
Dendrobium officinalis inhibited tumor growth in non-small cell lung cancer
Introduction
Lung cancer is always a severe disease (1) and is one of the most common malignant tumors in the world (2). In Chinese urban population, lung cancer has become the first cause of death of malignant tumors. It can be divided into 2 types, small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) (2). NSCLC accounts for about 80% of all lung cancers, and about 75% of patients have been found in the middle and advanced stage (3), with a meager 5-year survival rate. NSCLC can also be divided into 2 main histological subtypes: adenocarcinoma and squamous cell carcinoma (1). There is a higher prevalence of lung adenocarcinoma, and it accounts for 50% of all NSCLCs (4). Although there are many treatments for lung cancer, the results are generally unsatisfactory in terms of efficacy and survival (5). Thus, new treatments and drugs are urgently needed.
Dendrobium officinale is a valuable traditional medicine in China with very high medicinal value (6). According to a recent report, it exhibits modern effects in a few ways, including the hepatoprotective effect, antifatigue effect, hypoglycemic effect, gastric ulcer protective effect, etc. (7). There is increasing evidence that Dendrobium officinale can also inhibit tumor cell proliferation and induce apoptosis of tumor cells. It may be used as an adjuvant drug for chemotherapy (8-10). Thus, Dendrobium officinale has clear potential as a drug for improving cancer treatment.
In this study, we were interested in researching the role of Dendrobium officinale in lung cancer and whether it can effectively inhibit tumor development and reduce side effects. It will provide new idea for cancer treatment.
Methods
Experimental equipment
Experimental equipment included the flowing: a Model 5410 cryogenic high-speed centrifuge (Eppendorf, Germany); a BSC-1300 II B2 type bio-safety cabinet (Shanghai Bo Medical Instrument Co., Ltd.); a carbon dioxide incubator (Thermo Forma); an inverted microscope (Olympus, Japan); a 100,000th electronic balance (Mettler Toledo International Trading (Shanghai Co., Ltd.)
Medicinal materials and preparation
Fresh Dendrobium officinale was acquired from Shanglin County, Guangxi. It was prepared by dicing fresh dendrobium, adding pure water, and steaming. The dendrobium, was then centrifuged, concentrated, sterilized, and stored at 4 °C for later use (0.68 g fresh medicine per 1 mL).
Drugs and reagents
Cyclophosphamide (CTX, Baxter Medical Supplies Trading Co., Ltd.). Fetal bovine serum (FBS, Lonsa, South America), RNA extraction kit, and reverse transcription kit were purchased from Tiangen Biochemistry, with batch Numbers of RN05004M and fp205-02, respectively.
Cell culture
Human NSCLC A549 cells were purchased from CAS’ cell bank. Fetal calf serum was purchased from Lonsa (South America). A549 cells were cultured and retained at 37 °C in a carbon dioxide incubator in culture medium with 10% FBS, 0.1% penicillin-streptomycin solution, and 89.9% Dulbecco’s Modified Eagle’s Medium (DMEM). When cell growth reached critical mass (about 80% coverage), the cells were collected with the trypsin enzyme-digesting technique and passaged.
Cell collection
In the logarithmic phase of cell growth, cells are in a good state. Pancreatin was added to digestive cells, which were treated by centrifugation, and then resuspended in the phosphate buffer saline. The cells were centrifuged again and mixed with phosphate buffer saline to the desired levels (each nude mouse required 1×107 cells).
Establishment of the nude mice tumor model
Nude mice (specific-pathogen-free grade) were purchased from Hunan Laike Jingda experimental animals Co., Ltd [License number: SCXK (Xiang) 2016-0002]. We chose 5-week-old mice, and the study was started after the nude mice were allowed to acclimate for about 1 week (in SPF Animal Lab). There were a total of 30 nude mice that were given intraperitoneal injections of chloral hydrate (0.2 mL/20 g). With the mouse lying on the left side, a A549 single-cell suspension was subcutaneously injected into the nude mouse’s back (1×107 mL−1). The nude mice were observed until completely recovered.
Grouping and intervention methods
The tumor appeared after 4 days. The nude mice were randomly divided into 5 groups: the negative control group; the cytoxan group; and the high, medium, and low Dendrobium officinale concentration groups. When the mouse model started to appear anorexic and had reduced autonomic activity, it was prescribed drugs. The negative control group was only fed with normal saline (every day, 30 mL·kg−1), and a cyclophosphamide injection was regarded as a positive control (every other day, 0.0025 g/kg). Three different concentrations of Dendrobium doses were fed to nude mice (every day, 25, 12.5, 6.25/kg). The tumor volume of the mice was measured (tumor volume = length × width2 × 0.52) along with the bodyweight, every 3 days. The animals were sacrificed after 4 weeks.
Sample disposal
Half of the fresh tumor tissues were collected and stored at −80 °C for subsequent analysis. The other half were stored in formalin for hematoxylin-eosin staining.
The instructions for hematoxylin-eosin staining of samples were as follows.
Dewaxing
- Put in xylene dewaxing (5–10 min can be carried out in the two bottles).
- Move into anhydrous alcohol (100%) for about 2 min.
- Move into 90% alcohol for about 2 min.
- Move into 80% alcohol for about 2 min.
- Move into 70% alcohol for about 2 min.
- Move into water and wash away alcohol for about 2–3 min.
- Move into distilled water for about 2 min.
Staining
- Move into hematoxylin, and disseminate for 8–15 min.
- Move into the water, wash away the hematoxylin, and add color for about 1–2 min.
- Put into the 1% hydrochloric acid alcohol for a few seconds to 30 seconds.
- Move into the water, wash for 20 min.
- Move into 95% alcohol for about 30 s.
- Move into eosin with alcohol for about 30 s.
Dehydration
- Move into I 95% alcohol for about 30 s.
- Move into II 95% alcohol for about 30 s.
- Moved into I 100% alcohol for about 30 s.
- Move into II 100% alcohol for about 30 s.
Transparent
- Move into xylene I, transparent for about 3–5 min.
- Move into xylene II, transparent for about 5–10 min.
Sealing
Fix with neutral balsam.
Statistical analysis
All data used the Statistical Package for the Social Sciences (SPSS 25.0) for statistical analysis. T-test was used to find statistical significance. Analysis of variance (ANOVA) was used to analyze data for all experiments. Difference of statistical significance of the 5 groups was considered with a P value <0.05.
Results
Pathological gross and microscopic examination of nude mice after sampling
As shown in Figure 1, the size of the tumor can be seen with the naked eye; the size of tumor tissues in the negative control group was more extensive than that of the other drug groups. Under the microscope (Figure 2), compared with the negative control group, the area of necrosis in the drug group was significantly larger than that in the negative control group. In the group comparisons, the necrotic area in the high concentration of dendrobium officinale group and the cytoxan group reached more than half (Figure 2). The negative control and high concentration dendrobium officinale groups’ tumor size were compared to determine which holds the most contrast. The differences in the negative control and high concentration dendrobium officinale group were the largest (Figure 1).
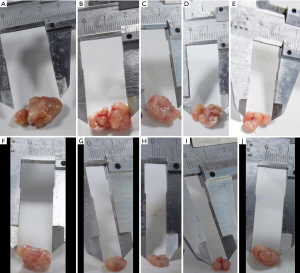
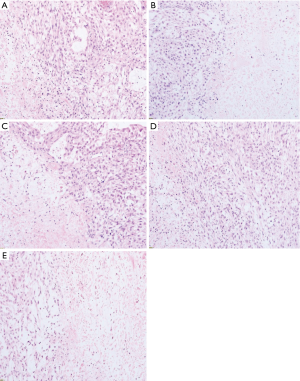
The gross tumor volume (GTV) change
Differences between pre-treatment and post-treatment GTV (Table 1) were evaluated. As seen in Table 1, different concentrations of dendrobium officinale could effectively inhibit the tumor. The tumor shrinkage and inhibitory growth rate in the drug group were greater compared to those in the negative control group. Compared to the negative control group, the tumor volume in high concentration dendrobium officinale group was smaller, and the anti-tumor rate was 56%. The tumor control rate in both the medium and low concentration dendrobium officinale groups were higher than 50%. The cure effect of the high concentration dendrobium officinale group and the cytoxan group were relatively better.
Table 1
| Group | GTV (mm3) () | Tumor weight (g) | Growth inhibitory rate (%) | |
|---|---|---|---|---|
| Pre-administration after administration | ||||
| Negative control group | 132.87±25.75 | 948.26±384.01 | 0.85 | 0 |
| High group | 114.53±17.03 | 412.67±239.31 | 0.37 | 56 |
| Medium group | 139.61±48.42 | 459.03±89.73 | 0.41 | 52 |
| Low group | 126.05±38.74 | 467.35±82.46 | 0.42 | 51 |
| Cytoxan group | 108.71±24.29 | 361.86±84.46 | 0.32 | 62 |
Tumor volume (V) =ab2/2, a = the longest tumor diameter, b = short tumor diameter. Volume(weight) inhibition rate: {V(weight)model – Vtreatment(weight)}/V(weight)model× 100%. GTV, gross tumor volume; high group, high concentration of Dendrobium officinale group; medium group, medium concentration of Dendrobium officinale group; low group, low concentration of Dendrobium officinale group; cytoxan group, positive control group.
SPSS statistical software was used to analyze the experimental data (Table 2). The GTV in the different concentration groups were significantly changed, as compared with those in the negative control group. The data were compared with a single-factor analysis of variance [pre-treatment: F=0.877, P=0.360 (P>0.05)]. The comparison of the first day with the 26th day was F=−16.61, with P=0.001 (P<0.05), and the tumor volume of the drug groups was lower than in the negative control group (P<0.05). The paired t-test was applied to compare the groups (Table 2); the high concentration dendrobium officinale group, the medium concentration of dendrobium officinale group and the low concentration of dendrobium officinale group had reduced tumor volume compared with the negative control group, with a significant difference (P<0.05). As for the different concentration groups, there was no statistical difference between the 4 groups (P>0.05).
Table 2
| Status | Group A | Group B | F/T value | P value |
|---|---|---|---|---|
| Pre-treatment | Negative control group | High group | F=0.877 | P=0.360 |
| Medium group | ||||
| Low group | ||||
| Cytoxan group | ||||
| After-treatment | Negative control group | High group | F=−16.61 | P=0.001 |
| Medium group | ||||
| Low group | ||||
| Cytoxan group | ||||
| After-treatment | Negative control group | High group | T=2.837 | P=0.047 |
| Medium group | T=4.258 | P=0.013 | ||
| Low group | T=4.569 | P=0.010 | ||
| Cytoxan group | T=3.914 | P=0.017 | ||
| After-treatment | High group | Medium group | T=0.310 | P=0.770 |
| Low group | T=0.282 | P=0.790 | ||
| Cytoxan group | T=0.688 | P=0.530 | ||
| Medium group | Low group | T=0.189 | P=0.860 | |
| Cytoxan group | T=0.684 | P=0.530 | ||
| Low group | Cytoxan group | T=0.284 | P=0.790 |
High group, high concentration of Dendrobium officinale group; medium group, medium concentration of Dendrobium officinale group; low group, low concentration of Dendrobium officinale group; cytoxan group, positive control group.
The volume of tumor trend line
The Figure 3 shows the tumor growth-curve of tumor volume according to time in every group. The tumor growth was slow in the drug groups, and the growth-curve of the high concentration of dendrobium officinale group and cytoxan group’s fluctuations were smaller than those of the other concentration groups. To some extent, the high concentration Dendrobium officinale group showed better efficacy.
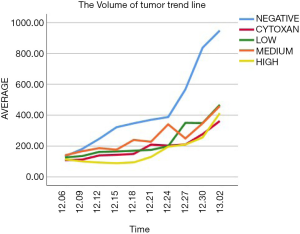
Discussion
Lung cancer is the most common type of cancer in the world (11). The primary subtype is NSCLC, which accounts for 80% of all lung cancers (12). One of the major subtypes of NSCLC is lung adenocarcinoma, and it has a high incidence (13). Surgery is usually not a good option for patients, because it typically has already spread at the time of diagnosis. Despite current advances in immunotherapy approaches, chemotherapy treatment, and target-specific drug treatment, the 5-year survival rate of NSCLC patients remains <15% (14-17). Therefore, looking for different new therapeutic approaches for lung cancer treatment is imperative.
Dendrobium officinale belongs to the Orchidaceae family. In China, dendrobium officinale has been used for more than 1,000 years (18,19). In previous research, the active substances of dendrobium officinale (numerous amino acids, alkaloids, and polysaccharides) showed anticancer effects on cervical cancer cells, liver cancer cells (20), gastric carcinogenesis (21), and breast cancer (22). Researchers discovered that dendrobium officinale also had a prophylactic effect on the formation of metastatic pulmonary tumors and colon cancer (23). Researchers also noted that Vicenin 2 extracted from dendrobium officinale showed anticancer effects on lung cancer cells (24). However, the degree to which dendrobium officinale regulates lung tumor invasion, angiogenesis, and tumor proliferation has not been sufficiently studied. Thus, this study aimed to investigate the inhibitory effect of dendrobium officinale in A549 lung cancer cells by adjusting the formation of the new vessels to slow tumor growth.
As far as we know, there is almost no correlative literature about dendrobium officinale for the treatment of pulmonary adenocarcinoma. In the present study, we were first to find that dendrobium officinale could adjust pulmonary adenocarcinoma tumor volume. Our preliminary study showed that dendrobium officinale can inhibit the tumor effectively. Based on our current experimental results, we consider that currently, dendrobium officinale may have crucial significance in regulating GTV. The treatment effect between the high concentration dendrobium officinale group and the cytoxan group had no clear differences, at least in the initial results. Although the other 2 different concentrations groups could also slow tumor growth, the extent of fluctuation was significant. So, we hold the opinion that the effect was more stable in the high concentration dendrobium officinale group, and we speculate that the tumor volume had a negative correlation with drug concentration. Thus, in follow-up experiments, we will continue to increase the number of nude mice and gradually adjust and optimize drug concentration to confirm the best medical concentration. One study reported (24) that the effective extraction of dendrobium officinale can reverse TGF-β1- to induce the transformation of lung adenocarcinoma cells. Most of the relevant research has focused on the cellular level, but the curative effect on animal models has seldom been tested.
Despite a shortage of research about dendrobium officinale therapy for A549 pulmonary adenocarcinoma, its regulatory mechanisms have been investigated in other tumors. For example, BAX and caspase-3 can promote apoptosis, and Zhao et al. (21) reported that dendrobium officinale can suppress the proliferation of gastric cancer through up-regulating the expression of BAX and caspase-3 factors. Wang et al. (25) reported that dendrobium officinale shows a preventive effect on colon cancer in mice through increasing BAX, Caspase-3, and Caspase-9 and decreasing Bcl-2. Xing et al. (20) discovered that dendrobium officinale can control and suppress TGF-β1-induced EMT phenotypes and inhibit the growth of liver cancer in nude mice. Sun et al. (22) reported that the effective extract of dendrobium officinale could increase BAX and decrease Bcl-2, and then suppress the breast tumor. Therefore, we can infer that dendrobium officinale can act on lung tumors and suppress lung tumor growth, and, based on findings from our preliminary study, we argue that our hypothesis has gained further credibility.
Conclusions
Above all, through this study, we found that the different concentrations of dendrobium officinale can slow the growth of lung tumor nude mice, and the best treatment concentration for the nude mice tumor model was a high concentration of dendrobium officinale. These findings suggest that dendrobium officinale has potent effects of inhibiting tumor on the nude mouse tumor model. Dendrobium officinale may be a new drug for to the treatment of lung cancer in future clinical application.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.79). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by Guangxi University of Chinese Medicine Institutional Review Board (No. DW20180829-33) and written informed consent was obtained from all patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Maj E, Filip-Psurska B, Milczarek M, et al. Vitamin D derivatives potentiate the anticancer and anti-angiogenic activity of tyrosine kinase inhibitors in combination with cytostatic drugs in an A549 non-small cell lung cancer model. Int J Oncol 2018;52:337-66. [PubMed]
- Blandin Knight S, Crosbie PA, Balata H, et al. Progress and prospects of early detection in lung cancer. Open Biol 2017; [Crossref] [PubMed]
- Liu QH, Li ZH, Li DB. The mechanism of acquired resistance to egfr tyrosine kinase inhibitors and its relationship with small RNAs in NSCLC patients. Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease 2019;(02):9-14.
- Liao M, Liu Q, Li B, et al. A group of long noncoding RNAs identified by data mining can predict the prognosis of lung adenocarcinoma. Cancer Sci 2018;109:4033-44. [Crossref] [PubMed]
- Chambers SK, Dunn J, Occhipinti S, et al. A systematic review of the impact of stigma and nihilism on lung cancer outcomes. BMC Cancer 2012;12:184. [Crossref] [PubMed]
- Cakova V, Bonte F, Lobstein A. Dendrobium: Sources of Active Ingredients to Treat Age-Related Pathologies. Aging Dis 2017;8:827-49. [Crossref] [PubMed]
- Tang H, Zhao T, Sheng Y, et al. Dendrobium officinale Kimura et Migo: A Review on Its Ethnopharmacology, Phytochemistry, Pharmacology, and Industrialization. Evid Based Complement Alternat Med 2017;2017:7436259.
- Wei Y, Wang L, Wang D, et al. Characterization and anti-tumor activity of a polysaccharide isolated from Dendrobium officinale grown in the Huoshan County. Chin Med 2018;13:47. [Crossref] [PubMed]
- Li F, Luo P, Liu H. A Potential Adjuvant Agent of Chemotherapy: Sepia Ink Polysaccharides. Mar Drugs 2018; [Crossref] [PubMed]
- Du Q, Jiang G, Li S, et al. Docetaxel increases the risk of severe infections in the treatment of non-small cell lung cancer: a meta-analysis. Oncoscience 2018;5:220-38. [PubMed]
- Choi JI. Medically inoperable stage I non-small cell lung cancer: best practices and long-term outcomes. Transl Lung Cancer Res 2019;8:32-47. [Crossref] [PubMed]
- Ahmmed B, Khan MN, Nisar MA, et al. Tunicamycin enhances the suppressive effects of cisplatin on lung cancer growth through PTX3 glycosylation via AKT/NF-κB signaling pathway. Int J Oncol 2019;54:431-42. [PubMed]
- Yochum ZA, Cades J, Wang H, et al. Targeting the EMT transcription factor TWIST1 overcomes resistance to EGFR inhibitors in EGFR-mutant non-small-cell lung cancer. Oncogene 2019;38:656-70. [Crossref] [PubMed]
- Tong L, Liu J, Yan W, et al. RDM1 plays an oncogenic role in human lung adenocarcinoma cells. Sci Rep 2018;8:11525. [Crossref] [PubMed]
- Ferrer I, Quintanal-Villalonga Á, Molina-Pinelo S, et al. MAP17 predicts sensitivity to platinum-based therapy, EGFR inhibitors and the proteasome inhibitor bortezomib in lung adenocarcinoma. J Exp Clin Cancer Res 2018;37:195. [Crossref] [PubMed]
- Chen RL, Zhao J, Zhang XC, et al. Crizotinib in advanced non-small-cell lung cancer with concomitant ALK rearrangement and c-Met overexpression. BMC Cancer 2018;18:1171. [Crossref] [PubMed]
- Zhang C, Leighl NB, Wu YL, et al. Emerging therapies for non-small cell lung cancer. J Hematol Oncol 2019;12:45. [Crossref] [PubMed]
- Shen C, Guo H, Chen H, et al. Identification and analysis of genes associated with the synthesis of bioactive constituents in Dendrobium officinale using RNA-Seq. Sci Rep 2017;7:187. [Crossref] [PubMed]
- Yu Z, Liao Y, Teixeira da Silva JA, et al. Differential Accumulation of Anthocyanins in Dendrobium officinale Stems with Red and Green Peels. Int J Mol Sci 2018; [Crossref] [PubMed]
- Xing S, Yu W, Zhang X, et al. Isoviolanthin Extracted from Dendrobium officinale Reverses TGF-β1-Mediated Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma Cells via Deactivating the TGF-β/Smad and PI3K/Akt/mTOR Signaling Pathways. Int J Mol Sci 2018; [Crossref] [PubMed]
- Zhao Y, Liu Y, Lan XM, et al. Effect of Dendrobium officinale Extraction on Gastric Carcinogenesis in Rats. Evid Based Complement Alternat Med 2016,2016:1213090.
- Sun J, Guo Y, Fu X, et al. Dendrobium candidum inhibits MCF-7 cells proliferation by inducing cell cycle arrest at G2/M phase and regulating key biomarkers. Onco Targets Ther 2015;9:21-30. [PubMed]
- Li G, Sun P, Zhou Y, et al. Preventive effects of Dendrobium candidum Wall ex Lindl. on the formation of lung metastases in BALB/c mice injected with 26-M3.1 colon carcinoma cells. Oncol Lett 2014;8:1879-85. [Crossref] [PubMed]
- Luo Y, Ren Z, Du B, et al. Structure Identification of ViceninII Extracted from Dendrobium officinale and the Reversal of TGF-β1-Induced Epithelial−Mesenchymal Transition in Lung Adenocarcinoma Cells through TGF-β/Smad and PI3K/Akt/mTOR Signaling Pathways. Molecules 2019; [Crossref]
- Wang Q, Sun P, Li G, et al. Inhibitory effects of Dendrobium candidum Wall ex Lindl. on azoxymethane- and dextran sulfate sodium-induced colon carcinogenesis in C57BL/6 mice. Oncol Lett 2014;7:493-8. [Crossref] [PubMed]


