This article has an erratum available at: http://dx.doi.org/10.21037/tcr-2023-06 the article has been update on 2023-10-18 at here.
Survival outcomes of pancreaticoduodenectomy versus extended pancreaticoduodenectomy procedure for pancreatic head carcinoma: a propensity score matching study
Introduction
Pancreatic carcinoma has ranked as one of the most lethal diseases and its 5-year overall survival (OS) is less than 8%, which will become the second most common cause in the USA of cancer-related deaths by the year (1-3). Pancreaticoduodenectomy (PD) is the widely used operation in approximately 75% of patients with pancreatic head carcinoma (PHC). Current treatment strategies need to be highlighted and improved due to its highly aggressive nature with poor prognosis. Nowadays, surgical resection is considered as the potentially curative therapy. However, because of its difficulty in definite early diagnosis, merely 20% of patients have the opportunity for successful resection when diagnosed (4). Therefore, the established and promising surgical procedures will be focused to improve postoperative recovery and survival.
The classic PD procedure was considered as the operation of choice for PHC, which was refined and popularized by Whipple et al. in 1935 (5). In this procedure, an en bloc resection of the pancreatic head, the common bile duct, the gallbladder, the duodenum, the upper jejunum, the distal portion of the stomach and the adjacent lymph nodes was encompassed (6). In addition, the extended PD procedure included extended lymphadenectomy, extended organic resection, and extended vascular resection and reconstruction (7-10).
The benefits of extended lymphadenectomy had not been specified, and many multicenter randomized controlled trials reported that radical PD combined with extended lymphadenectomy was not beneficial to long-term survival in resectable PHC patients, and on the contrary, it may lead to levels of morbidity and mortality when compared to patients with standard lymphadenectomy (8,10,11). Besides, surgery for PHC commonly required vascular resection and reconstruction, such as the superior mesenteric vein (SMV) and portal vein (PV), however, the splenic vein (SV) sometimes also needed to be incised when it was involved by tumor (12).
The evidence of evaluating the safety and efficacy of vascular resection mainly came from single institutional retrospective studies, which showed mixed results in both perioperative complications and survival. Among these cases, some have reported decreased survival and elevated perioperative complication rates with extended resection (13,14), while others have demonstrated similar short- and long-term survival with or without extended resection (15,16), which is controversial.
In this study, the objective was to assess whether the results of extended PD procedure were equal to those of the classic PD procedure in PHC patients, especially concerning survival outcomes.
Methods
Patients and clinical data
The data of patients were obtained from the Surveillance, Epidemiology, and End Results (SEER) database in our study and the selection criterion were as previously described (17). Besides, patients diagnosed from 2004 to 2014 with PD or extended PD surgical procedures with following chemotherapy and having available data for sex, race/ethnicity, age, grade, tumor size, liver metastasis, lung metastasis, TNM stage, regional lymph node examined, regional lymph node positive, insurance, marriage, and survival information were analyzed. Other patients with partial pancreatectomy, total pancreatectomy, and pylorus-preserving PD were all excluded. The AJCC 8th staging system was still evaluated as previous publications (4,17). Following the criteria, the whole cohort was finally included.
Statistical analysis
In this study, R project version 3.3.4 (http://www.r-project.org/) and SPSS 21.0 statistical package (SPSS Inc., Chicago, IL, USA) were used in statistical analyses. Propensity score matching (PSM) was applied to cut down selection bias. The propensity scores, which were defined as the probability of being assigned to the PD or extended PD groups by considering all potential confounders, were computed by the MatchIt package in R project with an algorithm of 2:1 matching by age, race/ethnicity, and AJCC 8th staging system. The nearest neighbor matching with a caliper width of 0.05 was employed. Correlations and differences between categorical variables were analyzed by the χ2 test and Fisher’s exact test, while continuous variables by Student’s t-test. The cancer-specific survival (CSS) and OS were depicted by Kaplan-Meier curves via Survminer package in the R project. CSS was calculated by the time from treatment to cancer-related death, while OS was defined as the time from treatment to the last follow-up or death. Univariate and multivariate analyses with Cox proportional hazards regression model were used to identify its independent prognostic factors. All P value <0.05 were considered the statistically significant difference.
Results
Demographic and clinicopathological characteristics
There were 7,084 histologically confirmed patients of PHC in this study, including 6,541 (92.3%) and 543 (7.7%) patients who received PD and extended PD surgical procedures, respectively. The mean ratio of extended PD procedure use was 7.7% (ranging from 5.9% in 2009 to 9.0% in 2005). There was no extensive application during these years; the detailed frequency of PD or extended PD procedure use is shown in Figure 1. In the whole cohort, there were 3,640 male and 3,444 female patients whose median age was 65.9 (ranging from 29 to 95) years old. Among these patients, liver metastasis was detected in 98 patients (1.4%) and lung metastasis in 24 patients (0.3%). The mean tumor size was 32.3±16.9 mm, and the average number of examined and positive lymph nodes was 15.9±10.3 and 2.6±3.3, respectively. For further analysis, the mean number of lymph nodes examined and positive with PD procedure was 15.9±10.3 and 2.6±3.3 respectively, while 16.1±10.1 and 2.6±3.1 respectively with extended PD procedure, which showed no significance between two groups. By the AJCC 8th TNM staging system, 659, 3,399, 2,499 and 527 patients were respectively classified into stage I, II, III and IV disease, respectively. The other detailed characteristics were displayed in Table 1.
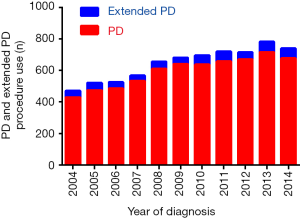
Table 1
| Variables | Unmatched | Matched (2:1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients (N) | Whipple, n (%) | Extended Whipple, n (%) | P value | Patients (N) | Whipple, n (%) | Extended Whipple, n (%) | P value | ||
| Age (years) | 0.044 | 0.949 | |||||||
| Mean ± STD | 65.99 | 65.9±10.5 | 66.8±10.4 | 66.8±10.4 | 66.8±10.4 | 66.8±10.4 | |||
| Race/ethnicity | 0.035 | 0.796 | |||||||
| White | 5,917 | 5,442 (92.0) | 475 (8.0) | 1,420 | 945 (66.5) | 475 (33.5) | |||
| Blank | 654 | 615 (94.0) | 39 (6.0) | 112 | 73 (65.2) | 39 (34.8) | |||
| Others | 513 | 484 (94.3) | 29 (5.7) | 95 | 66 (69.5) | 29 (30.5) | |||
| Sex | 0.593 | 0.207 | |||||||
| Male | 3,640 | 3,355 (92.2) | 285 (7.8) | 818 | 533 (65.2) | 285 (34.8) | |||
| Female | 3,444 | 3,186 (92.5) | 258 (7.5) | 809 | 551 (68.1) | 258 (31.9) | |||
| Grade | 0.265 | 0.206 | |||||||
| I | 659 | 609 (92.4) | 50 (7.6) | 151 | 101 (66.9) | 50 (33.1) | |||
| II | 3,399 | 3,159 (92.9) | 240 (7.1) | 776 | 536 (69.1) | 240 (30.9) | |||
| III or IV | 2,499 | 2,288 (91.6) | 211 (8.4) | 587 | 376 (64.1) | 211 (35.9) | |||
| Unknown | 527 | 485 (92.0) | 42 (8.0) | 113 | 71 (62.8) | 42 (37.2) | |||
| AJCC 8th stage | 0.042 | 0.160 | |||||||
| I | 1,590 | 1,493 (93.9) | 97 (6.1) | 330 | 233 (70.6) | 97 (29.4) | |||
| II | 2,621 | 2,403 (91.7) | 218 (8.3) | 599 | 381 (63.6) | 218 (36.4) | |||
| III | 2,524 | 2,328 (92.2) | 196 (7.8) | 594 | 398 (67.0) | 196 (33.0) | |||
| IV | 349 | 317 (90.8) | 32 (9.2) | 104 | 72 (69.2) | 32 (30.8) | |||
| Tumor size (mm) | 0.525 | 0.933 | |||||||
| Mean ± STD | 32.33±16.9 | 32.33±16.2 | 32.83±23.8 | 32.83±21.1 | 32.93±19.6 | 32.83±23.8 | |||
| Liver metastasis | 0.086 | 0.908 | |||||||
| Yes | 98 | 86 (87.8) | 12 (12.2) | 35 | 23 (65.7) | 12 (34.3) | |||
| No | 6,986 | 6,455 (92.4) | 531 (7.6) | 1,592 | 1,061 (66.6) | 531 (33.4) | |||
| Lung metastasis | 0.303 | 0.375 | |||||||
| Yes | 24 | 24 (100) | 0 (0) | 4 | 4 (100.0) | 0 (0) | |||
| No | 7,060 | 6,517 (92.3) | 543 (7.7) | 1,623 | 1,080 (66.5) | 543 (33.5) | |||
| Lesion number | 0.615 | 0.973 | |||||||
| 1 | 6,053 | 5,593 (92.4) | 460 (7.6) | 1,379 | 919 (66.6) | 460 (33.4) | |||
| ≥2 | 1,031 | 948 (91.9) | 83 (8.1) | 248 | 165 (66.5) | 83 (33.5) | |||
| Insurance | 0.132 | 0.126 | |||||||
| Yes | 5,383 | 4,988 (92.7) | 395 (7.3) | 1,230 | 835 (67.9) | 395 (32.1) | |||
| No | 123 | 110 (89.4) | 13 (10.6) | 30 | 17 (56.7) | 13 (43.3) | |||
| Unknown | 1,578 | 1,443 (91.4) | 135 (8.6) | 367 | 232 (63.2) | 135 (36.8) | |||
| Marriage | 0.742 | 0.670 | |||||||
| Married | 4,489 | 4,145 (92.3) | 344 (7.7) | 1,016 | 672 (66.1) | 344 (33.9) | |||
| Divorced | 1,655 | 1,526 (92.2) | 129 (7.8) | 404 | 275 (68.1) | 129 (31.9) | |||
| Single | 758 | 705 (93.0) | 53 (7.0) | 165 | 112 (67.9) | 53 (32.1) | |||
| Unknown | 182 | 165 (90.7) | 17 (9.3) | 42 | 25 (59.5) | 17 (40.5) | |||
PSM for PD and extended PD
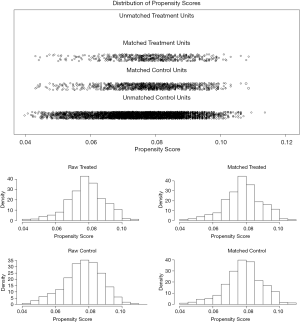
Patients who were older, of white ethnicity, and with higher TNM stage were more likely to receive extended PD procedure. To reduce confounder and reflect the nature of two surgical procedures, a PSM model based on these three indicators was built. As a result, 543 patients with extended PD procedure and 1,084 patients with PD procedure were completely matched using PSM. The distribution and histograms of propensity scores before and after matching were shown in Figure 2. The detailed characteristics of the unmatched and matched cohorts were listed in Table 1.
The prognostic factors before or after matching
The older age, male, higher grade (P<0.001), larger tumor size, liver metastasis, lung metastasis and advanced TNM stage were significantly considered as risk factors for CSS before matching in univariate analysis. Besides these factors, divorce was also a risk factor for OS before matching. However, after matching, older age, higher grade, lung metastasis, and advanced TNM stage were still considered as risk factors for CSS, as well as divorce for OS (Table 2). In multivariate analysis, age, sex, grade, the AJCC 8th stage, and tumor size remained as independent prognostic indicators for CSS before matching, while only age, grade, and the AJCC 8th stage were independent prognostic factors after matching. For OS before matching, age, sex, grade, tumor size, marriage, and the AJCC 8th stage were all considered as independent risk factors, while after matching, there were only age, grade, marriage, and the AJCC 8th stage remaining (Table 3). In the matched populations, the surgical procedures had no effect on CSS [hazard ratio (HR): 1.030; 95% confidence interval (CI): 0.906–1.172, P=0.648] or OS (HR: 1.017; 95% CI: 0.900–1.148, P=0.790).
Table 2
| Variables | Unmatched | Matched (2:1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CCS | OS | CCS | OS | ||||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||||
| Age (years) | |||||||||||
| <65 | 1 | 1 | 1 | 1 | |||||||
| ≥65 | 1.168 (1.101–1.239) | <0.001 | 1.208 (1.142–1.278) | <0.001 | 1.333 (1.177–1.509) | <0.001 | 1.400 (1.244–1.575) | <0.001 | |||
| Race/ethnicity | |||||||||||
| White | 1 | 1 | 1 | 1 | |||||||
| Blank | 0.956 (0.860–1.062) | 0.835 | 1.039 (0.944–1.143) | 0.436 | 0.869 (0.679–1.112) | 0.264 | 0.914 (0.728–1.147) | 0.437 | |||
| Others | 0.988 (0.878–1.110) | 0.400 | 0.983 (0.879–1.143) | 0.757 | 1.178 (0.905–1.533) | 0.222 | 1.174 (0.915–1.507) | 0.206 | |||
| Sex | |||||||||||
| Male | 1 | 1 | 1 | 1 | |||||||
| Female | 0.919 (0.867–0.975) | 0.005 | 0.901 (0.852–0.952) | <0.001 | 0.899 (0.796–1.105) | 0.085 | 0.881 (0.786–0.989) | 0.031 | |||
| Grade | |||||||||||
| I | 1 | 1 | 1 | 1 | |||||||
| II | 1.396 (1.247–1.564) | <0.001 | 1.340 (1.207–1.487) | <0.001 | 1.364 (1.071–1.737) | 0.012 | 1.229 (0.989–1.527) | 0.062 | |||
| III or IV | 1.945 (1.733–2.182) | <0.001 | 1.799 (1.617–2.001) | <0.001 | 1.976 (1.547–2.525) | <0.001 | 1.712 (1.372–2.135) | <0.001 | |||
| Unknown | 1.227 (1.049–1.435) | 0.011 | 1.193 (1.032–1.379) | 0.017 | 1.354 (0.974–1.884) | 0.072 | 1.199 (0.884–1.626) | 0.244 | |||
| AJCC 8th stage | |||||||||||
| I | 1 | 1 | 1 | 1 | |||||||
| II | 1.678 (1.541–1.826) | <0.001 | 1.545 (1.429–1.670) | <0.001 | 1.435 (1.202–1.712) | <0.001 | 1.377 (1.168–1.623) | <0.001 | |||
| III | 2.079 (1.909–2.264) | <0.001 | 1.888 (1.745–2.042) | <0.001 | 1.815 (1.519–2.167) | <0.001 | 1.725 (1.462–2.036) | <0.001 | |||
| IV | 3.208 (2.794–3.683) | <0.001 | 2.788 (2.444–3.181) | <0.001 | 2.541 (1.953–3.306) | <0.001 | 2.331 (1.814–2.995) | <0.001 | |||
| Tumor size (mm) | 1.004 (1.003–1.005) | <0.001 | 1.003 (1.002–1.004) | <0.001 | 1.002 (1.000–1.003) | 0.065 | 1.001 (1.000–1.003) | 0.097 | |||
| Liver metastasis | |||||||||||
| No | 1 | 1 | 1 | 1 | |||||||
| Yes | 1.964 (1.537–2.510) | <0.001 | 1.818 (1.431–2.310) | <0.001 | 1.326 (0.877–2.005) | 0.181 | 1.289 (0.867–1.916) | 0.210 | |||
| Lung metastasis | |||||||||||
| No | 1 | 1 | 1 | 1 | |||||||
| Yes | 2.113 (1.330–3.358) | 0.002 | 1.980 (1.262–3.107) | 0.003 | 2.771 (1.037–7.404) | 0.042 | 2.475 (0.927–6.609) | 0.071 | |||
| Lesion number | |||||||||||
| 1 | 1 | 1 | 1 | 1 | |||||||
| ≥2 | 0.991 (0.910–1.079) | 0.833 | 1.051 (0.972–1.137) | 0.211 | 1.052 (0.886–1.250) | 0.561 | 1.132 (0.966–1.326) | 0.125 | |||
| Insurance | |||||||||||
| No | 1 | 1 | 1 | 1 | |||||||
| Yes | 0.836 (0.667–1.046) | 0.117 | 0.820 (0.664–1.012) | 0.064 | 0.933 (0.577–1.510) | 0.778 | 0.804 (0.526–1.227) | 0.311 | |||
| Unknown | 0.948 (0.754–1.192) | 0.646 | 0.929 (0.750–1.151) | 0.502 | 1.049 (0.643–1.712) | 0.847 | 0.900 (0.584–1.386) | 0.631 | |||
| Marriage | |||||||||||
| Married | 1 | 1 | 1 | 1 | |||||||
| Divorced | 1.059 (0.987–1.137) | 0.111 | 1.098 (1.028–1.173) | 0.006 | 1.129 (0.979–1.303) | 0.095 | 1.174 (1.026–1.342) | 0.019 | |||
| Single | 1.007 (0.912–1.112) | 0.894 | 1.068 (0.974–1.170) | 0.163 | 1.110 (0.903–1.365) | 0.323 | 1.166 (0.962–1.414) | 0.118 | |||
| Unknown | 0.962 (0.789–1.174) | 0.706 | 0.943 (0.779–1.140) | 0.543 | 0.587 (0.367–0.938) | 0.026 | 0.584 (0.374–0.910) | 0.018 | |||
| Surgical procedure | |||||||||||
| Whipple | 1 | 1 | 1 | 1 | |||||||
| Extended Whipple | 1.106 (0.992–1.233) | 0.070 | 1.089 (0.982–1.208) | 0.106 | 1.030 (0.906–1.172) | 0.648 | 1.017 (0.900–1.148) | 0.790 | |||
CSS, cancer specific survival; OS, overall survival.
Table 3
| Variables | Unmatched | Matched (2:1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CCS | OS | CCS | OS | ||||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||||
| Age (years) | |||||||||||
| <65 | 1 | 1 | 1 | 1 | |||||||
| ≥65 | 1.220 (1.149–1.295) | <0.001 | 1.256 (1.187–1.329) | <0.001 | 1.397 (1.232–1.584) | <0.001 | 1.466 (1.300–1.652) | <0.001 | |||
| Sex | NA | NA | NA | NA | |||||||
| Male | 1 | 1 | |||||||||
| Female | 0.926 (0.873–0.982) | 0.011 | 0.878 (0.829–0.930) | <0.001 | |||||||
| Grade | |||||||||||
| I | 1 | 1 | 1 | 1 | |||||||
| II | 1.387 (1.238–1.554) | <0.001 | 1.331 (1.199–1.478) | <0.001 | 1.353 (1.062–1.725) | 0.014 | 1.224 (0.985–1.521) | 0.069 | |||
| III or IV | 1.870 (1.666–2.099) | <0.001 | 1.740 (1.564–1.937) | <0.001 | 1.958 (1.532–2.502) | <0.001 | 1.695 (1.359–2.115) | <0.001 | |||
| Unknown | 1.144 (0.977–1.339) | 0.094 | 1.122 (0.970–1.298) | 0.122 | 1.258 (0.903–1.753) | 0.174 | 1.125 (0.828–1.527) | 0.452 | |||
| AJCC 8th stage | |||||||||||
| I | 1 | 1 | 1 | ||||||||
| II | 1.608 (1.476–1.752) | <0.001 | 1.490 (1.376–1.612) | <0.001 | 1.457 (1.220–1.740) | <0.001 | 1.409 (1.194–1.662) | <0.001 | |||
| III | 2.005 (1.839–2.187) | <0.001 | 1.841 (1.700–1.995) | <0.001 | 1.919 (1.604–2.295) | <0.001 | 1.840 (1.557–2.175) | <0.001 | |||
| IV | 3.173 (2.710–3.716) | <0.001 | 2.799 (2.407–3.255) | <0.001 | 2.595 (1.984–3.395) | <0.001 | 2.459 (1.912–3.162) | <0.001 | |||
| Tumor size (mm) | 1.002 (1.001–1.004) | 0.001 | 1.002 (1.001–1.003) | 0.002 | NA | NA | NA | NA | |||
| Liver metastasis | NA | NA | NA | NA | |||||||
| No | 1 | 1 | |||||||||
| Yes | 1.037 (0.784–1.372) | 0.800 | 1.013 (0.771–1.331) | 0.925 | |||||||
| Lung metastasis | NA | NA | |||||||||
| No | 1 | 1 | 1 | ||||||||
| Yes | 0.973 (0.602–1.573) | 0.912 | 0.982 (0.616–1.567) | 0.940 | 1.843 (0.673–5.052) | 0.234 | |||||
| Marriage | NA | NA | |||||||||
| Married | 1 | 1 | 1 | ||||||||
| Divorced | 1.156 (1.080–1.238) | <0.001 | 1.134 (0.982–1.309) | 0.087 | 1.169 (1.022–1.339) | 0.023 | |||||
| Single | 1.123 (1.024–1.232) | 0.014 | 1.224 (0.993–1.507) | 0.058 | 1.284 (1.057–1.558) | 0.012 | |||||
| Unknown | 1.016 (0.840–1.229) | 0.871 | 0.665 (0.416–1.064) | 0.089 | 0.650 (0.416–1.016) | 0.059 | |||||
CSS, cancer specific survival; OS, overall survival; NA, not available.
CSS and OS
The median CSS and OS of the whole population was 20.0 and 18.0 months, respectively. The 1-, 3- and 5-year CSS rates were 69.7%, 29.0% and 15.8%, respectively, while the OS rates at 1, 3 and 5 years were 66.3%, 25.2%, and 15.8%, respectively. Stratified by surgical procedures, the median CSS of patients received PD procedure were a little larger than that of patients with extended PD procedure (20.0 vs. 19.0 months), so did median OS (19.0 vs. 18.0 months). However, it was still lack of significant differences (Figure 3A,B). The cumulative CSS rates of patients with PD procedure at 1, 3 and 5 years were 70.0%, 29.2% and 19.7%, whereas in extended PD group, they were 65.8%, 26.3% and 16.1%, respectively. The cumulative OS rates of patients with PD procedure at 1, 3 and 5 years were 66.6%, 25.5%, and 16.0%, whereas in extended PD group, they were 62.6%, 22.5% and 13.1%, respectively.
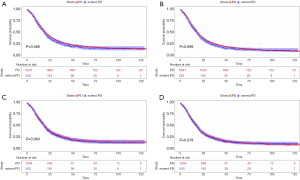
After PSM, the median CSS and OS changed to 19.0 and 18.0 months, respectively. The CSS rates of 1, 3 and 5 years in the matching group were 67.3%, 27.2%, and 17.1%, whereas the OS rates were 63.6%, 23.6% and 13.7%, respectively. For further analysis of surgical effects, the median CSS of both PD and extended PD procedure groups was 19.0 months. In PD procedure group, the 1-, 3- and 5-year CSS rates were 68.1%, 27.7%, and 17.5%, respectively, while in extended PD procedure group, they were 65.8%, 26.3% and 16.1%, respectively. Regarding the OS, the median OS of both groups was 18.0 months. The cumulative OS rates in PD procedure group at 1, 3 and 5 years were 64.1%, 24.2%, and 13.9%, whereas in extended PD procedure group, they were 62.6%, 22.5%, and 13.1%, respectively. Therefore, the CSS and OS were still not comparable between two surgical groups after PSM (Figure 3C,D).
Discussion
In this study, we analyzed the outcomes of different surgical procedures on PHC patients. A total of 7,084 PHC patients with PD or extended PD procedure were enrolled in the unmatched group. After the PSM of age, race and TNM stage, 1,627 matched patients were finally analyzed. The results showed that the survival outcomes were similar between PD and extended PD procedure in both unmatched and matched groups.
Through the above analysis, we found that the average of lymph nodes examined and positive with PD procedure was 15.9±10.3 and 2.6±3.3 respectively, while 16.1±10.1 and 2.6±3.1 respectively with extended PD procedure, which showed no difference between two populations. After PSM, the average of lymph nodes examined and positive with PD procedure changed into 15.6±9.5 and 2.8±3.5 respectively, while 15.8±8.9 and 2.6±3.1 with extended PD procedure respectively. The results meant that the extended PD procedure in this study mainly included the PD with extended resection and reconstruction instead of the PD with extended lymphadenectomy. As the results showed, the CSS rate of 5 years in both matched populations was 17.5% and 16.1%, respectively. Regarding the 5-year OS, it was respectively 13.9% and 13.1%. Patients had no significant difference in CSS or OS, which was following many previous studies (16,18,19). However, Kantor et al. (20) demonstrated that PD with major vascular resection increased the rate of severe adverse effects, readmissions, a total length of hospital stay and costs. Despite the rates of R0 resection and overall relapse were similar between two cohorts, decreased OS occurred in combination with vascular resection. Podda et al. (16) obtained similar conclusions that the median OS for standard PD was 21 months and for PD with venous resection was 18 months (P=0.588). However, patients undergoing PD procedure with venous and arterial resection had a median OS of 7 months, which was less than standard PD procedure significantly (P=0.044). In addition, the PD with venous resection had no increased risk of procedure specific postoperative complications compared to standard PD. On the other hand, no survival benefit was obtained in PD procedure with venous resection and arterial resection. All of these studies indicated that the extended PD procedure had no survival benefit and may lead to a higher risk of morbidity and mortality (14,21).
It was not clear about the potential etiology for the growing complications with the extended PD procedure. The cross-clamping of the PV may consist of the higher complication profile, and then might result in growing propensity for ileus, malnutrition, and delayed gastric emptying induced by transient intestinal ischemia. All of the complications occurred among populations with extended PD procedure in increased frequency (22,23), which contributed to the prolonged hospitalization and admission. In addition, longer length of hospital stay, a principal adverse contributor to patient debilitation and quality of life, was a widely known risk indicator for delays to adjuvant chemotherapy (20).
From the results of multivariate analysis, we found age, differentiated grade, and TNM stage level were independent prognostic factors for CSS. Intriguingly, marital status was an additional independent risk for OS. Asano et al. (24) reported that age contributed to the risk stratification in resected PC patients. In addition, differentiation grade, and TNM stage may reflect its biological activity highlighted in plenty of studies for its critical role in survival and some recent reports even combines these factors with other biomarkers to improve the predictive power (25-27). However, to our surprise, the single or divorced patients may have a poorer OS than those of being married. The reasons for that may include the lonely, anxious and depressed life, lack of enough care and high occurrence rate of accidents (28,29).
We recognize some possible limitations in this study. This study was limited as retrospective research, the data was lack of the morbidities and complications, and the extended PD was not classified specifically. Therefore, a multi-center and large-scale prospective study need be performed to eliminate the selective bias for further confirmation.
This population-based study shows that there is no distinctly different survival outcome between PHC patients with PD or extended PD procedure. The clinical indicators for the individual patient, such as age, differentiated grade, and TNM stage, may reveal a superior risk stratification to obtain an optimal treatment modality.
Acknowledgments
Funding: This work was granted by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.01.38). NP serves as an unpaid section editor of Translational Cancer Research from Jan 2020 to Dec 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Ethics Committees of Zhongshan Hospital, Fudan University, approved the design and analysis of this study (Ethical Committee No: B2017-006R). This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Pu N, Zhao G, Yin H, et al. CD25 and TGF-beta blockade based on predictive integrated immune ratio inhibits tumor growth in pancreatic cancer. J Transl Med 2018;16:294. [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Pu N, Yin L, Habib JR, et al. Optimized modification of the eighth edition of AJCC TNM staging system for resected pancreatic ductal adenocarcinoma. Future Oncol 2019;15:3457-65.
- Whipple AO, Parsons WB, Mullins CR. Treatment of Carcinoma of the Ampulla of Vater. Ann Surg 1935;102:763-79. [Crossref] [PubMed]
- Iqbal N, Lovegrove RE, Tilney HS, et al. A comparison of pancreaticoduodenectomy with pylorus preserving pancreaticoduodenectomy: a meta-analysis of 2822 patients. Eur J Surg Oncol 2008;34:1237-45. [Crossref] [PubMed]
- Wang WL, Ye S, Yan S, et al. Pancreaticoduodenectomy with portal vein/superior mesenteric vein resection for patients with pancreatic cancer with venous invasion. Hepatobiliary Pancreat Dis Int 2015;14:429-35. [Crossref] [PubMed]
- Dasari BV, Pasquali S, Vohra RS, et al. Extended Versus Standard Lymphadenectomy for Pancreatic Head Cancer: Meta-Analysis of Randomized Controlled Trials. J Gastrointest Surg 2015;19:1725-32. [Crossref] [PubMed]
- Liles JS, Katz MH. Pancreaticoduodenectomy with vascular resection for pancreatic head adenocarcinoma. Expert Rev Anticancer Ther 2014;14:919-29. [Crossref] [PubMed]
- Nimura Y, Nagino M, Takao S, et al. Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas: long-term results of a Japanese multicenter randomized controlled trial. J Hepatobiliary Pancreat Sci 2012;19:230-41. [Crossref] [PubMed]
- Ignjatovic I, Knezevic S, Knezevic D, et al. Standard versus extended lymphadenectomy in radical surgical treatment for pancreatic head carcinoma. J BUON 2017;22:232-8. [PubMed]
- Rosado ID, Bhalla S, Sanchez LA, et al. Pattern of Venous Collateral Development after Splenic Vein Occlusion in an Extended Whipple Procedure (Whipple at the Splenic Artery) and Long-Term Results. J Gastrointest Surg 2017;21:516-26. [Crossref] [PubMed]
- Hamidian Jahromi A, Jafarimehr E, Dabbous HM, et al. Curative resection of pancreatic adenocarcinoma with major venous resection/repair is safe procedure but will not improve survival. JOP 2014;15:433-41. [PubMed]
- Kantor O, Talamonti MS, Wang CH, et al. The extent of vascular resection is associated with perioperative outcome in patients undergoing pancreaticoduodenectomy. HPB (Oxford) 2018;20:140-6. [Crossref] [PubMed]
- Shyr BU, Chen SC, Shyr YM, et al. Surgical, survival, and oncological outcomes after vascular resection in robotic and open pancreaticoduodenectomy. Surg Endosc 2020;34:377-83. [Crossref] [PubMed]
- Podda M, Thompson J, Kulli CT, et al. Vascular resection in pancreaticoduodenectomy for periampullary cancers. A 10 year retrospective cohort study. Int J Surg 2017;39:37-44. [Crossref] [PubMed]
- Pu N, Li J, Xu Y, et al. Comparison of prognostic prediction between nomogram based on lymph node ratio and AJCC 8th staging system for patients with resected pancreatic head carcinoma: a SEER analysis. Cancer Manag Res 2018;10:227-38. [Crossref] [PubMed]
- Flis V, Potrc S, Kobilica N, et al. Pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head with venous resection. Radiol Oncol 2016;50:321-8. [Crossref] [PubMed]
- Menon VG, Puri VC, Annamalai AA, et al. Outcomes of vascular resection in pancreaticoduodenectomy: single-surgeon experience. Am Surg 2013;79:1064-7. [PubMed]
- Kantor O, Talamonti MS, Stocker SJ, et al. A Graded Evaluation of Outcomes Following Pancreaticoduodenectomy with Major Vascular Resection in Pancreatic Cancer. J Gastrointest Surg 2016;20:284-92. [Crossref] [PubMed]
- Castleberry AW, White RR, De La Fuente SG, et al. The impact of vascular resection on early postoperative outcomes after pancreaticoduodenectomy: an analysis of the American College of Surgeons National Surgical Quality Improvement Program database. Ann Surg Oncol 2012;19:4068-77. [Crossref] [PubMed]
- Merkow RP, Bilimoria KY, Tomlinson JS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg 2014;260:372-7. [Crossref] [PubMed]
- Müller SA, Hartel M, Mehrabi A, et al. Vascular resection in pancreatic cancer surgery: survival determinants. J Gastrointest Surg 2009;13:784-92. [Crossref] [PubMed]
- Asano T, Yamada S, Fujii T, et al. The Charlson age comorbidity index predicts prognosis in patients with resected pancreatic cancer. Int J Surg 2017;39:169-75. [Crossref] [PubMed]
- Pu N, Lv Y, Zhao G, et al. Survival prediction in pancreatic cancer patients with no distant metastasis: a large-scale population-based estimate. Future Oncol 2018;14:165-75. [Crossref] [PubMed]
- Pu N, Gao S, Yin H, et al. Cell-intrinsic PD-1 promotes proliferation in pancreatic cancer by targeting CYR61/CTGF via the hippo pathway. Cancer Lett 2019;460:42-53. [Crossref] [PubMed]
- Pu N, Yin H, Zhao G, et al. Independent effect of postoperative neutrophil-to-lymphocyte ratio on the survival of pancreatic ductal adenocarcinoma with open distal pancreatosplenectomy and its nomogram-based prediction. J Cancer 2019;10:5935-43. [Crossref] [PubMed]
- Reyngold M, Winter KA, Regine WF, et al. Marital Status and Overall Survival in Patients with Resectable Pancreatic Cancer: Results of an Ancillary Analysis of NRG Oncology/RTOG 9704. Oncologist 2020;25:e477-83. [Crossref] [PubMed]
- Wang XD, Qian JJ, Bai DS, et al. Marital status independently predicts pancreatic cancer survival in patients treated with surgical resection: an analysis of the SEER database. Oncotarget 2016;7:24880-7. [PubMed]