
Correlation analysis of multi-slice computed tomography (MSCT) findings, clinicopathological factors, and prognosis of gastric gastrointestinal stromal tumors
Introduction
Gastrointestinal stromal tumors (GISTs) are rare tumors, accounting for approximately 1% of the primary gastrointestinal (GI) tract malignancies. However, they are the most frequently diagnosed mesenchymal neoplasms involving the GI tract (1-4). GISTs originate from mesenchymal pluripotent stem cells, which are programmed to differentiate into interstitial Cajal cells (5). To distinguish these tumors, the term “stromal tumors” was first introduced by Mazur et al. (6) in 1983. They are located in the upper GI tract, mainly in the stomach (7). However, small bowel (8), esophageal, rectal (9), and extragastrointestinal GISTs are also observed less frequently. According to the revised National Institutes of Health (NIH) and Armed Forces Institute of Pathology (AFIP) risk classification criteria, primary GISTs are categorized into different risk groups, including very low-risk (15%), low-risk (30%), intermediate-risk (22%), and high-risk (33%). These groups were established based on tumor mitotic rate and size, tumor site, and tumor rupture. Accurate risk assessment is crucial for diagnosis, treatment selection, management, and the risk of GISTs recurrence after curative surgery (10,11).
Presently, the criteria for risk stratification of GISTs are based essentially on a combination of clinical and morphological features including tumor size (12,13) and tumor rupture. However, the reliability of these factors to predict prognosis cannot be assured. Therefore, the study aimed to evaluate the correlation between multi-slice computed tomography (MSCT) signs and clinicopathological characteristics in the prognosis of gastric GISTs. Furthermore, distinctive CT and clinicopathological characteristics that can assist in the prognostic evaluation of gastric GISTs were also elucidated.
Methods
Patients
The contrast-enhanced CT manifestations of 155 GISTs based on a consecutive cohort and confirmed by surgery and pathological analyses were retrospectively analyzed at our hospital from December 2010 to October 2016. Of these 155 patients with gastric GISTs, recurrence, metastasis, or death was observed in 30 patients.
This study was approved by the Institutional Ethics Committee of the Second Affiliated Hospital of Southwest Medical University (KY2010090) and the requirement for informed patient consent was waived. The inclusion criteria were (I) no tumor metastasis at the time of diagnosis, (II) the tumor should not have ruptured, (III) complete CT enhanced images should be available, and (IV) complete clinical and pathological data should be available. The NIH consensus classification system, based on a GIST Workshop convened by the NIH in April 2001, stratifies the risk of an aggressive clinical course based on tumor size and mitotic count (Table 1) (14). Unfortunately, we did not find CT classification criteria related to gastric stromal tumors. The patients were followed up till August 2018, with an average follow-up duration of 48 months.
Table 1
| Risk category | Tumor size in largest dimension (cm) | Mitotic count (per 50 HPFs) (cm) |
|---|---|---|
| Very low | <2 | <5 |
| Low | 2–5 | <5 |
| Intermediate | <5 | 6–10 |
| 5–10 | <5 | |
| High | >5 | >5 |
| >10 | Any mitotic rate | |
| Any size | >10 |
CT acquisition
All patients were examined by a plain CT scan and a contrast-enhanced CT scan. MSCT was performed using a 64-spiral CT scanner and a Somatom Definition dual-source scanner (Siemens Sensation 128, Munich, Germany). The patients fasted for at least 8 h before the CT examination. Patients undergoing scanning were in an inhale condition, and the scan range was the entire abdomen at a tube voltage of 120 kV and a tube current of 250 mA. The slice thickness and spacing were 5.0 mm. The contrast-enhanced scans used a nonionic iodinated intravenous contrast agent (300 mL/mg), with an injection volume of 80–100 mL and an injection rate of 3.0 mL/s. Data from the enhanced scan were sent to an advantage workstation (syngo via 2008G) for analysis.
Image analysis
Two radiologists with adequate experience in GI imaging, who were blinded to the histopathological findings, independently interpreted the abdominal CT images of 155 patients. The scans were reviewed to determine the maximum size, shape, margin, growth pattern, enhancement pattern, and necrosis or cystic degeneration of the tumor. The necrotic or cystic areas were avoided and the solid portion was set as the maximum. The corresponding parameters, including the CT values of the plain scan and the contrast enhancement pattern, were measured three times and the average values were calculated.
Pathological examination
The pathological examination included formaldehyde fixation, routine sectioning, hematoxylin-eosin (HE) staining, and observation under a microscope. CD117, CD34, smooth muscle actin (SMA), and S-100 protein were labeled by Envision staining (immunohistochemical staining: Beijing Zhongshan, LLC).
Statistical analysis
The statistical analyses were performed with Statistical Package for Social Sciences (SPSS) version 21.0 software packages (SPSS Inc, Chicago, IL, USA). Survival curves were estimated using the Kaplan–Meier method and significant differences between the survival curves were assessed by the log-rank test. The independent variables were first analyzed by univariate analysis. The variables determined to be significantly associated by univariate analysis were then subjected to a Cox proportional hazards regression model for multivariate analysis to determine the independent factors affecting recurrence. Frequency tables were analyzed using the χ2 test. A two-sided P value of <0.05 was considered statistically significant.
Progression-free survival (PFS) was calculated as the length of time during and after disease treatment for which the patient lived with the disease without the disease getting worse.
Results
CT findings
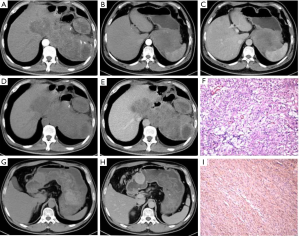
The average tumor size in patients was 7.2±3.9 cm, based on the CT results. In 69 (44.5%) patients, the tumors were round or oval and in 86 (55.5%), they were lobulated or irregular. Further, a majority of patients exhibited internal necrosis or cystic degeneration (99/155, 63.9%) and presented with well-defined and smooth (81.3%) and endophytic (85.2%) masses. However, following contrast enhancement, most of them showed heterogeneous enhancement (76.1%) (Figure 1).
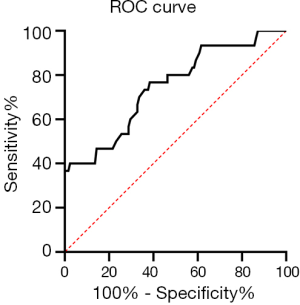
Receiver operating characteristic (ROC) curve calculation and analysis
When the tumor size diagnostic threshold was 7.85 cm, the maximum Youden index was 0.615, and the area under the ROC curve (AUC) was 0.746 (Figure 2). Based on this cutoff value, patients with tumors diameter greater than 7.85 cm were considered to have large-sized tumors, which were more likely to lead to relapse, metastasis, or death.
Results of univariate analysis
The clinicopathological and CT characteristics, including age, sex, and tumor size, shape, margin, growth pattern, mitotic rate, enhancement pattern, necrosis or cystic degeneration, and Ki-67 index were analyzed in the different risk stratification groups. The degree of correlation between clinicopathological and CT characteristics and prognosis was evaluated based on these different risk groups (Table 2). Univariate analysis revealed that tumor size, shape, margin, growth pattern, mitotic rate, enhancement pattern, necrosis or cystic degeneration, and Ki-67 index were significantly associated with CT and clinicopathological characteristics (Figure 3, P=0.005, 0.004, 0.000, 0.000, 0.016, 0.015, and 0.023, respectively). However, no significant association was found between the age and sex of the patients and gastric GISTs (P=0.313 and 0.836, respectively) (Table 2).
Table 2
| Index | Cases | Recurrence, metastasis or death, n (%) | P value | |
|---|---|---|---|---|
| Yes | No | |||
| Age (years) | 0.313 | |||
| <60 years | 81 | 13 (16.0) | 68 (84.0) | |
| ≥60 years | 74 | 17 (23.0) | 57 (77.0) | |
| Gender | 0.836 | |||
| Male | 93 | 19 (20.4) | 74 (79.6) | |
| Female | 62 | 11 (17.7) | 51 (82.3) | |
| Size (cm) | 0.005 | |||
| <5 | 49 | 3 (6.1) | 46 (93.9) | |
| 5–10 | 78 | 17 (21.8) | 61 (78.2) | |
| >10 | 28 | 10 (35.7) | 18 (64.3) | |
| Shape | 0.004 | |||
| Round/oval | 69 | 6 (8.7) | 61 (91.3) | |
| Lobulated/irregular | 86 | 24 (27.9) | 62 (72.1) | |
| Necrosis or cystic degeneration | 0.003 | |||
| Yes | 99 | 26 (26.3) | 73 (73.7) | |
| No | 56 | 4 (7.1) | 52 (92.9) | |
| Margin | <0.001 | |||
| Defined/smooth | 126 | 12 (9.5) | 114 (90.5) | |
| 0 rough/Ill-defined | 29 | 18 (62.1) | 11 (37.9) | |
| Growth pattern | <0.001 | |||
| Endophytic | 132 | 17 (12.9) | 115 (87.1) | |
| Exophytic | 23 | 13 (56.5) | 10 (43.5) | |
| Enhancement pattern | 0.016 | |||
| Homogeneous | 37 | 2 (5.4) | 35 (94.6) | |
| Heterogeneous | 118 | 28 (23.7) | 90 (76.3) | |
| Mitotic rate | 0.015 | |||
| <5 | 83 | 10 (12.0) | 73 (88.0) | |
| ≥5 | 72 | 20 (27.8) | 52 (72.2) | |
| Ki-67 index | 0.023 | |||
| <10 | 123 | 19 (15.4) | 104 (84.6) | |
| ≥10 | 32 | 11 (34.4) | 21 (65.6) | |

Multivariate Cox regression analysis
Combined with the clinicopathological and CT characteristics of gastric GISTs, the correlation between the indexes and gastric GIST prognosis was analyzed with Kaplan-Meier single factor analysis. The significant factors were further analyzed by Cox regression analysis to determine the independent factors affecting prognosis. The results suggested that tumor size [P=0.005, Exp(B) =1.307], margin [P=0.008, Exp(B) =5.351], and Ki-67 index [P=0.046, Exp(B) =0.330] were significant independent predictive factors affecting the prognosis of gastric GISTs (Table 3).
Table 3
| Index | Exp(B) | 95% CI | P value |
|---|---|---|---|
| Size | 1.307 | 1.084–1.577 | 0.005 |
| Shape | 0.844 | 0.271–2.629 | 0.770 |
| Necrosis or cystic degeneration | 0.490 | 0.118–2.042 | 0.328 |
| Margin | 5.351 | 1.556–18.403 | 0.008 |
| Growth pattern | 1.561 | 0.619–3.939 | 0.346 |
| Enhancement pattern | 0.836 | 0.187–3.737 | 0.815 |
| Mitotic rate | 0.492 | 0.168–1.443 | 0.196 |
| Ki-67 | 0.330 | 0.111–0.980 | 0.046 |
Discussion
Accurate diagnosis and assessment of the biological behavior of GISTs are highly desirable for the appropriate management of GISTs, which remains clinically challenging. The identification of diagnostic and prognostic factors to differentiate between benign and malignant tumors is extremely important in the prognosis and treatment selection of this tumor. Furthermore, the classic NIH risk stratification criteria to determine the biological behavior of tumors have been questioned by several researchers (15) and consequently, revised systems of classification have been proposed. Because of the histological similarity between gastric GISTs and other spindle cell tumors, it is not appropriate to rely entirely on pathological diagnosis to make a definite diagnosis.
Univariate analysis revealed that the prognosis of GISTs was independent of age and sex in the present study. Further, the shape, growth pattern, enhancement pattern, necrosis or cystic degeneration, and mitotic rate of GISTs had a significant influence on prognosis. However, these factors, which were significant prognostic factors in univariate analysis, were not independent variables influencing prognosis by multivariate Cox regression analysis. The count was significantly affected by multiple factors, but with poor repeatability. Consequently, they cannot conclusively determine the prognosis of GISTs. Furthermore, by univariate analysis and multivariate Cox regression analysis, tumor size, tumor margin, and Ki-67 index were determined to be significant independent predictors affecting the prognosis of gastric GISTs.
Recently, a study (16) suggested that the differences in growth pattern, tumor size, shape, enhancement pattern, and tumor margin of gastric GISTs were significantly associated with different risk grades of gastric GISTs. In this study, the univariate analysis also showed that tumor size, growth pattern, shape, margin, and enhancement pattern were significant predictors of gastric GISTs. Consistent with these results, a study by O’Neill et al. (17) of more than 140 patients with GISTs reported that the independent predictors of metastasis were irregular or lobulated outline, size >10 cm, and necrosis or cystic degeneration. However, the findings in the present study revealed that tumor shape and necrosis or cystic degeneration were not independent predictors of recurrence and metastasis. However, enhancement pattern plays an important role in differentiating between other tumors, such as gastric schwannoma (18) and GISTs. Furthermore, the study also demonstrated that low invasive risk gastric GISTs were the predominant growth type with small tumor size, regular shape, defined margin, and homogeneous enhancement. In terms of the present study, a large tumor size, exophytic growth, lobulated/irregular outline, ill-defined/rough margin, necrosis or cystic degeneration, or heterogeneous enhancement of gastric GISTs were factors associated with recurrence and metastasis.
Because of the high speed, high spatial resolution, and density, MSCT scanning can accurately demonstrate (4,16,19,20) the tumor size, shape, margin, growth pattern, necrosis or cystic degeneration, and enhancement pattern. Moreover, it can reveal tumors with or without necrosis and cystic degeneration with peripheral tissue relationships, enhancement patterns, and degree, as well as lymph node metastasis. All these critical features make MSCT the most extensively used and one of the best detection methods for the prognosis of GISTs (21,22).
The Ki-67 index, also designated mki67, is a nuclear marker that labels the nuclei and chromosomes of cells explicitly actively undergoing proliferation. The Ki-67 index is considered to be the most reliable immunohistochemical marker (23) for evaluating tumor cell proliferation. Noticeably, the mitotic index accurately reflects the number of cells that complete the cell cycle. However, as Ki-67 can recognize most cell proliferation stages except for G0, it is considered to be highly appropriate for detecting cell proliferation, a significant feature of the malignancy in GISTs. Thus far, many studies have reported an association of Ki-67 expression and GISTs with the risk of developing a malignancy (16,24,25). The findings of the present study also indicated that the rates of recurrence and metastasis were significantly higher in the group with Ki-67 >5% than in the group with Ki-67 ≤5% (P<0.05). Besides, univariate analysis and multivariate Cox regression analysis demonstrated that the Ki-67 index was a significant independent risk factor affecting the prognosis of patients with gastric GISTs.
The present study has several limitations. First, the findings of the study were limited by the small number of cases. Second, we did not have complete information on the patients’ previous treatments and did not have enough information on whether the patients experienced metastasis, recurrence, or death because of the short follow-up period. In addition, the NIH risk classification criteria and a previous study (26) reported that tumor rupture was an independent prognostic factor for GISTs. However, in our group of cases, we did not include it.
In conclusion, tumor shape, growth pattern, mitotic rate, enhancement pattern, and necrosis or cystic degeneration were non-negligible criteria for improving the accuracy of prognosis for patients with gastric GISTs, whereas tumor size, margin, and Ki-67 index were significant independent predictors for identifying high-risk patients, and this might facilitate personalized treatment to improve the prognosis of patients with gastric GISTs. Thus, the combination of MSCT findings and clinicopathological features may be a valuable tool for the prognostic assessment of patients with gastric GISTs.
Acknowledgments
We would like to acknowledge Professors Yi Yuan and Bo Chen for their technical assistance.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.26). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Ethics Committee of the Second Affiliated Hospital of Southwest Medical University (KY2010090) and the requirement for informed patient consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bachmann R, Strohäker J, Kraume J, et al. Surgical treatment of gastrointestinal stromal tumours combined with imatinib treatment: a retrospective cohort analysis. Transl Gastroenterol Hepatol 2018;3:108. [Crossref] [PubMed]
- Nishida T, Sakai Y, Takagi M, et al. Adherence to the guidelines and the pathological diagnosis of high risk gastrointestinal stromal tumors in the real world. Gastric Cancer 2020;23:118-25. [Crossref] [PubMed]
- Faiyaz-Ul-Haque M, Al-Dayel F, Tulba A, et al. Spectrum of the KIT Gene Mutations in Gastrointestinal Stromal Tumors in Arab Patients. Asian Pac J Cancer Prev 2018;19:2905-10. [PubMed]
- Huh CW, Jung DH, Kim JS, et al. CT Versus Endoscopic Ultrasound for Differentiating Small (2-5 cm) Gastrointestinal Stromal Tumors From Leiomyomas. AJR Am J Roentgenol 2019;213:586-91. [Crossref] [PubMed]
- Xu L, Zhang M, Xu M. Primary hepatic gastrointestinal stromal tumor with right adrenal gland invasion A case report and systematic literature review. Medicine (Baltimore) 2019;98:e15482. [Crossref] [PubMed]
- Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol 1983;7:507-19. [Crossref] [PubMed]
- Lillemoe HA, Brudvik KW, Vauthey JN. Treatment Options for Metastatic Gastrointestinal Stromal Tumors to the Liver: A Review. Semin Liver Dis 2019;39:395-402. [Crossref] [PubMed]
- Boonstra PA, Steeghs N, Farag S, et al. Surgical and medical management of small bowel gastrointestinal stromal tumors: A report of the Dutch GIST registry. Eur J Surg Oncol 2019;45:410-5. [Crossref] [PubMed]
- Nepal P, Mori S, Kita Y, et al. Management of a case of high-risk gastrointestinal stromal tumor in rectum by transanal minimal invasive surgery. World J Surg Oncol 2018;16:165. [Crossref] [PubMed]
- Khoo CY, Goh BKP, Eng AKH, et al. Laparoscopic wedge resection for suspected large (≥5 cm) gastric gastrointestinal stromal tumors. Surg Endosc 2017;31:2271-9. [Crossref] [PubMed]
- Cui JX, Gao YH, Xi HQ, et al. Comparison between laparoscopic and open surgery for large gastrointestinal stromal tumors: A meta-analysis. World J Gastrointest Oncol 2018;10:48-55. [Crossref] [PubMed]
- Mazzei MA, Cioffi N, Vindigni C, et al. Gastrointestinal stromal tumors (GIST): a proposal of a “CT-based predictive model of Miettinen index” in predicting the risk of malignancy. Abdom Radiol (NY) 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Hølmebakk T, Bjerkehagen B, Lobmaier IVK. Is Peritoneal Tumor Penetration of Prognostic Importance in Gastrointestinal Stromal Tumors? Ann Surg Oncol 2019;26:4730-6. [Crossref] [PubMed]
- Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39:1411-9. [Crossref] [PubMed]
- Chen T, Xu L, Ye L, et al. A new nomogram for recurrence-free survival prediction of gastrointestinal stromal tumors: Comparison with current risk classification methods. Eur J Surg Oncol 2019;45:1109-14. [PubMed]
- Li H, Ren G, Cai R, et al. A correlation research of Ki67 index, CT features, and risk stratification in gastrointestinal stromal tumor. Cancer Med 2018;7:4467-74. [Crossref] [PubMed]
- O’Neill AC, Shinagare AB, Kurra V, et al. Assessment of metastatic risk of gastric GIST based on treatment-naive CT features. Eur J Surg Oncol 2016;42:1222-8. [Crossref] [PubMed]
- Chen Z, Yang J, Sun J, et al. Gastric gastrointestinal stromal tumours (2-5 cm): Correlation of CT features with malignancy and differential diagnosis. Eur J radiol 2020;123:108783. [Crossref] [PubMed]
- Theiss L, Contreras CM. Gastrointestinal Stromal Tumors of the Stomach and Esophagus. Surg Clin North Am 2019;99:543-553. [Crossref] [PubMed]
- Ma X, Ling W, Xia F, et al. Application of Contrast-Enhanced Ultrasound (CEUS) in Lymphomatous Lymph Nodes: A Comparison between PET/CT and Contrast-Enhanced CT. Contrast Media Mol Imaging 2019;2019:5709698.
- Xu F, Ma X, Wang Y, et al. CT texture analysis can be a potential tool to differentiate gastrointestinal stromal tumors without KIT exon 11 mutation. Eur J Radiol 2018;107:90-97. [Crossref] [PubMed]
- Vaz S, Oliveira C, Castanheira JC, et al. Gastric GIST Incidentally Detected on 68Ga-PSMA-PET/CT: Correlation Between Functional Imaging and Histology. Clin Nucl Med 2018;43:e488-91. [Crossref] [PubMed]
- Büscheck F, Sulimankhil M, Melling N, et al. Loss of cytoplasmic survivin expression is an independent predictor of poor prognosis in radically operated prostate cancer patients. Cancer Med 2020;9:1409-18. [Crossref] [PubMed]
- Lewitowicz P, Matykiewicz J, Chrapek M, et al. Tumor Digital Masking Allows Precise Patient Triaging: A Study Based on Ki-67 Scoring in Gastrointestinal Stromal Tumors. Scanning 2018;2018:7807416.
- Li P, Li M, Wang K, et al. Genetic alterations in cell cycle regulation-associated genes may promote primary progress of gastrointestinal stromal tumors. Lab Invest 2019; [Epub ahead of print].
- Joensuu H, Vehtari A, Riihimaki J, et al. Risk of recurrence of gastrointestinal stromal tumour aher surgery: an analysis of pooled population-based cohorts. Lancet Oncol 2012;13:265-74. [Crossref] [PubMed]


