Pazopanib as salvage therapy in metastatic renal cell carcinoma with hypercalcemic crisis and renal insufficiency: a case report and literature review
Introduction
The number of newly diagnosed renal cell carcinoma (RCC) cases is about 338,000 every year, making it the 12th most common cancer in incidence rate in the world. Furthermore, about 40% of patients with locoregional RCC experience recurrence and develop metastasis after surgery (1,2). Up to 20–30% of metastatic RCC (mRCC) patients present with hypercalcemia and renal function failure, but only 1.6% patients experience crisis (3,4). Some systemic symptoms resulting from metastatic disease, such as fever, weight loss, anemia, local bone pain, and pulmonary symptoms have been associated with a poor prognosis. In current therapy, gaining control of the disease and improving the quality of life (QOL) of unresectable mRCC patients is still challenging. Pazopanib is an oral multiple receptor tyrosine kinase inhibitor approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) as a first-line therapy for clear cell carcinoma for mRCC patients (5). Based on clinical evidence, few studies have reported on salvage therapy in mRCC with hypercalcemic crisis and renal insufficiency. Here, we share the experience of an mRCC case with concurrent hypercalcemic crisis and renal insufficiency who responded to individual salvage therapy of only 200 mg daily oral pazopanib.
Case presentation
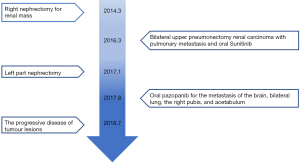
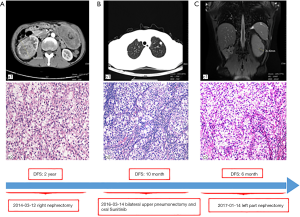
The patient gave written informed consent for the inclusion of material about himself, and he acknowledged that he could not be identified via the article. A 53-year-old man suffered from a 6.6 cm × 6.2 cm right renal mass with hematuria. His past medical history, family history, and systematic physical examination was not particular. There was no distant metastasis reported by a full-body computed tomography (CT) scan, and a right nephrectomy was performed on March 12, 2014 (Figure 1A). Histopathology revealed stage pT1bN0M0 renal clear-cell carcinoma (according to AJCC 7th ed.). On March 14, 2016, approximately 2 years after the operation, another CT scan indicated a relapse of the disease with bilateral pulmonary metastatic lesions (Figure 1B). He underwent bilateral upper pneumonectomy and was administered oral sunitinib 25 mg twice a day from April 1, 2016, to August 7, 2017, as a first-line treatment. Unfortunately, he experienced further deterioration from the disease and underwent left partial nephrectomy (Figure 1C).
The patient was noted as experiencing nausea, anorexia, and debilitation in May 2017. The Karnofsky Performance Status Score (KPS) was 60. There was no diagnostic challenges for the patient. Positron emission tomography/CT (PET/CT) scans revealed that there were nodular lesions with an increased 18 F-fluorodeoxyglucose (FDG) uptake in the brain, bilateral lung, the right pubis, and acetabulum (Figure 2). Moreover, hypercalcemic crisis, moderate anemia, and renal insufficiency occurred concurrently with serum calcium level 3.84 mmol/L, serum hemoglobin 82 g/L, and serum creatinine 211 µmol/L with the endogenous creatinine clearance rate being 24 mL/min (Figure 3). Three of the five adverse prognostic factors were present in the patient, which included a KPS of lower than 80%, serum calcium greater than the upper limit of normal (ULN), and serum hemoglobin less than the lower limit of normal (LLN) which classified the patient into the poor-risk group according to Memorial Sloan Kettering Cancer Center (MSKCC) criteria. Firstly, the patient received treatments with diuretic therapy, including diphosphonate and calcitonin for 2 months. However, the levels of serum calcium and serum creatinine were high, with both fluctuating from 3.3 to 3.8 mmol/L and 198 to 217 µmol/L, respectively. The symptoms of nausea, anorexia, and debilitation improved little. We evaluated his poor response in order to choose the best supportive care.
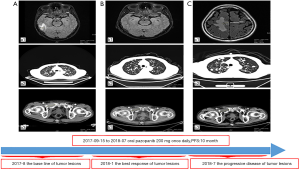
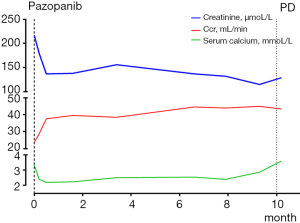
Given the evidence of the symptomatology, poor prognosis, and renal insufficiency (CKD4) for the patient, anti-tumor treatment was ruled out. Nevertheless, the patient was individually provided treatment with a reduced dose of oral pazopanib 200 mg once daily on September 10, 2017 as salvage therapy, and the symptoms of nausea, anorexia, and debilitation were improved in 2 weeks. Meanwhile, the level of the serum calcium dropped to a dangerously low level of 2.24 mmol/L. The creatinine and the creatinine clearance rate were 114 µmol/L and 49 mL/min at best response, respectively (Figure 3). Serial CT and magnetic resonance imaging (MRI) scans revealed evidence of objective disease response and subsequent disease stabilization for the focuses of the bilateral lung, the right pubis, and acetabulum (Figure 2). There was no recurrence of these symptoms, QOL improved, and PFS was 10 months. The treatment was well tolerated with minimal toxicity symptoms, such as leukotrichia and hypertension. Blood pressure (BP) ranged from 150–120/80–90 mmHg, which is classified as stage 2 hypertension (CTCAE 4.0). Neither rashes nor diarrhea was found in the patient.
Unfortunately, on July 25, 2018, we evaluated the progression of disease (PD), and pazopanib was discontinued due to the recurrence of anorexia and patient debilitation. The serum calcium was elevated to 3.59 mmol/L, while the creatinine reached 129 µmol/L (Figure 3). The CT scans confirmed that the focuses of the bilateral lung, the right pubis, and acetabulum progressed slowly. The cerebral metastases were obviously enlarged on MRI scan (Figure 2). Overall, the patient achieved progression-free survival (PFS) for 10 months. The timeline picture of the patient was shown in Figure 4.
Discussion
In our case, the patient was evaluated to be in the poor-risk category by MSKCC criteria with a KPS of <60, hypercalcemia, and anemia. Furthermore, this was coupled with renal insufficiency, in which the endogenous creatinine clearance rate was only 24 mL/min. Combination therapy, including diuretic therapy, diphosphonate, and calcitonin, was administered but received a poor response. The main aims of the therapy were the best supportive care according to the National Comprehensive Cancer Network (NCCN) and the European Association of Urology (EAU) guidelines, which represent the gold standard. However, how to control the primary disease and concurrently relieve the symptoms in clinic to improve QOL and prolong the individual patient’s PFS and overall survival (OS) is the challenging task. The theme of the American Society of Clinical Oncology (ASCO) in 2019 focused on caring and learning for every patient.
The first problem for the patient was that the creatinine clearance rate was only 24 mL/min. As is well-known, pazopanib is a tyrosine kinase inhibitor that targets vascular endothelial growth factor receptors (VEGFR-1, VEGFR-2, VEGFR-3), platelet-derived growth factor receptors (PDGFR-α, PDGFR-β), fibroblast growth factor receptors (FGFR-1, FGFR-3), and c-kit (5,6). The primary route of metabolism is hepatic, and excretion is through feces (7), with the kidney excreting less than 4% of the dosage. Even so, caution is recommended in patients with creatinine clearance below 30 mL/min (8). Therefore, we needed to decide whether to start the anti-tumor treatment for him given the creatinine clearance rate.
The second problem for the patient was determining how many doses he should take. By going through the recent retrospective reviews, most patients showed only partial response (PR) or stable disease as the best possible response (9). In a global, randomized, double-blind, placebo-controlled phase III study of the 435 treatment-naive and cytokine-pretreated patients with advanced RCC, pazopanib improved PFS when compared with placebo (median, 9.2 vs. 4.2 months; hazard ratio, 0.46) (10). In the real-world, median PFS with pazopanib as first-line therapy was 10.6 months (from 5.3 to 13.7 months) (11). In the subgroup analysis of the COMPARTZ phase 3 randomized trial for 209 Chinese patients, the PFS was 8.0 months, and the objective response rate (ORR) was 35% (12). The safety and quality-of-life profiles favor pazopanib. The dose of these trials is not appropriate for the Chinese patient with poor performance status. Whether dose reduction affects the efficacy is controversial. In a phase I trial of pazopanib, a clinical benefit was observed in patients who received doses of ≥800 mg once daily or 300 mg twice daily (13). Cecere et al. reported that dose reduction was not associated with decreased efficacy and confirmed that a personalized drug schedule, along with persistence in therapy, may enhance the therapeutic benefit (14).
The third problem for the patient was whether pazopanib could be used as salvage therapy and if it would have therapeutic effect. Osawa et al. reported that regorafenib prolonged the OS of patients with mCRC in the salvage setting compared with the best supportive care (15). Therefore, although mRCC means higher prognostic risk scores after the first-line treatment, especially for renal insufficiency and hypercalcemic crisis, we individually treated him with oral pazopanib at a dose modification of 200 mg once daily, given these problems and reports from the literature. The patient achieved PR and PFS for 10 months which was far more than the relevant literature reported. The treatment-related toxicities were grade 1 leukotrichia and hypertension (CTCAE 4.0). The patient received treatment on an ongoing basis while maintaining a good QOL, and experienced no rashes, diarrhea, or hand-foot syndrome.
Conclusions
The results show that the lower dose of pazopanib achieved better efficacy in a patient with poor KPS, hypercalcemic crisis, and renal insufficiency. This finding is promising and may provide critical data for the further development of this therapeutic strategy. Individual administration of pazopanib as salvage therapy in mRCC is worth exploring in a pilot trial. As Noritz reports, clinician-researchers can easily access clinical and research data for caring and learning from patients (16). There are some limitations in our report. We could combine this treatment with immune checkpoint inhibitors or increase the dose of pazopanib as the condition of the patient was good. These adjustments to the therapy may achieve greater PFS and OS.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.73). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this case report and any accompanying images. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sun M, Marconi L, Eisen T, et al. Adjuvant Vascular Endothelial Growth Factor-targeted Therapy in Renal Cell Carcinoma: A Systematic Review and Pooled Analysis. Eur Urol 2018;74:611-620. [Crossref] [PubMed]
- Janzen NK, Kim HL, Figlin RA, et al. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am 2003;30:843-52. [Crossref] [PubMed]
- Gomes Lda S, Kulak CA, Costa TM, et al. Association of primary hyperparathyroidism and humoral hypercalcemia of malignancy in a patient with clear cell renal carcinoma. Arch Endocrinol Metab 2015;59:84-8. [Crossref] [PubMed]
- Ahmad S, Kuraganti G, Steenkamp D. Hypercalcemic crisis: a clinical review. Am J Med 2015;128:239-45. [Crossref] [PubMed]
- Sternberg CN, Hawkins RE, Wagstaff J, et al. A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: final overall survival results and safety update. Eur J Cancer 2013;49:1287-96. [Crossref] [PubMed]
- Miaris N, Maltezou M, Papaxoinis G, et al. Posterior Reversible Encephalopathy Syndrome With Concurrent Nephrotic Syndrome in a Patient Treated With Pazopanib for Metastatic Renal Cell Carcinoma: Case Report and Review of the Literature. Clin Genitourin Cancer 2017;15:e99-103. [Crossref] [PubMed]
- Shibata SI, Chung V, Synold TW, et al. Phase I study of pazopanib in patients with advanced solid tumors and hepatic dysfunction: a National Cancer Institute Organ Dysfunction Working Group study. Clin Cancer Res 2013;19:3631-9. [Crossref] [PubMed]
- Hutson TE, Davis ID, Machiels JP, et al. Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. J Clin Oncol 2010;28:475-80. [Crossref] [PubMed]
- Hainsworth JD, Rubin MS, Arrowsmith ER, et al. Pazopanib as second-line treatment after sunitinib or bevacizumab in patients with advanced renal cell carcinoma: a Sarah Cannon Oncology Research Consortium Phase II Trial. Clin Genitourin Cancer 2013;11:270-5. [Crossref] [PubMed]
- Porta C, Ferrari A, Czarnecka AM, et al. Pazopanib in Patients with Clear-Cell Renal Cell Carcinoma: Seeking the Right Patient. Front Pharmacol 2017;8:329. [Crossref] [PubMed]
- Rossetti S, D'Aniello C, Iovane G, et al. Sequential Treatment with Pazopanib and Everolimus in Metastatic Renal Cell Carcinoma. Front Pharmacol 2017;8:484. [Crossref] [PubMed]
- Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013;369:722-31. [Crossref] [PubMed]
- Hurwitz HI, Dowlati A, Saini S, et al. Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res 2009;15:4220-7. [Crossref] [PubMed]
- Cecere SC, Rossetti S, Cavaliere C, et al. Corrigendum: Pazopanib in Metastatic Renal Cancer: A "Real-World" Experience at National Cancer Institute "Fondazione G. Pascale". Front Pharmacol 2016;7:468. [PubMed]
- Osawa H. Response to regorafenib at an initial dose of 120 mg as salvage therapy for metastatic colorectal cancer. Mol Clin Oncol 2017;6:365-72. [Crossref] [PubMed]
- Noritz G, Boggs A, Lowes LP, et al. "Learn From Every Patient": How a Learning Health System Can Improve Patient Care. Pediatr Qual Saf 2018;3:e100. [Crossref] [PubMed]