
Anti-HER2 therapies: when more is more
Human epidermal growth factor receptor 2 (HER2) is a member of the epidermal growth factor receptor (EGFR) family of receptor tyrosine kinases (RTKs), which includes EGFR (HER1), c-erbB2 (HER2), c-erbB3 (HER3), and c-erbB4 (HER4) (1). These four receptors share an extracellular domain in N-terminal position corresponding to the ligand binding site, a transmembrane domain, and an intracellular domain in C-terminal position having tyrosine kinase (TK) activity, except for HER3, whose kinase domain is inactive. Apart for HER2, for which no ligand has been identified, for other members of the HER family, ligands bind to the receptor leading to homo- or hetero-dimerisation, and self-activation of tyrosine residues in the C terminal domain, and transduction of signal via the Ras/Raf/MEK/ERK and the PI3K/AKT/mTOR pathways (2,3) involved in cell survival, migration, apoptosis and proliferation.
Approximately 10-15% of early breast cancers overexpress HER2 and/or harbour HER2 gene amplification (2,3). HER2 protein overexpression has been shown to be underpinned by HER2 gene amplification in >90% of cases (4). Importantly, HER2 overexpression and gene amplification have been shown to constitute drivers of breast cancer in in vitro and in vivo models (5,6), and are independent prognostic factors (2). A number of therapeutic approaches have been developed against HER2, including Trastuzumab (Herceptin™, Roche-Genentech, CA, USA), Pertuzumab (Omnitarg™, Roche-Genentech, CA, USA) and Lapatinib (Tykerb™/Tyverb™, GlaxoSmithKline, UK), which have either been incorporated into clinical practice or are being tested in the context of clinical trials (Tables 1,2).
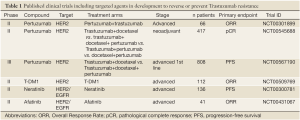
Full table
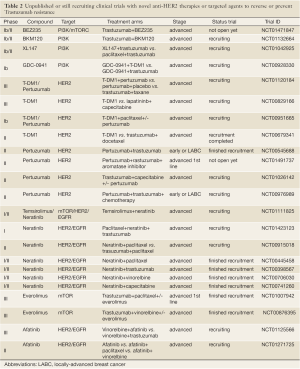
Full table
Trastuzumab is a humanised monoclonal antibody (mAb) that binds to the extracellular, juxtamembrane portion of the HER2 receptor and suppresses HER2 signalling activity, resulting in inhibition of downstream signalling pathways, cell cycle arrest and a reduction in angiogenesis. As a result of antibody binding to the HER2 extracellular domain, Trastuzumab also leads to antibody-dependent cell-mediated cytotoxicity (ADCC), and prevents HER2 receptor extracellular domain cleavage, leading to tumour cell stasis and/or death (7-9). In patients with advanced HER2-amplified breast cancer, Trastuzumab has shown antitumour activity (10,11) and improves overall survival (OS) when given in combination with chemotherapy in the first-line setting (12,13). It has, however, become clear that a subset of patients with HER2-positive disease fails to benefit from Trastuzumab treatment, either due to primary (also known as de novo) resistance, or the acquisition of resistance during the course of treatment (secondary resistance) (14).
Pertuzumab is a humanised monoclonal antibody that binds to a distinct epitope of HER2 (domain II), preventing HER2 from dimerisation with other ligand-activated HER2 receptors, mostly HER3 (15). As a single agent Pertuzumab was assessed in HER2-negative breast cancer advanced patients showing only modest activity (16).
Given that Pertuzumab and Trastuzumab bind to different HER2 epitopes and have complementary mechanisms of action (15), preclinical studies have shown a synergistic effect when these two agents were administered together (17,18). In a phase 2 study in the advanced setting a benefit was observed for the Trastuzumab-Pertuzumab combination in patients who had failed Trastuzumab (19). In early breast cancer, the results of the Neoadjuvant Study of Pertuzumab and Herceptin in an Early Regimen Evaluation (NEOSPHERE; NCT00545688) have shown a markedly higher pathological complete response rate with the triple therapy of docetaxel, Trastuzumab and Pertuzumab compared to docetaxel with either one of the anti-HER2 therapies alone (20). However, the true clinical significance of pathological complete response, or lack of, in oestrogen receptor-positive HER2-positive breast cancers remains to be fully established.
The recently published Clinical Evaluation of Pertuzumab and Trastuzumab study (CLEOPATRA; NCT00567190) (21) has provided direct evidence to demonstrate that the addition of Pertuzumab to Trastuzumab plus docetaxel improved progression-free survival (PFS) by 6.1 months (18.5 vs. 12.4 months for Pertuzumab arm vs. control, respectively; HR, 0.62; 95% CI, 0.51-0.75; P<0.001). This benefit was observed in all pre-defined subgroups based on previous neoadjuvant or adjuvant treatment (chemotherapy+/-Trastuzumab), geographic region, age, race, visceral involvement, hormone receptor status and if HER2-positivity had been determined by immunohistochemistry or fluorescence in situ hybridisation. There was a non-significant trend in benefit in overall survival (P=0.05), although the analysis was performed after only 43% of the pre-specified total number of events requested for the final analysis. These results are therefore consistent with those observed in the previously described phase II studies. The fact that the benefit was observed in both Trastuzumab pre-treated and Trastuzumab-naive patients suggests that Trastuzumab-resistant HER2-positive breast cancers may still depend on HER2 signalling. The observed clinical benefit of Pertuzumab is likely to stem from the different mechanisms of action of Trastuzumab and Pertuzumab. Unlike Trastuzumab, Pertuzumab-mediated blockade of the epitope II, involved in heterodimer formation, prevents the formation of HER2-HER3 heterodimers, which are the most potent in activating the PI3K pathway (22). This mechanisms of action would add to the previously described mechanism of Trastuzumab benefit, which are not solely linked to a direct HER2 inhibition (9). Importantly, no significant increase in toxicity, especially in terms of cardiac events, was observed with the combination of the two monoclonal antibodies compared to Trastuzumab alone and no significant differences in treatment exposure to docetaxel occurred between the two treatment groups. This is of crucial importance, given that one of the main challenges in combinatorial therapies where one of the agents is a targeted agent, is the fact that full dose chemotherapy treatment cannot be delivered due to compounded toxicity [e.g., Sunitinib in combination to docetaxel (23)].
In addition to the direct inhibition of HER2, alternative approaches for targeting the overexpression of this transmembrane receptor have emerged. Trastuzumab-maytansine (DM1) (T-DM1, Roche-Genentech, CA, USA) is an immunoconjugate agent that combines Trastuzumab with DM1, an antimicrotubule cytotoxic agent. A phase II study (NCT00509769) has now shown significant antitumour activity in patients with HER2-positive metastatic breast cancer who had progressed on anti-HER2 plus chemotherapy (24). Several clinical trials are currently testing T-DM1 in the metastatic setting at several stages of the disease: after progression on Trastuzumab in a head-to-head comparison with Lapatinib plus capecitabine (EMILIA, NCT00829166) or with Pertuzumab and paclitaxel (NCT00951665). A phase II clinical trial comparing PFS of T-DM1 vs. Trastuzumab plus docetaxel in first line metastatic HER2-positive has completed recruitment (NCT00679341). The impact of this trial on clinical practice will be affected to some extent by the publication of the CLEOPATRA study (21), since Trastuzumab plus docetaxel can no longer be considered as standard first line therapy.
There is clear interest in developing other strategies to deal with progressive HER2-positive metastatic breast cancer. As discussed above Lapatinib, an oral, small-molecule tyrosine kinase inhibitor that inhibits the kinase activity of both HER1 (EGFR) and HER2, has been approved for clinical use in combination with capecitabine after progression on Trastuzumab (25). Furthermore, several oral small-molecule, tyrosine kinase inhibitors are also in development. Afatinib (BIBW 2992, Boehringer Ingelheim, Germany) is an oral, irreversible HER family inhibitor that targets EGFR, HER2 and HER4. Initial reports confirmed antitumour activity of this compound alone or in combination with chemotherapy in patients who have progressed on anti-HER2 therapy (26). Neratinib (Pfizer, NY, USA) is an irreversible EGFR/HER2 kinase inhibitor that covalently binds the target kinase as part of its mechanism of action. A phase I dose - escalation study (NCT00146172) reported a response rate of 24% and clinical benefit rate of 38% in patients with HER2-amplified metastatic breast cancer, all of whom had progressed on prior anthracycline, taxane and Trastuzumab therapy (27). In a large phase II clinical trial in patients with HER2-amplified breast cancer (NCT00300781), Neratinib therapy resulted in a response rate of 24% (95% CI: 14-36%) for patients with prior Trastuzumab and a response rate of 56% (95% CI: 43-69%) for patients with no prior Trastuzumab (28). Several phase I/II trials of Neratinib combinations are in ongoing (Table 2) and preliminary safety and efficacy data have been presented [reviewed in (29)]. Phase III clinical trials utilising this agent in combination with other regimens have been planned for patients with metastatic or locally-advanced breast cancer.
In addition to targeting HER2 itself, alternative therapeutic approaches targeting pathways downstream of HER2 are currently being tested to overcome primary and/or acquired resistance to anti-HER2 agents in HER2-positive breast cancers (Table 2). For instance, one of the mechanisms of resistance to anti-HER2 agents is activation of the PI3K pathway through activating PIK3CA mutations and PTEN loss of function (29,30). An ongoing phase I study is currently investigating the activity of a PI3K inhibitor (GDC-0941; Roche-Genentech) in combination with either T-DM1 or Trastuzumab in an attempt to reverse Trastuzumab resistance (NCT00928330). Given the potential cumulative toxicity and cost implications of these various combinations, the identification of robust biomarkers to guide for the best approach is imperative.
Studying HER2-positive breast cancers has shifted the paradigm of how subgroups of breast cancer patients can be treated effectively. The trials performed so far have objectively demonstrated that contrary to the maxim sometimes more is more: dual blockade is better than Trastuzumab only treatment and this should need to be initiated at early stages of the disease. Furthermore, from a conceptual standpoint, the results of the clinical trials discussed in this editorial lend further credence to the notion that HER2 is a bona fide driver of a subset of breast cancers and to the concept of ‘oncogene addiction’ (31) (i.e. that despite the multiple genetic aberrations found in HER2-positive cancers, these cancers are dependent on the activity of this oncogenes for the maintenance of their malignant phenotype).
From a clinical standpoint, despite the changes in clinical practice, CLEOPATRA and the other trials discussed above raise numerous questions: (I) what are the best therapeutic agents for patients with HER2-positive disease: dual or multiple HER2 inhibition? (II) is dual inhibition required in all HER2-positive breast cancers? (III) at what stage of disease progression should Pertuzumab, T-DM1, Lapatinib and other novel anti-HER2 agents be introduced? (IV) does the order of the agents affects the likelihood of sustained responses? (V) how many different anti-HER2 and targeted agents should be administered in combination? (VI) should they be used concurrently or sequentially? With the panoply of valid therapeutic approaches available, clearly, answers to most of the questions above will be required for the full realisation of the potentials of personalised medicine, maximising the chances of preventing, delaying or reversing resistance to HER2-blockade.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2012.03.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 2005;5:341-54. [PubMed]
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177-82. [PubMed]
- Arteaga CL, Sliwkowski MX, Osborne CK, et al. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol 2011;9:16-32. [PubMed]
- Jacobs TW, Gown AM, Yaziji H, et al. Comparison of fluorescence in situ hybridization and immunohistochemistry for the evaluation of HER-2/neu in breast cancer. J Clin Oncol 1999;17:1974-82. [PubMed]
- Benz CC, Scott GK, Sarup JC, et al. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat 1992;24:85-95. [PubMed]
- Stancovski I, Hurwitz E, Leitner O, et al. Mechanistic aspects of the opposing effects of monoclonal antibodies to the ERBB2 receptor on tumor growth. Proc Natl Acad Sci U S A 1991;88:8691-5. [PubMed]
- Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med 2007;357:39-51. [PubMed]
- Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2009;27:5838-47. [PubMed]
- Park S, Jiang Z, Mortenson ED, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell 2010;18:160-70. [PubMed]
- Baselga J, Tripathy D, Mendelsohn J, et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol 1996;14:737-44. [PubMed]
- Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999;17:2639-48. [PubMed]
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. [PubMed]
- Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 2005;23:4265-74. [PubMed]
- Bailey TA, Luan H, Clubb RJ, et al. Mechanisms of Trastuzumab resistance in ErbB2-driven breast cancer and newer opportunities to overcome therapy resistance. J Carcinog 2011;10:28. [PubMed]
- Franklin MC, Carey KD, Vajdos FF, et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 2004;5:317-28. [PubMed]
- Gianni L, Lladó A, Bianchi G, et al. Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of Pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 2010;28:1131-7. [PubMed]
- Scheuer W, Friess T, Burtscher H, et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res 2009;69:9330-6. [PubMed]
- Fuentes G, Scaltriti M, Baselga J, et al. Synergy between trastuzumab and pertuzumab for human epidermal growth factor 2 (Her2) from colocalization: an in silico based mechanism. Breast Cancer Res 2011;13:R54. [PubMed]
- Baselga J, Gelmon KA, Verma S, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol 2010;28:1138-44. [PubMed]
- Gianni L, Pienkowski T, Im Y-H, et al. Neoadjuvant Pertuzumab (P) and Trastuzumab (H): Antitumor and Safety Analysis of a Randomized Phase II Study (‘NeoSphere’). Cancer Res 2010;70:Abstract S3-3.
- Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012;366:109-19. [PubMed]
- Soltoff SP, Carraway KL 3rd, Prigent SA, et al. ErbB3 is involved in activation of phosphatidylinositol 3-kinase by epidermal growth factor. Mol Cell Biol 1994;14:3550-8. [PubMed]
- Bergh J, Bondarenko IM, Lichinitser MR, et al. First-Line Treatment of Advanced Breast Cancer With Sunitinib in Combination With Docetaxel Versus Docetaxel Alone: Results of a Prospective, Randomized Phase III Study. J Clin Oncol 2012;30:921-9. [PubMed]
- Burris HA 3rd, Rugo HS, Vukelja SJ, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol 2011;29:398-405. [PubMed]
- Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006;355:2733-43. [PubMed]
- Hickish T, Wheatley D, Lin N, et al. Use of BIBW 2992, a Novel Irreversible EGFR/HER1 and HER2 Tyrosine Kinase Inhibitor To Treat Patients with HER2-Positive Metastatic Breast Cancer after Failure of Treatment with Trastuzumab. Cancer Res 2009;69. Abstract 5060.
- Wong KK, Fracasso PM, Bukowski RM, et al. A phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clin Cancer Res 2009;15:2552-8. [PubMed]
- Burstein HJ, Sun Y, Dirix LY, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol 2010;28:1301-7. [PubMed]
- Gajria D, Chandarlapaty S. HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev Anticancer Ther 2011;11:263-75. [PubMed]
- Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 2007;12:395-402. [PubMed]
- Ashworth A, Lord CJ, Reis-Filho JS. Genetic interactions in cancer progression and treatment. Cell 2011;145:30-8. [PubMed]


