Assessing the potential association between Epstein-Barr virus and oral squamous cell carcinoma: a systematic review and meta-analysis
Introduction
Oral squamous cell carcinoma (OSCC) is a multifactorial disease, whose risk factors have been well documented in the past. The most well-established risk factors of OSCC include tobacco and alcohol (1-5). There are independent risk factors in that they can cause oral cancer on their own and together (alcohol and tobacco) have a synergistic effect augmenting the overall cancer risk (4-6). The World Health Organisation (WHO) and other health agencies around the globe have constantly implemented regulations to prohibit the use of these known risk factors of oral cancer. Despite strict regulations on known risk factors, the incidence of OSCC has not been curbed (7,8). The cause of the persisting high OSCC incidence can largely be attributed to the potential risk factors. Unlike tobacco, and alcohol the causal nature of potential risk factors including environmental agents (e.g., household air pollution), infections (e.g., HPV, EBV, candida); immune status, etc. to oral cancer remains controversial (9-15). Studies have shown contrasting results which in turn is largely attributed to the variations in the sensitivity and specificity of the diagnostic tools used. Among microbial factors, much importance is given to HPV high-risk types 16 and 18 (14) and candida, including non-candida albicans species (13). Other microbial agents including EBV have been relatively less explored in OSCC. Most EBV based studies are on nasopharyngeal carcinoma wherein the causal nature is well-established. Similar to HPV, the carcinogenic potential of EBV remains controversial with respect to the oral cavity. Although several studies have isolated EBV from OSCC and oral potentially malignant tissues (OPMDs), many of these studies have significant methodological flaws including a lack of control group; sampling of saliva or exfoliated oral cells for detecting EBV; use of different sampling methods between study and the control groups; lack of potential confounder (age, gender and habit, etc.) matching between the comparison groups (16-34). In addition, even the diagnostic targets have often varied between the studies. In EBV based studies the most commonly assessed targets include latent membrane protein (LMP)-1 (an EBV associated protein implicated in activating EBV associated signaling pathway), Epstein-Barr virus (EBV)-encoded small non-polyadenylated RNA (EBER)-2, and EBV determined nuclear antigen (EBNA)-1 (implicated in regulating genetic expression) (17,21,23,25,27-31,34). Most EBV based studies on OSCC have assessed only one of these targets using modalities with different sensitivity and specificity, thus causing further discrepancies in the results obtained. The present manuscript attempts to assess the association between EBV and OSCC by systematically reviewing the literature for published case-control studies investigating the prevalence of EBV in OSCC tissues. Apart from the qualitative and quantitative assessment of EBV associations to OSCC, the present review also focuses on addressing the methodological flaws in the published studies.
Methods
Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) protocol was strictly adhered in the systematic review.
Inclusion criteria
- Case-control observational studies in English language.
- The control samples must be clinically assessed to have normal oral mucosa (NOM).
- The OSCC samples must be histopathologically confirmed cases.
- The samples must be conventional (incisional or excisional) tissue biopsies.
Exclusion criteria
- Narrative review, systematic review, meta-analysis, case reports/series, letter to the editor, conference papers.
- Articles in languages other than English.
- Microbes other than EBV.
- Cancers other than OSCC.
Focused question
“Is there an association between EBV and OSCC?
The framework of the population (P), intervention (I), comparison (C), outcome (O), studies (S) were used for this focused question. P represents histopathologically confirmed cases of OSCC; I represent the presence of Epstein Barr virus (EBV); C represents clinically determined cases of NOM, O represents the prevalence of EBV in OSCC compared to NOM, S represents case-control observational studies.
Search strategy
Data mining was done using Web of Science, Scopus and PubMed databases. The search included articles published until August 2019. The following keywords were used for data mining “EBV or Epstein Barr virus and Oral cancer or Oral squamous cell carcinoma”.
Studies selection and data extraction
Two reviewers (SS and ATR) used the above selection criteria to select the cases for this systematic review independently. Two steps were involved in this selection process. First, the reviewers screened the titles and abstract of the identified articles to remove potential duplicates and irrelevant articles. In the second step, the full text of the articles selected in the first step was assessed by the two reviewers using the inclusion criteria.
Risk of biased assessment
Newcastle Ottawa scale (NOS) was used to score the quality of the articles such as selection outcome/exposure and comparability. The maximum score for selection was 4, comparability was 2, and outcome/exposure was 4. Thus, a total of 10 points can be given for a single study. A score above 7 was considered good.
Statistical analysis
As they were two reviewers, potential inter-observer bias was assessed by the Kappa coefficient. Confidence interval (CI) and odds ratio (OR) was calculated for each of the studies included in the meta-analysis. WinPepi version 11.38 was used for generating the forest plot from the estimated CI and OR.
Results
Study selection
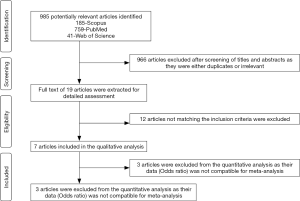
Using the keywords “EBV or Epstein Barr virus and Oral cancer or Oral squamous cell carcinoma”, a total of 985 articles were identified (185-Scopus, 759-PubMed, 41-Web of Science). A total of 966 articles were excluded after screening the titles and abstracts as they were irrelevant and/or duplicates. The full text of the remaining 19 articles was assessed using the inclusion criteria. Out of the 19 articles, only 7 satisfied the selection criteria and were included in the systematic review (17,21,23,28-31). Figure 1 summarizes the search strategy of the review in the form of a PRISMA flowchart. Kappa coefficient between the two reviewers (SS and ATR) for the first and second steps of the review was 0.98 and 1 respectively. Table 1 summarizes the data extracted from the included studies.
Table 1
| S.no | First authors name/year/country (reference number) | Nature of the tissue sample examined | Sample size (n) | Parameters matched between the comparison groups | Diagnostic modality/diagnostic target | Results-positive cases (n) | ||
|---|---|---|---|---|---|---|---|---|
| OSCC | NOM | OSCC | NOM | |||||
| 1 | Reddy SS/2017/India (28) | FFPE | 25 | 25 | Age, gender, tobacco chewing habit were matched | IHC/LMP-1 | 2 | 2 |
| 2 | Kikuchi K/2016/Japan (23) | FFPE | 150 | 30 | Matching details were not provided | PCR/EBNA-2 (EBV DNA) | 78 | 25 |
| PCR/LMP-1 | 16 | 7 | ||||||
| 3 | Sand LP/2002/Sweden (30) | FBS placed in 99% alcohol for 24 hours at room temperature, followed by storage at −20 °C | 29 | 67 | Only 12 of the 67 controls were age and tobacco habit matched | PCR/EBV DNA | 11 | 4 |
| 4 | Bagan JV/2008/Spain (17) | FBS frozen at −80 °C | 11 | 5 | Age and gender were not matched | PCR/EBV DNA | 6 | 0 |
| 5 | Gonzalez-Moles/2002/Spain (21) | FFPE | 78 | 50 | Age and gender were matched | PCR/EBV DNA | 15 | 0 |
| IHC/LMP-1 | 12 | 0 | ||||||
| ISH/EBER (EBV RNA) | 0 | 0 | ||||||
| 6 | Rahman R/2019/Thailand (29) | FFPE | 36 | 10 | Matching details were not provided | IHC/LMP-1 | 21 | 3 |
| 7 | Talacko AA/1991/UK (31) | FBS frozen at −70 °C | 20 | 18 | Matching details were not provided | ISH/EBV DNA | 0 | 0 |
All included articles were case-control observational studies. All case and control samples were biopsied oral cancer and normal oral mucosal tissues. OSCC, oral squamous cell carcinoma; NOM, normal oral mucosa; EBNA, Epstein-Barr virus determined nuclear antigen; LMP, latent membrane protein; EBER, Epstein-Barr virus-encoded small non-polyadenylated RNA; FFPE, formalin-fixed paraffin-embedded samples; FBS, fresh biopsy specimens; UK, The United Kingdom; DNA, deoxyribonucleic acid; RNA, ribonucleic acid.
Study characteristics
All the included articles were case-control observational studies. Two studies were from Spain (17,21), one each from India (28), Sweden (30), Japan (23), Thailand (29), and The United Kingdom (31). The diagnostic modality used in the studies ranged from immunohistochemistry (IHC), in situ hybridization (ISH) to polymerase chain reaction (PCR). The tissue samples were either formalin-fixed paraffin-embedded (FFPE) tissues or frozen biopsy specimens, except for one study (30) wherein the biopsy specimen was placed in 99% alcohol for 24 hours at room temperature, followed by storage at −20 °C.
Newcastle-Ottawa scale
In the included studies matching details of confounding factors (age, gender, associated habits, etc.) between the comparison groups (OSCC and NOM) were assessed. Reddy et al. (28) matched for age, gender and habit history, while only age and habit history was matched by Sand et al. (30) for a small portion of the control sample. Gonzalez-Moles et al. (21) matched only age and gender. In the remaining studies either matching was not done or the matching information was not provided. Of the 7 included studies, only Gonzalez-Moles et al. (21) used more than 1 diagnostic modality to 3 diagnostic targets ((PCR-EBV DNA, IHC-LMP-1 and ISH- EBER). Kikuchi et al. (23) used one diagnostic modality (PCR) for assessing two markers (EBNA-1 and LMP-1). The rest of the studies assessed only one diagnostic target using one diagnostic modality. The highest sample size was noted in Kikuchi et al. study (150 OSCC and 30 NOM cases) followed by Gonzalez-Moles et al. (78 OSCC and 50 NOM cases), Sand et al. (29 OSCC and 67 NOM cases), Reddy et al. (25 OSCC and 25 NOM cases), Rahman et al. (36 OSCC and 10 NOM cases), and Talacko et al. (20 OSCC and 18 NOM). The lowest sample size was noted in Bagan et al. (11 OSCC and 5 NOM cases). The Newcastle-Ottawa scores for the studies included in the systematic review are in Table 2.
Table 2
| First author (reference number) | Selection | Comparability | Exposure | Total score | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case definition | Case representativeness | Control selection | Control definition | Matching known confounding factor | Matching potential confounding factor | Secure patient records | Interviewer blinded to cases and control | Similarity in case and control ascertainment | Non-response rate | ||||
| Reddy SS (28) | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 6 | ||
| Kikuchi K (23) | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 5 | ||
| Sand LP (30) | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 5 | ||
| Bagan JV (17) | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 5 | ||
| Gonzalez-Moles (21) | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 5 | ||
| Rahman R (29) | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 5 | ||
| Talacko AA (31) | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 5 | ||
Vital details including that blinding information on cases and controls were not provided in the manuscript. Thus, without confirmation, score of 0 was rendered in such studies.
Prevalence of EBV in OSCC compared to the NOM
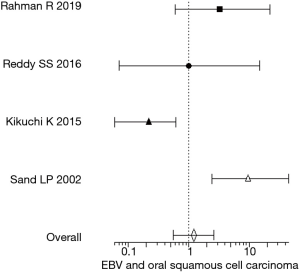
The prevalence of EBV in OSCC in the included studies ranged from 0 to 58.3%. The vast differences in the estimates were due to the variations in the sensitivity and specificity of the diagnostic modalities used and the type of diagnostic targets investigated. While the diagnostic modalities included PCR, IHC, and ISH, the targets included EBNA-1, EBER, LMP-1. In order to estimate an overall EBV prevalence, the studies were subjected to meta-analysis for which the confidence interval and odds ratio were calculated for the included studies individually. Four (23,28-30) out of the 7 studies in the qualitative analysis had no EBV prevalence in controls, thus their odds ratio was arriving at infinity and were excluded from the meta-analysis. The confidence interval and odds ratio of the remaining 4 studies are shown in Table 3. Figure 2 shows the forest plot depicting the overall estimate of the EBV association with OSCC from the 4 included studies. The meta-analysis (forest plot) shows an association between EBV and OSCC, although, the number and the quality of studies (based on the Newcastle-Ottawa scale) contributing to the meta-analysis data are below par.
Table 3
Discussion
The most common and well-established risk factors for OSCC are tobacco and alcohol (1-6). Most of the remaining risk factors in OSCC are designated the term potential risk factor to indicate that there is a lack of conclusive evidence for causal inference. Cases of OSCC presenting with no history of known risk factors have often been attributed to these potential risk factors ranging from environmental factors such as household air pollution to infections, immune status, genetic profile, etc. (9-15). Among this much importance is being given to microbial agents as they have shown to possess the carcinogenic potential (presence of oncogenes) and have shown to have a causal association with other forms of carcinoma including HPV with cervical and oropharyngeal cancer and EBV with nasopharyngeal cancer (35-38). Despite years of research, establishing microbial etiology for OSCC as an independent risk factor has been largely controversial (14).
The major reason for the lack of conclusive evidence is due to both the multi-factorial nature of the disease and that most OSCC cases included in studies carry a history of known risk factors such as tobacco and alcohol. Even in studies including only OSCC cases with no known risk factors, there seem to be large scale variations in the sensitivity and specificity of the diagnostic tools used. In addition to the varying diagnostic modalities used, there also seems to be variations in the diagnostic targets. While some studies have focused on identifying the presence of the microbe in the tissue sample (EBV DNA/RNA), others have largely focused on assessing the presence of surrogate markers (LMP-1) which could implicate a causal association for the agent in question (17,21,23,28-31). Very few studies have investigated both the presence of the microbial entity and its nature of association with the disease. In the present systematic review, only 2 (21,23) of the 7 included studies assessed the presence of more than one diagnostic target.
Another major factor to be considered is the samples to be collected. Several studies were excluded in the present systematic review as they had collected samples from saliva and exfoliated oral cells. This due to the fact that specific populations irrespective of the presence or absence of the disease might be microbial carriers. Thus, in such cases, the presence of the microbial agents in saliva or exfoliated cells would not be related to the OSCC. Identifying the microbe within the oral cancer tissue would be relatively more specific (39). Thus, only studies using biopsied tissue specimens were included in the review. Further several studies were also excluded as they failed to provide a comparison control group. Within those studies providing the control groups, there were several major limitations including use of alternative sample collections between the OSCC and the control specimens. While the OSCC cases were subjected to biopsy, samples from the controls (NOM) subjects were often collected through saliva or exfoliated oral cells/brush biopsy. Thus, the present review only included those studies which had analyzed the biopsied tissues from both the OSCC and the NOM groups.
As with many case-control studies, it is vital that the comparison groups (OSCC and NOM) are matched with respect to potential confounders such as age, gender and in cases of oral cancer, the associated habit history. Although the lack of matching would reduce the overall quality of the study, it was not considered as an exclusion criterion in the present systematic review. Apart from defining the inclusion and exclusion criteria one major factor determining the relevance of the case-control study is the sample size, which in the included studies varied significantly from 180 (150 OSCC cases and 30 NOM) to 16 (11 OSCC cases and 5 NOM). Thus, in such cases, the CI of the odds ratio also varied largely between the studies as shown in Table 3. The forest plot in Figure 2 was based on the calculated OD and CI from the included studies.
Based on the meta-analysis (forest plot) there is an association between EBV and OSCC. The association has to be viewed carefully as both the number and the quality of the studies (based on the Newcastle-Ottawa scale) contributing to the meta-analysis is below par. Further, there were large scale discrepancies between the included studies ranging from sensitivity and specificity of the diagnostic modalities used, diagnostic targets selected, sample size and potential confounder matching between the comparison groups.
Conclusions
The aim of the present systematic review was to answer the focused question: Is there an association between EBV and OSCC? According to the meta-analysis, there is an association between EBV and OSCC. The significance of the meta-analysis data obtained depends on the quality and the number of individual studies (their sample size) contributing to the data. The systematic review included only seven studies, out of which only four studies were compatible with the meta-analysis. Even within the limited number of studies included, there were major limitations. Two out of the 4 studies failed to provide information on matching between the OSCC and the NOM groups, while 1 study matched only age and habit for small proportion of the control samples. Further only one of the 4 studies assessed more than one diagnostic target. Thus, given the several major limitations of the included studies, the association noted between the EBV and OSCC in the present meta-analysis would require further validation for any conclusive inference.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Oral Pre-cancer and Cancer”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.01.09). The series “Oral Pre-cancer and Cancer” was commissioned by the editorial office without any funding or sponsorship. KHA served as an unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of Carcinogenic Risk in Humans. Tobacco Smoke and Involuntary Smoking. Volume 83. Lyon, France: IARC Press, 2004.
- Gandini S, Botteri E, Iodice S, et al. Tobacco smoking and cancer: a meta-analysis. Int J Cancer 2008;122:155-64. [Crossref] [PubMed]
- Gupta B, Johnson NW. Systematic review and meta-analysis of association of smokeless tobacco and of betel quid without tobacco with incidence of oral cancer in South Asia and the Pacific. PLoS One 2014;9:e113385. [Crossref] [PubMed]
- Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res 1988;48:3282-7. [PubMed]
- International Agency for Research on Cancer (IARC). Section 2.2. Cancer of the oral cavity and pharynx. In: IARC WorkingGroup on the Evaluation of Carcinogenic Risks to Humans, eds. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Alcohol Consumption and Ethylcarbamate. Volume 96. Lyon, France: IARC Press, 2010:237-329.
- Raj AT, Patil S, Awan KH, et al. Odds Ratio for Oral Cancer is directly Proportional to the Number of associated Habits. World J Dent 2017;8:351. [Crossref]
- Centers for Disease Control and Prevention (CDC). State smoking restrictions for private-sector worksites, restaurants, and bars--United States, 2004 and 2007. MMWR Morb Mortal Wkly Rep 2008;57:549-52. [PubMed]
- Biener L, Nyman AL, Stepanov I, et al. Public education about the relative harm of tobacco products: an intervention for tobacco control professionals. Tob Control 2014;23:385-8. [Crossref] [PubMed]
- Raj AT, Patil S, Sarode S, et al. Evaluating the association between household air pollution and oral cancer. Oral Oncol 2017;75:178-9. [Crossref] [PubMed]
- Josyula S, Lin J, Xue X, et al. Household air pollution and cancers other than lung: a meta-analysis. Environ Health 2015;14:24. [Crossref] [PubMed]
- Kerr AR. The oral microbiome and cancer. American Dental Hygienists' Association 2015;89:20-3.
- Schwabe RF, Jobin C. The microbiome and cancer. Nature Reviews Cancer 2013;13:800. [Crossref] [PubMed]
- Raj AT, Patil S, Awan KH. Assessing the Carcinogenic Potential of Non-Candida albicans in Cancer Therapy-induced Oral Mucositis. World J Dent 2018;9:79. [Crossref]
- Raj AT, Patil S, Gupta AA, et al. Reviewing the role of human papillomavirus in oral cancer using the Bradford Hill criteria of causation. Dis Mon 2019;65:155-63. [Crossref] [PubMed]
- Chakraborty P, Karmakar T, Arora N, et al. Immune and genomic signatures in oral (head and neck) cancer. Heliyon 2018;4:e00880. [Crossref] [PubMed]
- Acharya S, Ekalaksananan T, Vatanasapt P, et al. Association of Epstein-Barr virus infection with oral squamous cell carcinoma in a case-control study. J Oral Pathol Med 2015;44:252-7. [Crossref] [PubMed]
- Bagan JV, Jiménez Y, Murillo J, et al. Epstein-Barr virus in oral proliferative verrucous leukoplakia and squamous cell carcinoma: A preliminary study. Med Oral Patol Oral Cir Bucal 2008;13:E110-3. [PubMed]
- Bagan L, Ocete-Monchon MD, Leopoldo-Rodado M, et al. Prevalence of salivary Epstein-Barr virus in potentially malignant oral disorders and oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal 2016;21:e157-60. [Crossref] [PubMed]
- Cruz I, Van den Brule AJ, Steenbergen RD, et al. Prevalence of Epstein-Barr virus in oral squamous cell carcinomas, premalignant lesions and normal mucosa--a study using the polymerase chain reaction. Oral Oncol 1997;33:182-8. [Crossref] [PubMed]
- D'Costa J, Saranath D, Sanghvi V, et al. Epstein-Barr virus in tobacco-induced oral cancers and oral lesions in patients from India. J Oral Pathol Med 1998;27:78-82. [Crossref] [PubMed]
- Gonzalez-Moles MA, Gutierrez J, Rodriguez MJ, et al. Epstein-Barr virus latent membrane protein-1 (LMP-1) expression in oral squamous cell carcinoma. Laryngoscope 2002;112:482-7. [Crossref] [PubMed]
- Jalouli J, Ibrahim SO, Sapkota D, et al. Presence of human papilloma virus, herpes simplex virus and Epstein-Barr virus DNA in oral biopsies from Sudanese patients with regard to toombak use. J Oral Pathol Med 2010;39:599-604. [Crossref] [PubMed]
- Kikuchi K, Noguchi Y, de Rivera MW, et al. Detection of Epstein-Barr virus genome and latent infection gene expression in normal epithelia, epithelial dysplasia, and squamous cell carcinoma of the oral cavity. Tumour Biol 2016;37:3389-404. [Crossref] [PubMed]
- Kis A, Feher E, Gall T, et al. Epstein-Barr virus prevalence in oral squamous cell cancer and in potentially malignant oral disorders in an eastern Hungarian population. Eur J Oral Sci 2009;117:536-40. [Crossref] [PubMed]
- Mao EJ, Smith CJ. Detection of Epstein-Barr virus (EBV) DNA by the polymerase chain reaction (PCR) in oral smears from healthy individuals and patients with squamous cell carcinoma. J Oral Pathol Med 1993;22:12-7. [Crossref] [PubMed]
- Nasher AT, Al-Hebshi NN, Al-Moayad EE, et al. Viral infection and oral habits as risk factors for oral squamous cell carcinoma in Yemen: a case-control study. Oral Surg Oral Med Oral Pathol Oral Radiol 2014;118:566-72.e1. [Crossref] [PubMed]
- Nola-Fuchs P, Boras VV, Plecko V, et al. The prevalence of human papillomavirus 16 and Epstein-Barr virus in patients with oral squamous cell carcinoma. Acta Clin Croat 2012;51:609-14. [PubMed]
- Reddy SS, Sharma S, Mysorekar V. Expression of Epstein-Barr virus among oral potentially malignant disorders and oral squamous cell carcinomas in the South Indian tobacco-chewing population. J Oral Pathol Med 2017;46:454-9. [Crossref] [PubMed]
- Rahman R, Poomsawat S, Juengsomjit R, et al. Overexpression of Epstein-Barr virus-encoded latent membrane protein-1 (LMP-1) in oral squamous cell carcinoma. BMC Oral Health 2019;19:142. [Crossref] [PubMed]
- Sand LP, Jalouli J, Larsson PA, et al. Prevalence of Epstein-Barr virus in oral squamous cell carcinoma, oral lichen planus, and normal oral mucosa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;93:586-92. [Crossref] [PubMed]
- Talacko AA, Teo CG, Griffin BE, et al. Epstein-Barr virus receptors but not viral DNA are present in normal and malignant oral epithelium. J Oral Pathol Med 1991;20:20-5. [Crossref] [PubMed]
- Sharma U, Singhal P, Bandil K, et al. Genetic variations of TLRs and their association with HPV/EBV, co-infection along with nicotine exposure in the development of premalignant/malignant lesions of the oral cavity in Indian population. Cancer Epidemiol 2019;61:38-49. [Crossref] [PubMed]
- van Heerden WE, van Rensburg EJ, Engelbrecht S, et al. Prevalence of EBV in oral squamous cell carcinomas in young patients. Anticancer Res 1995;15:2335-9. [PubMed]
- Van Rensburg EJ, Engelbrecht S, Van Heerden W, et al. Detection of EBV DNA in oral squamous cell carcinomas in a black African population sample. In Vivo 1995;9:199-202. [PubMed]
- Han BL, Xu XY, Zhang CZ, et al. Systematic review on Epstein-Barr virus (EBV) DNA in diagnosis of nasopharyngeal carcinoma in Asian populations. Asian Pac J Cancer Prev 2012;13:2577-81. [Crossref] [PubMed]
- da Costa VG, Marques-Silva AC, Moreli ML. The Epstein-Barr virus latent membrane protein-1 (LMP1) 30-bp deletion and XhoI-polymorphism in nasopharyngeal carcinoma: a meta-analysis of observational studies. Syst Rev 2015;4:46. [Crossref] [PubMed]
- Kjaer SK, Van den Brule AJC, Boch JE, et al. Human papillomavirus—the most significant risk determinant of cervical intraepithelial neoplasia. Int J Cancer 1996;65:601-6. [Crossref] [PubMed]
- Bosch FX, Lorincz A, Munoz N, et al. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002;55:244-65. [Crossref] [PubMed]
- She Y, Nong X, Zhang M, et al. Epstein-Barr virus infection and oral squamous cell carcinoma risk: A meta-analysis. PLoS One 2017;12:e0186860. [Crossref] [PubMed]