
Oncolytic virus-mediated tumor radiosensitization in mice through DNA-PKcs-specific shRNA
Introduction
Radiation therapy is commonly used in the primary or adjuvant setting for treatment of various solid tumors. In some circumstances, it is also used as the primary treatment for surgically inoperable patients with localized diseases (1). One of the main advantages of radiation therapy is that delivery of radiation to the tumor can be achieved in a targeted manner, especially with new technologies such as Intensity Modulated Radiation Therapy (IMRT), which makes delivery of radiation therapy even more accurate. However, even with increased precision radiotherapy has its limits. The total radiation dose that can be delivered to cancer is limited by damage to normal tissues that are irradiated during radiotherapy (2). Therefore, new agents that can selectively increase the radiation sensitivity of tumor cells are highly desired because they may enhance the efficacy of radiotherapy significantly without increasing damage to adjacent normal tissues.
There have been many previous efforts in developing radiosensitizing agents. Examples include those that are targeted to tumor hypoxia (3) and those that are aimed at disrupting DNA structure such as DNA nucleotide homologs iododeoxyuridine (IrdU) and bromodeoxyuridine (BrdU) (4). Limited success has been associated with those types of radiosensitizers.
The deciphering of molecular mechanisms for DNA double strand break (DSB) repair in recent years offered exciting opportunities to develop novel radiosensitizing agents that are mechanism-based. One of the most important molecular factors for DSB repair DNA-dependent protein kinase (DNA-PK). It is a holoenzyme with three subunits: the two regulatory subunits Ku70, Ku80 and the catalytic subunit DNA-PKcs. Ku70 and Ku80 are regulatory subunits that are responsible for binding DNA double strand breaks and recruiting of DNA-PKcs, which is the catalytic subunit that phosphorylates a variety of downstream factors that collectively are responsible for coordinating cellular response to induction of DSBs. Previous studies have indicated that deficiencies in any one of the three subunits of the DNA-PK holoenzyme will make the host cells extremely sensitive to the cell-killing effects of ionizing radiation (5-7). These findings indicate that DNA-PK subunits may provide promising targets to enhance radiation therapy. Similarly, other DSB repair and DNA damage response genes, such as ATM (8), XRCC1 (9), NBS1 (10), DNA Ligase IV (11), are also good molecular targets for radiosensitization. Indeed, there have been recent studies indicating that inhibition of DNA-PK, ATM/ATR with small molecule inhibitors can enhance the therapeutic efficacy of radiation therapy (12-15). A potential drawback of the chemically based approach, however, is that such strategies will sensitize both normal and tumor tissues to radiation therapy. Therefore, novel approaches that can deliver these sensitizers selectively to tumor tissues are highly desirable for small molecule-based radiosensitizers targeted to DNA repair proteins.
Another promising approach to achieve tumor specific targeting of DNA repair proteins for the purpose of sensitizing tumor cells to radiotherapy is tumor-specific delivery of shRNA (small hairpin RNA) that are targeted at the DNA repair proteins. The main advantage of such a strategy is that DSB repair proteins could be inhibited with high specificity and thus achieving a wider therapeutic ratio between normal and tumor tissues. A key to the success of such an strategy is the availability of gene delivery vectors that can reach tumor cells specifically. One strategy to achieve this goal is to use a vector that will selectively replicate in tumor cells. For example, an adenovirus vector that can selectively replicate in a telomerase-dependent manner would be highly specific to tumor cells because the vast majority of normal tissues do not express telomerase (16).
In the present study, we evaluated the efficacy of tumor-specific delivery of an DNA-PKcs targeted shRNA to sensitize human xenograft tumors to radiation therapy. A conditionally replicative adenovirus that selectively target telomerase-positive tumor cells (17) was combined with a replication-deficient adenovirus vector to efficiently deliver a shRNA-encoding minigene that targeted the DNA-PKcs subunit of the DNA-PK holoenzyme. Our strategy was based previous publications indicating that targeting of DNA-PK genetically through the use of siRNA or shRNA can effectively sensitize tumor cells to ionizing radiation (18,19). It is also based on our prelimary data that combining a replicative and a non-replicative virus vector could enable the non-replicative virus to replicate in a manner that is dependent on the replicative virus. Our results indicated a significant enhancement of radiation therapy efficacy by this approach.
Methods
Cell culture
We have used the following cell lines in this study: (I) HEK 293, an adenovirus E1 gene transduced human embryonic kidney cell line for adenovirus packaging and expansion; (II) HCT116, a human colon cancer cell line obtained from the Tissue Culture Facility at Duke University Medical Center. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen Inc.) with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 µg/mL streptomycin at 37 °C, 5% CO2.
Designing shRNA-encoding minigenes targeted at DNA-PKcs
To design the shRNA-encoding minigenes, we utilized an Internet-based program available at the website of Ambion Inc (Austin, TX). Oligonucleotide DNA sequences based on these targeting sequences were then synthesized by commercial sources. These oligos contain two 19-mer complimentary targeting sequences with a loop sequence separating them and a polythymidine tract to terminate transcription. In addition, they were engineered to possess Bam HI- and Hind III-compatible overhangs that facilitate their ligation into the expression vector pSilencer2.0 (Ambion Inc., Austin, TX), which is a plasmid with a human U6 gene based RNA polymerease III promoter. The derived DNA-PKcs-targeted minigene-encoding plasmid was pSilencer-shDNA-PKcs. The targeting sequence is: GAACACTTGTACCAGTGTT, which correlates the nucleotide 381-399 of DNA-PKcs gene (accession#U47077). The control plasmid was pSilencer-shNT (for non-targeted, obtained from Ambion), which is a plasmid with a similar structure but encoding a nonsense minigene with no homology to any known sequences in the human genome. The sequence of the scrambled minigene is: AATTCTCCGAACGTGTCACGT.
Adenovirus production
The AdEasy system of adenovirus packaging, including plasmid pAdtrack, pAdeasy-1 and the packaging E. coli BJ5183 cells was commercially purchased from Stratagen Corporation. The shRNA-encoding gene expression cassette (with the U6 gene promoter) was then excised by Pvu II/ Hind III from pSilencer-shDNA-PKcs and subcloned into the EcoRV/HindIII sites of pAdTrack. The resulting plasmid was pAdTrack-shDNA-PKcs. Packaging and production of the adenovirus that carries the DNA-PKcs targeted shRNA gene was carried out by following manufacturer’s protocol. Briefly, the pAdtrack-U6-shDNA-PKcs plasmid was linearized by Pme I and then recombined with pAdeasy-1 plasmid in recA+ bacteria BJ5183. The resultant pAdeasy-shDNA-PKcs was then transfected into relatively low passage (passage#<30) 293 cells after linearization by Pac I. After 7-10 days, we obtained infectious adenovirus vector, AdshDNA-PKcs. Large-scale preparation of the virus particles was carried out subsequently following established protocols.
Quantitative PCR assessment of DNA-PKcs messenger RNA level
To measure the level DNA-PKcs mRNA in cells that have been infected with the shRNA-encoding adenovirus vectors, quantitative PCR (Q-PCR) technology was used. Forty hours after infection by adenovirus vectors (AdshNT or AdshDNA-PKcs), total RNA from the infected cells were extracted by use of the RNAeasy kit (Qiagen, Valencia, CA). Afterwards, cDNA from the mRNA were synthesized by use of the SuperScript first-strand synthesis system for RT-PCR (Invitrogen, Carlbad, CA). The cDNA was then used as templates in Q-PCR reactions. The Q-PCR reactions were carried out by use of the QuantiTech SYBR Green PCR kit (Qiagen, Valencia, CA) in the Chromo 4 Four-Color-Real-Time System (MJ research, Waltham, MA). Relative quantification of DNA-PKcs was done by comparative CT method. The relative amount of target (DNA-PKcs), normalized to an endogenous sequence, is given by 2–△△CT.
The primers used for the amplification of the DNA-PKcs gene were:
- (forward) 5'- GTGGCTTTTAGCTCATTGTGG-3'
- (reverse): 5'- CCACAAATTAGGGGATCTGTTG-3'
The sequence for the β-actin primers were:
- (forward) 5'-TCAAGATCATTGCTCCTCCTG-3'
- (reverse) 5'-CTGCTTGCTGATCCACATCTG-3'.
Irradiation
Irradiation of cells were carried out using an experimental, 320 KeV x-ray irradiator (PXI, XRAD320, North Branford, CT). The dose rate used was 1 Gy/minute. The dose levels were chosen to be clinically relevant and/or to achieve significant cell killing.
Tumor growth delay studies
About 5×106 of HCT116 cells (in 50 µL PBS) were inoculated subcutaneously into nude mice. After tumor mass reach 5-7 mm in diameter, recombinant adenovirus viruses were injected into the tumor mass. Twenty-four hours later, the tumors were irradiated with an experimental X-irradiator (XRAD320). The irradiated dose was chosen to achieve significant tumor growth delay yet at sub-eradication level to allow the radiosensitizing effect of the shRNA to be evaluated. Each treatment group consisted of 8-10 animals. Growth curves are plotted as the mean relative treatment group tumor volume ± standard error (SE). The following formula was used to calculate tumor volume (20): V=(1/2) W2 × L (W, the shortest dimension; L, the longest dimension).
Immunohistochemistry staining for DNA-PKcs
Immunohistochemistry was performed using 8-10 µm serial sections of frozen tissue placed onto positively charged glass slides using a single-staining procedure. Sections were fixed in 4 °C cold acetone for 10 min. After endogenous peroxidase activity was quenched with 3% hydrogen peroxide for 15 min, tumor sections were blocked with 10% donkey serum for 15 minutes. Sections were incubated in DNA-PK-specific monoclonal antibodies (2 µg/mL) (Roche Diagnostics, Indianapolis, IN), overnight at 4 °C. After rinsing 3×5 minutes with Tris-buffered saline (TBS), biotinylated donkey anti-mouse antibody (Jackson Immunoresearch, PA, USA) was applied for 30 min at room temperature. The slides were washed with TBS for 3×5 minutes, followed by application of an avidin-biotin complex (Vectastain ABC kit, Vector Lab, Inc., Burlingame, USA). Location of the reaction was visualized with 3, 3'-diaminobenzidine tetrahydrochloride (DAB chromogen, Vector Lab, Inc., Burlingame, USA). Slides were counterstained with hematoxylin. Omission of the primary antibody served as negative control. Staining was evaluated at lower magnification (×400) by acquiring digital images of 5 randomly chosen fields and averaged.
Ethics Statement
All animals were handled in strict accordance with good animal practice as defined by the relevant national and/or local animal welfare bodies, and all animal work was approved Duke University Animal Care and Use Committee.
Results and discussion
Effective down-regulation of the DNA-PKcs protein by use of adenovirus-mediated shRNA delivery
To effectively down-regulate the levels of the DNA-PKcs gene, an shRNA minigene was designed. It contains the 21-bp sense targeting sequence domain, the 9bp loop domain, and a 21-bp anti-sense targeting sequence domain (Figure 1A). The 21-bp targeting domain is identical to nucleotide 381-399 of DNA-PKcs gene (accession#U47077). The minigene was placed under the control of the pol III promoter U6 and the expression cassette was cloned into the adenovirus vector pAdTrack. It was then packaged into adenovirus vectors in 293 cells by use of the AdEasy system.
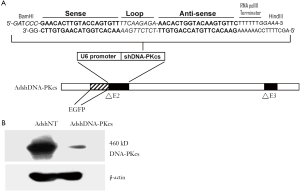
After amplification and maxiprep, the purified replication-deficient virus AdshDNA-PKcs was used to infect HCT116 cells at an MOI(multiplicity of infection) 10. About forty-eight hours after infection of the cells, they were harvested and analyzed. Quantitative PCR analysis of the mRNA indicated that the mRNA levels for the DNA-PKcs gene was only 18% of the control, indicating effective degradation of mRNA.
Western blot analysis showed significant (over 90%) suppression of the DNA-PKcs protein level (Figure 1B). Therefore effective attenuation of the DNA-PKcs gene was achieved both at the messenger RNA and protein levels.
AdshDNA-PKcs-mediated in vitro sensitization of HCT116 cells to the cell killing effects of ionizing radiation
To evaluate whether the down-regulation of the DNA-PKcs gene is sufficient to sensitize tumor cells to the cell-killing effects of ionizing radiation, HCT116 cells were infected with AdshDNA-PKcs and the viability of the cells before and after radiation was evaluated with the MTT assay according to published protocols (21). It is clear that significant (P<0.05, t-test, n=3) radiosensitization was achieved by infection of AdshDNA-PKcs (Figure 2A). Interestingly, our data also showed that infection of the cells with shRNA targeted to DNA-PKcs alone can cause a significant growth delay of the HCT116 cells (Figure 2A). At present we do not understand the underlying molecular mechanism yet.

In addition, a standard clonogenic assay (22) was used to evaluate the effect of the DNA-PKcs suppression (Figure 2B). A small but significant sensitization (1.24 at 10% survival, P<0.05, n=3, t-test) of the cells was achieved in this assay as well (P<0.05 for 2, 4, 6, and 8 Gy data points, t-test, n=3 for each data point). The discrepancy between the clonogenic assay and the MTT assay are probably caused by the very different nature of the two assay methods. These data show that adenovirus-mediated, DNA-PKcs-targeted shRNA can significantly sensitize tumor cells to the cell-killing effects of ionizing radiation.
Lack of efficacy of AdshDNA-PKcs in enhancing radiation therapy in a xenograft model of colon cancer
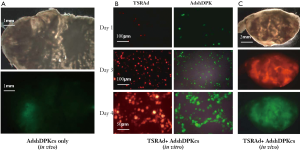
In vivo xenograft tumor models were used to evaluate the efficacy of AdshDNA-PKcs. HCT116 xenograft tumors were established in nude mice subcutaneously. When tumors reach 5-7 mm in diameter, AdshDNA-PKcs was injected into the tumors and radiotherapy was delivered. Virus injection itself has no effect on tumor growth. Combined radiation and AdshDNA-PKcs treatment had minimal effect on tumor growth (data not shown). This was surprising, given the clear radiosensitizing effect of the treatment in vitro (Figure 2). We hypothesized that this might be caused by the lack of gene transduction efficiency of the intra-tumorally transduced vectors. We therefore sectioned some of the tumors that had been injected with AdshDNA-PKcs. Virus spread in the tumors were then measured by examining green fluorescence protein (GFP) gene expression in the sections because AdshDNA-PKcs also encodes a constitutively active GFP gene (Figure 1). When measuring the areas of GFP expression against the whole tumor sections, our results indicate that only 5-10% of various tumor sections was infected with AdshDNA-PKcs (Figure 3A). Therefore, inefficient intratumoral adenovirus spread was the most likely cause for the lack of in vivo radiosensitizing effects of AdshDNA-PKcs.
A telomerase-specific, replicative adenovirus significantly enhances the ability of AdshDNA-PKcs to sensitize radiation therapy of xenograft tumors
We sought to enhance the delivery of the AdshDNA-PKcs to tumors by use of a recently developed conditionally replicative adenovirus that can selectively replicate in telomerase-positive cancer cells. We have shown previously that this virus can effectively infect and spread in the tumor mass (17). Therefore, we reasoned that we may achieve efficient delivery of the shRNA into the tumor mass with this vector.
We experimented with a novel strategy where a telomerase-specific replicative adenovirus (TSRAd) was combined with AdshDNA-PKcs to delivery shDNA-PCcs to the tumors. It was reasoned that co-infection of the two viruses would allow both vectors to selectively replicate in telomerase-positive cancer cells because the E1 proteins produced by the replicative AdTERT-E1-dsRed would support the replication of AdshDND-PKcs in trans. This was indeed the case (Figures 3B&C). In vitro, HCT116 cells infected with both AdTERT-E1-dsRed and AdshDNA-PKcs at low titers had significant virus replication activities for both TSRAd, which encodes a red fluorescent protein, and AdshDNA-PKcs, which encodes a green fluorescent protein (Figure 3B). When the two vectors were injected together in vivo, significantly more virus spread (60-100%) for both vectors was observed in various tumor sections (Figure 3C), indicating the success of the combined vector strategy.
Subsequently, a tumor growth delay study was conducted with the combined vectors approach in conjunction with radiation therapy according to the scheme shown in Figure 4A. It is clear that a significant in vivo radio-sensitizing effect was observed when radiation was combined with co-delivery of telomerase-specific replicative adenovirus (TSRAd) and AdshDNA-PKcs (Figure 4B) (P<0.05 from day 9 for comparison between the treatment vs. all other groups). The treatment group showed the greatest degree of tumor suppression among the four experimental groups. The tumor growth delay was also very obvious when Kaplan-Meier survival curve was plotted for each of the experimental groups (Figure 4C) using relative tumor volume >5 × initial volume as the surrogate endpoint). These results also show that the increased therapeutic efficacy observed was primarily due to the significant down-regulation of DNA-PKcs mediated by the efficient replication and intra-tumoral spread of AdshDNA-PKcs.
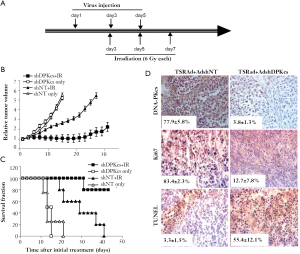
Immunohistochemical characterization was carried out to characterize DNA-PKcs expression and therapeutic efficacy (Figure 4D). DNA-PKcs expression was significantly attenuated compared to those in the control (P<0.05, n=5, t-test). In addition, TUNEL assay showed significantly increased apoptotic rate (P<0.05, n=5, t-test), and decreased Ki67 expression (P<0.05, n=5, t-test), which indicated reduced tumor cell proliferation, in the combination treatment group, consistent with the observed anti-tumor efficacy.
One of key advantages of cancer gene therapy is the ability to target specific genes in tumors at will, especially with the advent of the RNA interference technology. While results from in vitro experiment have been generally excellent, in vivo delivery of RNAi has been less than ideal due the inefficiency of vectors systems. The strategy adopted in this study combined the advantages of two powerful approaches, the gene-targeting specificity/efficacy of shRNA and the delivery efficiency of conditionally replicative adenovirus specific to telomerase, to achieve a highly effective delivery of shRNA into the tumor mass. The combined strategy makes it possible to deliver multiple therapeutic genes located within several replication-deficient vectors, significantly expanding the capacity of adenovirus-mediated therapeutic gene delivery. The excellent results achieved here indicate potential applicability of this approach to solid tumors in general. Of course, more studies in different models are needed to verify the general validity of this approach. With increasing number of tumor-specific gene targets being identified, the approach highlighted in this study clearly has the potential to be expanded to include more target shRNAs.
Acknowledgments
Funding: This study was supported in part by grants CA131408, CA136748, CA155270 from the US National Cancer Institute (to C-Y Li), and grant NNX12AB88G (to C-Y Li) from NASA Space Radiation Biology Research Program; and grants 30428015, 30325043 from the Nation Science Foundation of China, grant 2004CB518804 from Ministry of Science of China “973 project” to Q Huang. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2012.05.02). CYL serves as an unpaid editorial board member of Translational Cancer Research. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki. Institutional ethical approval and informed consent were waived. All animals were handled in strict accordance with good animal practice as defined by the relevant national and/or local animal welfare bodies, and all animal work was approved Duke University Animal Care and Use Committee.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rodriguez-Bigas MA, Lin EH, Crane CH. Adenocarcinoma of the colon and rectum. In Cancer Medicince 6 (D. W. Kufe, R. E. Pollock, R. R. Weichselbaum, R. C. Bast, T. S. Gansler, J. F. Holland and E. Frei, Eds.), pp. 1635-65. BC Decker Inc, Hamilton. London, 2003.
- Mundt AJ, Roeske JC, Chung TD, et al. In Cancer Medicine 6 (D. W. Kuff, R. E. Pollock, R. R. Weichselbaum, R. C. Bast, T. S. Gansler, J. F. Holland and E. Frei, Eds.), pp. 585-604. BC Decker Inc, Hamilton. London, 2003.
- Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer 2002;2:38-47. [PubMed]
- Coleman CN. Modification of radiotherapy by radiosensitizers and cancer chemotherapy agents. I. Radiosensitizers. Semin Oncol 1989;16:169-75. [PubMed]
- Gu Y, Jin S, Gao Y, Weaver DT, et al. Ku70-deficient embryonic stem cells have increased ionizing radiosensitivity, defective DNA end-binding activity, and inability to support V(D)J recombination. Proc Natl Acad Sci USA 1997;94:8076-81. [PubMed]
- Taccioli GE, Amatucci AG, Beamish HJ, et al. Targeted disruption of the catalytic subunit of the DNA-PK gene in mice confers severe combined immunodeficiency and radiosensitivity. Immunity 1998;9:355-66. [PubMed]
- Kurimasa A, Ouyang H, Dong LJ, et al. Catalytic subunit of DNA-dependent protein kinase: impact on lymphocyte development and tumorigenesis. Proc Natl Acad Sci USA 1999;96:1403-8. [PubMed]
- Lavin MF, Khanna KK. ATM: the protein encoded by the gene mutated in the radiosensitive syndrome ataxia-telangiectasia. Int J Radiat Biol 1999;75:1201-14. [PubMed]
- Thompson LH, Brookman KW, Jones NJ, et al. Molecular cloning of the human XRCC1 gene, which corrects defective DNA strand break repair and sister chromatid exchange. Mol Cell Biol 1990;10:6160-71. [PubMed]
- Paull TT, Gellert M. Nbs1 potentiates ATP-driven DNA unwinding and endonuclease cleavage by the Mre11/Rad50 complex. Genes Dev 1999;13:1276-88. [PubMed]
- Frank KM, Sekiguchi JM, Seidl KJ, et al. Late embryonic lethality and impaired V(D)J recombination in mice lacking DNA ligase IV. Nature 1998;396:173-7. [PubMed]
- Veuger SJ, Curtin NJ, Richardson CJ, et al. Radiosensitization and DNA repair inhibition by the combined use of novel inhibitors of DNA-dependent protein kinase and poly(ADP-ribose) polymerase-1. Cancer Res 2003;63:6008-15. [PubMed]
- Shinohara ET, Geng L, Tan J, et al. DNA-dependent protein kinase is a molecular target for the development of noncytotoxic radiation-sensitizing drugs. Cancer Res 2005;65:4987-92. [PubMed]
- Hardcastle IR, Cockcroft X, Curtin NJ, et al. Discovery of potent chromen-4-one inhibitors of the DNA-dependent protein kinase (DNA-PK) using a small-molecule library approach. J Med Chem 2005;48:7829-46. [PubMed]
- Svirnovski AI, Serhiyenka TF, Kustanovich AM, et al. ATM and MDR proteins inhibitors in overcoming fludarabine resistance in CLL cells. Exp Oncol 2010;32:258-62. [PubMed]
- Mo Y, Gan Y, Song S, et al. Simultaneous targeting of telomeres and telomerase as a cancer therapeutic approach. Cancer Res 2003;63:579-85. [PubMed]
- Huang Q, Zhang X, Wang H, et al. A novel conditionally replicative adenovirus vector targeting telomerase-positive tumor cells. Clin Cancer Res 2004;10:1439-45. [PubMed]
- Peng Y, Zhang Q, Nagasawa H, et al. Silencing expression of the catalytic subunit of DNA-dependent protein kinase by small interfering RNA sensitizes human cells for radiation- induced chromosome damage, cell killing, and mutation. Cancer Res 2002;62:6400-4. [PubMed]
- Collis SJ, Swartz MJ, Nelson WG, et al. Enhanced radiation and chemotherapy-mediated cell killing of human cancer cells by small inhibitory RNA silencing of DNA repair factors. Cancer Res 2003;63:1550-4. [PubMed]
- Huang Q, Hu JK, Lohr F, et al. Heat-induced gene expression as a novel targeted cancer gene therapy strategy. Cancer Res 2000;60:3435-9. [PubMed]
- Garn H, Krause H, Enzmann V, et al. An improved MTT assay using the electron-coupling agent menadione. J Immunol Methods 1994;168:253-6. [PubMed]
- Tsang NM, Nagasawa H, Li C, et al. Abrogation of p53 function by transfection of HPV16 E6 gene enhances the resistance of human diploid fibroblasts to ionizing radiation. Oncogene 1995;10:2403-8. [PubMed]


