | 6 (5.3) | 2.5 (0.7, 8.6) | 8 (7.1) | 1.5 (0.5, 4) | |||||
| No (N=249) | 1 (0.4) | 1 | 9 (3.6) | 1 | 18 (7.2) | 1 | |||||
| Postoperative radiation therapy | 0.02 | 0.07 | 0.36 | ||||||||
| Yes | 2 (0.7) | 0.1 (0.0, 0.7) | 9 (3.2) | 0.4 (0.1, 1.1) | 19 (6.8) | 0.6 (0.2, 1.7) | |||||
| No | 3 (3.6) | 1 | 6 (7.1) | – | 7 (8.3) | – | |||||
*, one patient died due to non-cancerous cause during the second year and was not counted into the analysis. †, OR and P for neoadjuvant chemotherapy were adjusted for tumor differentiation, clinical stage, vascular tumor thrombus, and postoperative radiation therapy.
Table 3
| Variables | First year | First two years* | First three years* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Death, N (%) | Adjusted OR (95% CI)† | Adjusted P† | Death, N (%) | Adjusted OR (95% CI)† | Adjusted P† | Death, N (%) | Adjusted OR (95% CI)† | Adjusted P† | |||
| Tumor differentiation | 0.64 | 0.12 | 0.75 | ||||||||
| Poorly differentiated | 1 (2.8) | 5.3 (0.3, 86.5) | 4 (11.1) | 4.6 (1.2, 16.8) | 4 (11.1) | 1.9 (0.6, 6.2) | |||||
| Well differentiated | 0 (0.0) | – | 0 (0.0) | – | 0 (0.0) | 1 | |||||
| Undifferentiated | 1 (4.2) | 2.9 (0.2, 41.7) | 2 (8.3) | 2.8 (0.5, 16.1) | 2 (8.3) | 1.4 (0.3, 7.7) | |||||
| Moderately differentiated | 3 (1.0) | 1 | 9 (3.0) | 1 | 20 (6.7) | 1 | |||||
| Clinical stage | 0.53 | 0.57 | 0.06 | ||||||||
| IB2 | 2 (2.3) | 4.2 (0.2, 80.8) | 5 (5.7) | 2.4 (0.4, 14.5) | 11 (12.5) | 3.2 (0.8, 13.2) | |||||
| IIA1 | 2 (0.9) | 1.4 (0.1, 23.9) | 8 (3.7) | 1.4 (0.2, 8.2) | 12 (5.5) | 1.2 (0.3, 4.8) | |||||
| IIA2 | 1 (1.8) | 1 | 2 (3.6) | 1 | 3 (5.5) | 1 | |||||
| Vascular tumor thrombus | 0.17 | 0.06 | <0.01 | ||||||||
| Yes | 3 (1.7) | 4.6 (0.5, 41.0) | 10 (5.6) | 3.2 (1.0, 10.4) | 20 (11.2) | 4.6 (1.7, 12.4) | |||||
| No | 2 (1.1) | 1 | 5 (2.7) | 1 | 6 (3.3) | – | |||||
*, one patient died due to non-cancerous cause during the second year and was not counted into the analysis. †, OR and P for neoadjuvant chemotherapy were adjusted for tumor differentiation, clinical stage, vascular tumor thrombus, and postoperative radiation therapy.
Deterioration comparison between patients with or without NACT
Compared to patients who did not receive NACT, patients who received NACT had a statistically significantly increased chance of deterioration during the 3-year follow-up period, after adjusted for tumor differentiation, clinical stages, vascular tumor thrombus, and postoperative radiotherapy (Table 4).
Table 4
| Variables | Deterioration, N (%)* | Adjusted OR (95% CI)† | Adjusted P† |
|---|---|---|---|
| Neoadjuvant | 0.01 | ||
| Yes (N=114) | 15 (13.2) | 2.9 (1.3, 6.5) | |
| No (N=249) | 20 (8.0) | 1 | |
| Tumor differentiation | 0.63 | ||
| Poorly differentiated | 5 (13.9) | 2.0 (0.7, 5.7) | |
| Well differentiated | 0 (0.0) | - | |
| Undifferentiated | 2 (8.3) | 0.7 (0.1, 3.5) | |
| Moderately differentiated | 28 (9.4) | 1 | |
| Clinical stage | 0.17 | ||
| IB2 | 12 (13.6) | 2.5 (0.8, 8.1) | |
| IIA1 | 18 (8.2) | 1.2 (0.4, 3.8) | |
| IIA2 | 5 (9.1) | 1 | |
| Vascular tumor thrombus | < 0.01 | ||
| Yes | 26 (14.5) | 4.2 (1.8, 9.7) | |
| No | 9 (4.9) | 1 | |
| Postoperative radiation therapy | 0.59 | ||
| Yes | 27 (9.7) | 0.8 (0.3, 1.9) | |
| No | 8 (9.5) | 1 |
*, one patient died due to non-cancerous cause during the second year and was not counted into the analysis. †, OR and P for neoadjuvant chemotherapy were adjusted for tumor differentiation, clinical stage, vascular tumor thrombus, and postoperative radiation therapy.
Discussion
Locally advanced cervical cancer commonly occurs in developing countries. It imposes a serious threat to the health of women. Controversies still exist on its diagnosis and treatments. The report from FIGO suggested that NACT could shrink the tumor mass to facilitate the radical surgical resection of tumor. In addition, the NACT could also remove the lesions in the lymph nodes and periuterine tissues and reduce the risk factors for postoperative adjuvant therapy (11). Another study showed that NACT could improve the tumor resectability, which led to better overall survival and progression-free survival rates compared to simple radiotherapy in patients with IB2 or IIB cervical cancer. A meta-analysis by Kim et al. also showed that NACT could shrink the tumor mass and reduce the chance of lymph node and distant metastasis in patients with stage IB1 to IIA cervical cancer (12). However, there were also studies reported no significant beneficial effects of the NACT compared to patients who did not receive it (13,14). Therefore, there has been no consensus on the efficacy and safety of NACT worldwide. Therefore, more studies are needed to investigate the benefit of NACT on patients with locally advanced cervical cancer (15).
In the current study, we retrospectively analyzed and compared the postoperative pathology, treatment strategy, and prognosis of patients who received preoperative NACT, with patients who received only routine surgery and postoperative RCT. Our study included a relatively large number of patients who visited the Radiotherapy Department of Oncology at the Second Hospital of Jilin University between 2014 and 2016. All the patients received relatively uniform health care, which could facilitate the comparisons between patients who received different preoperative chemotherapies. Our study showed statistically significant lower 1-year survival rate in the NACT group than those who did not receive NACT, after adjusting for tumor differentiation, clinical stages, vascular tumor thrombus, and postoperative radiotherapy. During the 3-year follow-up period, the mortality rate tended to be similar between two groups. In terms of clinical deterioration, higher percentage of patients had clinical deterioration in the NACT group than non-NACT group. Our results did not show benefits of neoadjuvant therapy. This was different from the previous reports (9,16,17). Previous studies have shown that NACT could shrink tumors and reduce tumor metastasis (18) which might facilitate minimally invasive treatment (19). However, this seemed not to be converted entirely into long-term survival benefits (20). There were also reports that minimally invasive surgery might increase the chance of tumor spreads and reduce the survival rate, which led to poor prognosis (21).
The American Gynecologic Oncology Group (GOG)-141 performed a randomized controlled trial in 28 patients with IB2 cervical cancer who underwent extensive hysterectomy + pelvic para-aortic lymphadenectomy or simple surgery in 1996. It was found that there was no statistically significant difference in 3 and 5 years of tumor-free survival and overall survival between two groups of patients (4). Another study that was performed in 2010 also suggested that there was currently no evidence to support NACT+ radical hand rubs over surgery alone. Lee et al. compared 85 cervical cancer patients who underwent NACT and 358 cervical cancer patients who underwent radical resection, and found that there was no significant difference in tumor-free survival (75.6% vs. 74.0%) and the overall survival (92.1% vs. 84.9%) after 5 years of treatment (11). Katsumata et al. performed a prospective clinical randomized trial in 134 patients with stage IB2 to IIB (67 in the NACT group and 67 in the simple surgery group) cervical cancer. Their results also did not show any benefit of NACT to improve the survival chance (22). Recent studies have shown that NACT + radical surgery did not significantly improve the overall survival and disease-free survival when compared with simple surgery or NACT combined with cervical cancer radical surgery (23,24). Our study reached conclusions similar to these previous studies. Preoperative NACT might decrease patient's tolerance for the subsequent surgery and postoperative CRT. Therefore, the efficacy of NACT needs to be verified by further clinical trials.
Current protocols for the NACT for cervical cancer include cisplatin + 5-FU (PF), cisplatin + vincristine + bleomycin (PVB), cisplatin + paclitaxel (TP), and carboplatin + paclitaxel (TC). The study from the GOG-204 trial showed that a certain advantage to give classic paclitaxel + cisplatin in patients with advanced cervical cancer (25). The overall remission rate could reach 20–40% in patients who received platinum-based dual-drug therapy (26). It was reported that chemotherapy alone could prolong the survival chance in patients with high-risk factors after surgery (27-30). Chemotherapy alone is less toxic and does not cause side effects such as intestinal injury, renal insufficiency, and vaginal cramps (31-33). Therefore, we recommended post-operative chemotherapy in intermediate-risk or high-risk patients who had indications for radiotherapy or CRT but refused to undergo radiotherapy. In addition, although there is currently no large-scale clinical trial to prove the benefits of postoperative chemotherapy, some retrospective studies have shown that postoperative chemotherapy could reduce the risk of long-term metastasis and recurrence rate. In our department, we recommended postoperative adjuvant chemotherapy to patients with intermediate risks, especially for young patients.
Being a single hospital center study is one of limitations of the current study. The retrospective study design also had its inherent biases. The postoperative adjuvant CRT was partially determined based on the desire of the patients and their families, as well as their economic situations. These biases could also affect our research results.
Conclusions
Our current study did not show any benefit of NACT in patients with stage I B2 and IIA cervical cancer. More prospective clinical trials should be performed to further study this chemotherapy method.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.27). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Second Hospital of Jilin University ethics committee (Ethic approval ID: 2018-227) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China in 2013: an analysis based on urbanization level. Chin J Cancer Res 2017;29:1-10. [Crossref] [PubMed]
- Song B, Ding C, Chen W, et al. Incidence and mortality of cervical cancer in China, 2013. Chin J Cancer Res 2017;29:471-6. [Crossref] [PubMed]
- Lai JC, Chou YJ, Huang N, et al. Survival analysis of Stage IIA1 and IIA2 cervical cancer patients. Taiwan J Obstet Gynecol 2013;52:33-8. [Crossref] [PubMed]
- Rydzewska L, Tierney J, Vale CL, et al. Neoadjuvant chemotherapy plus surgery versus surgery for cervical cancer. Cochrane Database Syst Rev 2012;12:CD007406. [PubMed]
- de la Torre M. Neoadjuvant chemotherapy in woman with early or locally advanced cervical cancer. Rep Pract Oncol Radiother 2018;23:528-32. [Crossref] [PubMed]
- de Azevedo CR, Thuler LCS, de Mello MJG, et al. Phase II trial of neoadjuvant chemotherapy followed by chemoradiation in locally advanced cervical cancer. Gynecol Oncol 2017;146:560-5. [Crossref] [PubMed]
- Cai HB, Chen HZ, Yin HH. Randomized study of preoperative chemotherapy versus primary surgery for stage IB cervical cancer. J Obstet Gynaecol Res 2006;32:315-23. [Crossref] [PubMed]
- Hasiuk MM, Brown D, Cooney C, et al. Application of fast-track surgery principles to evaluate effects of atipamezole on recovery and analgesia following ovariohysterectomy in cats anesthetized with dexmedetomidine-ketamine-hydromorphone. J Am Vet Med Assoc 2015;246:645-53. [Crossref] [PubMed]
- Benson R, Pathy S, Kumar L, et al. Locally advanced cervical cancer - neoadjuvant chemotherapy followed by concurrent chemoradiation and targeted therapy as maintenance: A phase II study. J Cancer Res Ther 2019;15:1359-64. [Crossref] [PubMed]
- Tsikouras P, Zervoudis S, Manav B, et al. Cervical cancer: screening, diagnosis and staging. J BUON 2016;21:320-5. [PubMed]
- Lee J, Kim TH, Kim GE, et al. Neoadjuvant chemotherapy followed by surgery has no therapeutic advantages over concurrent chemoradiotherapy in International Federation of Gynecology and Obstetrics stage IB-IIB cervical cancer. J Gynecol Oncol 2016;27:e52. [Crossref] [PubMed]
- Kim HS, Sardi JE, Katsumata N, et al. Efficacy of neoadjuvant chemotherapy in patients with FIGO stage IB1 to IIA cervical cancer: an international collaborative meta-analysis. Eur J Surg Oncol 2013;39:115-24. [Crossref] [PubMed]
- Eddy GL, Bundy BN, Creasman WT, et al. Treatment of ("bulky") stage IB cervical cancer with or without neoadjuvant vincristine and cisplatin prior to radical hysterectomy and pelvic/para-aortic lymphadenectomy: a phase III trial of the gynecologic oncology group. Gynecol Oncol 2007;106:362-9. [Crossref] [PubMed]
- Marchetti C, Fagotti A, Tombolini V, et al. Survival and toxicity in neoadjuvant chemotherapy plus surgery versus definitive chemoradiotherapy for cervical cancer: A systematic review and meta-analysis. Cancer Treat Rev 2020;83:101945. [Crossref] [PubMed]
- Furuta Y, Todo Y, Yamazaki H, et al. Radiation therapy versus surgery for patients with cervical squamous cell carcinoma who have undergone neoadjuvant chemotherapy revisited. Int J Clin Oncol 2018;23:126-33. [Crossref] [PubMed]
- Harsh KK, Kapoor A, Paramanandhan M, et al. Induction Chemotherapy Followed by Concurrent Chemoradiation in the Management of Different Stages of Cervical Carcinoma: 5-year Retrospective Study. J Obstet Gynaecol India 2016;66:372-8. [Crossref] [PubMed]
- Narayan S, Sharma N, Kapoor A, et al. Pros and Cons of Adding of Neoadjuvant Chemotherapy to Standard Concurrent Chemoradiotherapy in Cervical Cancer: A Regional Cancer Center Experience. J Obstet Gynaecol India 2016;66:385-90. [Crossref] [PubMed]
- Hauerberg L, Hogdall C, Loft A, et al. Vaginal Radical Trachelectomy for early stage cervical cancer. Results of the Danish National Single Center Strategy. Gynecol Oncol 2015;138:304-10. [Crossref] [PubMed]
- Rob L, Skapa P, Robova H. Fertility-sparing surgery in patients with cervical cancer. Lancet Oncol 2011;12:192-200. [Crossref] [PubMed]
- Liu SP, Yang JX, Cao DY, et al. Efficacy of neoadjuvant cisplatin and 5-flourouracil prior to surgery in FIGO stage IB2/IIA2 cervical cancer. Mol Clin Oncol 2014;2:240-4. [Crossref] [PubMed]
- Tjalma WAA. The survival after a radical hysterectomy for cervical cancer by open surgery is significantly better then after minimal invasive surgery: Evidence beats gut feeling! Eur J Obstet Gynecol Reprod Biol 2018;229:195-7. [Crossref] [PubMed]
- Katsumata N, Yoshikawa H, Kobayashi H, et al. Phase III randomised controlled trial of neoadjuvant chemotherapy plus radical surgery vs radical surgery alone for stages IB2, IIA2, and IIB cervical cancer: a Japan Clinical Oncology Group trial (JCOG 0102). Br J Cancer 2013;108:1957-63. [Crossref] [PubMed]
- Gupta S, Maheshwari A, Parab P, et al. Neoadjuvant Chemotherapy Followed by Radical Surgery Versus Concomitant Chemotherapy and Radiotherapy in Patients With Stage IB2, IIA, or IIB Squamous Cervical Cancer: A Randomized Controlled Trial. J Clin Oncol 2018;36:1548-55. [Crossref] [PubMed]
- Zhao H, He Y, Yang SL, et al. Neoadjuvant chemotherapy with radical surgery vs radical surgery alone for cervical cancer: a systematic review and meta-analysis. Onco Targets Ther 2019;12:1881-91. [Crossref] [PubMed]
- Yang Z, Chen D, Zhang J, et al. The efficacy and safety of neoadjuvant chemotherapy in the treatment of locally advanced cervical cancer: A randomized multicenter study. Gynecol Oncol 2016;141:231-9. [Crossref] [PubMed]
- Elit LM, Hirte H. Management of advanced or recurrent cervical cancer: chemotherapy and beyond. Expert Rev Anticancer Ther 2014;14:319-32. [Crossref] [PubMed]
- Greimel ER, Winter R, Kapp KS, et al. Quality of life and sexual functioning after cervical cancer treatment: a long-term follow-up study. Psychooncology 2009;18:476-82. [Crossref] [PubMed]
- Hosaka M, Watari H, Takeda M, et al. Treatment of cervical cancer with adjuvant chemotherapy versus adjuvant radiotherapy after radical hysterectomy and systematic lymphadenectomy. J Obstet Gynaecol Res 2008;34:552-6. [Crossref] [PubMed]
- Mikami M, Aoki Y, Sakamoto M, et al. Surgical principles for managing stage IB2, IIA2, and IIB uterine cervical cancer (Bulky Tumors) in Japan: a survey of the Japanese Gynecologic Oncology Group. Int J Gynecol Cancer 2014;24:1333-40. [Crossref] [PubMed]
- Curtin JP, Hoskins WJ, Venkatraman ES, et al. Adjuvant chemotherapy versus chemotherapy plus pelvic irradiation for high-risk cervical cancer patients after radical hysterectomy and pelvic lymphadenectomy (RH-PLND): a randomized phase III trial. Gynecol Oncol 1996;61:3-10. [Crossref] [PubMed]
- Takekuma M, Kasamatsu Y, Kado N, et al. Reconsideration of postoperative concurrent chemoradiotherapy with fluorouracil and cisplatin for uterine cervical cancer. J Obstet Gynaecol Res 2015;41:1638-43. [Crossref] [PubMed]
- Takekuma M, Kasamatsu Y, Kado N, et al. The issues regarding postoperative adjuvant therapy and prognostic risk factors for patients with stage I-II cervical cancer: A review. J Obstet Gynaecol Res 2017;43:617-26. [Crossref] [PubMed]
- Jung PS, Kim DY, Lee SW, et al. Clinical Role of Adjuvant Chemotherapy after Radical Hysterectomy for FIGO Stage IB-IIA Cervical Cancer: Comparison with Adjuvant RT/CCRT Using Inverse-Probability-of-Treatment Weighting. PLoS One 2015;10:e0132298. [Crossref] [PubMed]


