
CK1α-targeting inhibits primary and metastatic colorectal cancer in vitro, ex vivo, in cell-line-derived and patient-derived tumor xenograft mice models
Introduction
With an incidence of 1,849,518 and mortality of 880,792, colorectal cancer (CRC) accounted for 10.2% and 9.2% of all new cancer cases and cancer-related deaths, respectively, for both sexes, globally in the 2018 alone, thus, ranking as the third most diagnosed and second most fatal malignancy in the world (1,2). Regardless of the significant advances made in CRC diagnosis and documented progression in the effectiveness of contemporary multimodal anti-CRC therapeutic strategies in the last 3 decades, however, their off-target toxicity against rapidly-dividing colorectal cells and acquisition of resistant phenotype continue to limit achievement of optimal therapeutic effect; thus, CRC remains associated with poor clinical outcome, with high rates of metastasis and recurrence, and relatively low overall survival (OS) in advanced-stage cases (3-7).
In the last three decades, better understanding of CRC biology and emerging kinase-related concepts reveal that the enhanced metastatic and recurrent phenotype of CRC may be associated with the enhanced activities of protein kinases, which are known to catalyze several reversible post-translational modifications, including the phosphorylation and nucleotidylation of several receptors or substrates (8-10). The human protein kinase family is sub-divided into 13 classes based on their substrates including the receptor tyrosine and serine/threonine kinases which are critical regulators of most bioactivities including cell division, proliferation, cell fate determination, signal transduction, and cell death (8-10).
Like receptor tyrosine kinases, the transmembrane receptor serine/threonine kinases contain extracellular ligand-binding domains and cytoplasmic kinase domains. Casein kinase 1α (CK1α), encoded by CSNK1A1 in humans, is an isoform of the CK1 protein, exhibits broad serine/threonine kinase activity, and is a critical effector of the canonical Wnt signaling pathway (11). CK1α has been shown to induce β-catenin phosphorylation at Ser45 and constitutes an active part of the β-catenin multiprotein destruction complex which subsequently facilitates the ubiquitination and proteasomal degradation of β-catenin (11,12). In other studies, CK1α has been shown to negatively regulate the tumor suppressor p53 in concert with the ubiquitin ligase murine double minute clone 2 (MDM2) while enhancing E2F-1 activity in undamaged cells, thus playing a central role in p53 and E2F-1 protein stability (13). These β-catenin-MDM2-E2F-1-p53 modulating role of CK1α do suggest its probable role in the constitutive or acquired insensitivity of metastatic CRC cells to conventional CRC multimodal therapy. Thus, we hypothesized that the aberrant expression and enhanced activity of CK1α is actively involved in CRC initiation, viability, proliferation, metastasis and recurrence, with the need to develop or identify therapeutic strategies that target and eliminate these CK1α enriched CRC cells.
Recently, Epiblastin A, through the mediation of adenosine triphosphate (ATP), has been shown to competitively and dose-dependently inhibit CK1 at concentrations <10 µM, as well as induce the dedifferentiation of mouse epiblast stem cells into embryonic stem cells several fold more efficiently than the sodium channel inhibitor, triamterene (14). While the kinase-inhibiting role of Epiblastin A has attracted much scientific attention in recent years, very little is documented on its anticancer and cancer stem cells (CSCs)-targeting effect. Thus, we hypothesized based on it CK1α-targeting activity, that Epiblastin A suppresses cancer cell division and proliferation, enhances apoptosis, inhibits tumor metastasis and recurrence, and ultimately facilitate better prognosis. This would be consistent with our increasing understanding of the critical role of protein kinase modulation and dysfunction in the progression, metastasis and recurrence of many malignancies (15) and clinically relevant considering that the single- or multiple- agent targeting of kinases is increasingly explored as an attractive therapeutic strategy for many human cancer types (8-10,15).
Testing our hypotheses, in the present study, we investigated the anticancer effect of CK1α in CRC in vitro (cell lines), ex vivo (clinical samples and primary culture) and in vivo [patient-derived xenograft (PDX) and tumor xenograft] models, and demonstrated that Epiblastin A inhibits primary and metastatic CRC by targeting CK1α and Ki-67.
Methods
Patients clinicopathological characteristics
This study was conducted on a cohort of patients with CRC at Hwa Mei Hospital, University of Chinese Academy of Sciences. The protocol for the study was reviewed and approved by the Hwa Mei Hospital, University of Chinese Academy of Sciences review board. In our CRC samples (n=78) from patients with CRC stage I to IV, patients aged from 29 to 72 with median age of 50.3; 69 (88.46%) were male, while 9 (11.54%) were female. The median follow-up time to CRC-related mortalities were recorded during the follow-up.
Reagents and chemicals
Epiblastin A (≥98% HPLC purity, CAS #16470-02-3, Cat. #22758) was purchased from Cayman Chemicals (Ann Arbor, MI, USA) and the anti-human EGFR monoclonal antibody, Cetuximab/Erbitux was purchased from Merck (ImClone LLC, Merck KGaA, Darmstadt, Germany). Epiblastin A or Cetuximab were reconstituted following the manufacturers’ instruction, stored at –20 °C, and further diluted in sterile culture medium just before use. Antibodies against CK1α (sc-74582), Ki-67 (sc-23900) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (sc-47724) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Goat Alexa Fluor 488 anti-mouse IgG H & L (ab 150113) were obtained from Abcam (Aibo Trading Co., Ltd., Shanghai, China).
Cells and cell culture
Human normal fetal colonic epithelial cell line fetal human normal colonic mucosa (FHC), colorectal carcinoma cell lines HCT116, HT29, and DLD1 were obtained from ATCC (American Type Culture Collection, Manassas, VA, USA). The cells were cultured in RPMI-1640 medium containing 5% fetal bovine serum (#F2442, Sigma-Aldrich Inc., St. Louis, MO, USA), streptomycin (200 µg/mL), and penicillin (500 units/mL: Life Technologies, Grand Island, NY, USA) at 37 °C in 5% CO2 incubator.
Western blotting
After trypsinizing, harvesting and lysing CRC cells, the obtained protein lysates were then heated and used for immunoblotting. The polyvinylidene fluoride (PVDF) membranes with blots were blocked with 5% low fat milk in Tris-buffered saline (TBST) for 1 h and incubated with primary antibodies against CK1α (1:1,000), Ki-67 (1:1,000), and GAPDH (1:1,000) overnight in 4 °C refrigerator, washed three times with TBST, incubated with horseradish peroxidase (HRP)-labeled anti-mouse secondary antibody for 1 h at room temperature, washed again 3 times with TBST, before protein band detection was performed using enhanced chemiluminescence (ECL) western blotting reagents and the c600 Western Blot Imaging System (Azure Biosystems Inc., Dublin, CA, USA).
Sulforhodamine B (SRB) cell viability assay
Cell viability was evaluated using SRB assay as described previously (16). Briefly, after seeding 3,500 CRC cells in complete medium in each well of 96-well plates, the cells were incubated at 37 °C in humidified 5% CO2 for 24 h, followed by treatment with various concentrations of Epiblastin A, with untreated CRC cells serving as control. Our quantification was performed three times in triplicates at an optical density (OD) of 495 nm using SpectraMax iD3 multi-mode microplate reader (Molecular devices LLC., Shanghai Office, Shanghai, China).
Quantitative real-time polymerase chain reaction (qRT-PCR)
After total RNA isolation from tissue sections of CRC patients was performed using the RNeasy Mini Kit (QIAGEN, Hilden, Germany), reverse transcription of 1 µg of total RNA into cDNA using the QuantiTect reverse transcription kit (QIAGEN, Hilden, Germany). GAPDH was used as house-keeping gene and its PCR amplification helped verify RNA integrity and cDNA synthesis fidelity based on the primer for GAPDH (forward) 5'-AGCACCAGGTGGTCTCCTCT-3' and (reverse) 5'-TGAGGTCCACCACCCTGTTG-3', and CK1α (forward) 5'-AGTGGCAGTGAAGCTAGAATCT-3' and (reverse) 5'-CGCCCAATACCCATTAGGAAGTT-3'. RT-PCR was performed using the PowerUp SYBR Green Master Mix Kit (Thermo Fisher Scientific, Waltham, MA, USA) in a SimpliAmp thermal cycler (Thermo Fisher Scientific, Waltham, MA, USA) under the cycling conditions: initiation—95 °C for 60 sec, 40 cycles of 95 °C for 15 sec, followed by annealing/extension for 60 °C for 16 sec, and then reaction concluded with a melting curve analysis spanning 65–95 °C in 0.5 °C increments at 5 seconds per step.
Immunohistochemistry assay
Frozen paraffin-embedded samples cut into 5-µm sections were deparaffinized by soaking in xylene thrice for 5 min each, rehydrated in 100% to 70% graded ethanol, and the slides then rinsed with distilled water for 5 min. For hematoxylin and eosin (H&E) staining, CRC tissues were fixed at 4 °C with 10% neutral buffered formalin overnight, transferred to 70% ethanol before being processed, paraffin-embedded, and then stained with H&E. For immunochemistry, paraffin-embedded sections were incubated at 60 °C for 15 min, deparaffinized, hydrated in 100%, 95% and 80% ethanol for 5 min each, rinsed in distilled water, then the slides incubated in DAKO citrate buffer (pH 6.0; Agilent Technologies Inc., Santa Clara, CA, USA) were microwaved for 20 min to enhance antigen retrieval. Three percent hydrogen peroxide (H2O2) in methanol was used to quench endogenous peroxidase activity. After the blocking process, sections were incubated at 4 °C overnight with antibodies against: CK1α (1:1,000), and Ki-67 (1:200), followed by incubation in HRP-conjugated anti-mouse secondary antibodies. UltraBrite Green IHC chromogen (#M1309, BioVision Inc., Milpitas, CA, USA) was used for detection, while hematoxylin was counterstain. Slides were read under microscope and Image analyzed using the ImageJ software.
PDX and tumor xenograft assays
Forty thousand DLD1 cells (for the tumor xenograft assays) or CRC-001/002 primary culture cells (for PDX assays) were subcutaneously injected into 5–6 weeks old NOD.CB17-Prkdcscid/NcrCrl (NOD/SCID) mice (Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., China). All mice experiments were approved by the Hwa Mei Hospital, University of Chinese Academy of Sciences Animal Care and Use Committee and performed according to the institution’s guidelines (HMH-2019-001). Each treatment group consisted of 10 mice. Mice were treated with 5 mg/kg Epiblastin A and/or 5 mg/kg Cetuximab by intraperitoneal injection every 72 h after injection of tumor cells. Normal saline was used as control. The mice were sacrificed a week after the last treatment was carried out and the tumors size and weight assessed. Tumor size (in mm3) was calculated using the formula: x × y2 × 0.52, where 0.52 is a constant to calculate ellipsoid volume, x is longest diameter and y is the shortest diameter of tumors. Tumors from each treatment group were removed, fixed in 10% formalin and photo-images taken.
Statistical analysis
All experiments were performed three times in triplicates and data represent mean ± standard deviation (SD). Comparison between groups was done using the student’s t-test and one-way ANOVA with Dunnett’s post hoc test. All statistical analyses were performed on the GraphPad Prism version 6.0 (GraphPad Software Inc., La Jolla, CA, USA). P value <0.05 was considered statistically significant.
Results
CRC cells aberrantly express CK1α at mRNA and protein levels
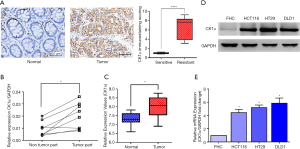
To understand the role of CK1α in CRC, we compared its expression profile in paired human colorectal tumor and normal samples, and observed that compared to its expression in normal tissues, CK1α was overexpressed in the CRC samples, as demonstrated by significantly higher positive nuclear immunostaining of CK1α in the tumor compared to the normal mucosal colon tissues (P<0.001) (Figure 1A). Using the q-PCR of oral cancer and their matched normal colon tissues from eight randomly-selected CRC patients, we also showed that the expression of CK1α mRNA was upregulated in the CRC samples in comparison to the matched normal colon tissues (P<0.05) (Figure 1B). This was validated by the findings from our analysis of the array expression profiling data of human colon cancer cohort freely obtained from the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) profiles (http://www.ncbi.nlm.nih.gov/geoprofiles) which showed that the relative median CK1α mRNA expression in human colon cancer was about 1.3-fold higher than in the normal colon samples (P<0.0001) (Figure 1C). In addition, we also demonstrate that compared to its expression in the human normal fetal colonic epithelial cell line FHC, the expression of CK1α was significantly upregulated in CRC cell lines, HCT116, HT29, and DLD1 both at the protein (Figure 1D) and mRNA (Figure 1E) levels, using western blot analysis and q-PCR, respectively.
High CK1α expression is implicated in the poor clinical outcome of patients with CRC
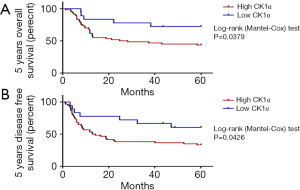
Because of the aberrant expression of CK1α in CRC cell line and tissue samples, we hypothesized that CK1α probably plays a role in the clinical outcome of patients with CRC. Thus, using the Kaplan-Meier survival curves followed by the time-stratified Mantel-Cox log-rank test, we demonstrated that patients with high CK1α expression were more likely to have significantly worse 5-year OS (1.58-fold, P=0.0379) or disease-free survival (DFS) (1.83-fold, P=0.0426), compared to their counterparts with low CK1α expression levels (Figure 2A,B). Median CK1α expression was used for defining high or low CK1α.
Pharmacological inhibition of CK1α using Epiblastin A significantly suppressed the viability of CRC cells without cytotoxicity to normal colonic cells, in vitro
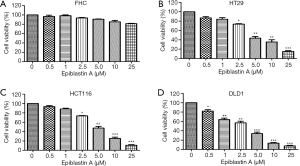
Having demonstrated that CK1α aberrantly expressed in CRC cells and implicated in the poor clinical outcome of patients with CRC, we examined the likely anticancer effect of pharmacologically targeting CK1α in CRC cells. Using FHC, HT29, HCT116 or DLD1 cells subjected to treatment with or without 0.5–25 µM for 48 h, we demonstrated that while Epiblastin A had no apparent effect on the viability of FHC cells (Figure 3A), it significantly suppressed the viability of HT29 (13.7–62.3%, P<0.001) (Figure 3B), HCT116 (5.4–9.9%, P<0.001) (Figure 3C), and DLD1 (20–94.6%, P<0.001) cells (Figure 3D).
Epiblastin A effectively inhibit CK1α expression in CRC clinical and PDX tumor samples, ex vivo
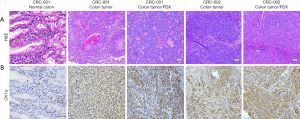
Evaluating the translational relevance of our findings, we performed a comparative evaluation of the expression profile of CK1α in normal colon (CRC-001) versus CRC tissue (CRC-001 or CRC-002), or PDX tumor (CRC-001 or CRC-002) samples. We observed that in comparison to its no/very weak expression in the CRC-001 normal colon samples, the expression of CK1α in the CRC-001 clinical or PDX tumor samples, and CRC-002 clinical or PDX tumor samples was significantly enhanced (Figure 4A,B). Subsequently, we examined the effect of treatment with 5 mg/kg Epiblastin A in tissue samples derived from the PDX tumor mice. We demonstrated that treatment with 5 mg/kg Epiblastin A concurrently suppressed the expression of CK1α and Ki-67 proteins in the treated mice, compared to their untreated control counterparts (Figure 5).
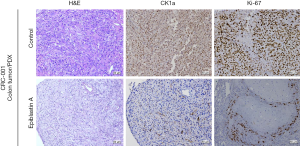
Epiblastin A inhibits tumor growth in CRC PDX and cell-line-derived tumor xenograft models by suppressing CK1α expression, in vivo
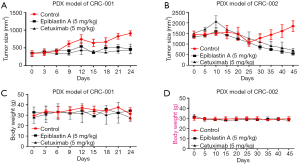
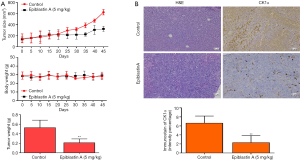
In addition, for translational significance, we evaluated the therapeutic efficacy of Epiblastin A compared to the treatment of choice for patients with metastatic CRC, by using PDX tumor mice models treated with 5 mg/kg Epiblastin A or same concentration of Cetuximab, After inoculation with 40,000 cells derived from CRC patient 001 or 002, we demonstrated that without any apparently significant effect on the mice body weights, the size of tumors in mice treated with 5 mg/kg Epiblastin A alone was comparable with that in the mice treated with 5 mg/kg Cetuximab alone, and significantly smaller than in the vehicle-treated mice at same time-point (P<0.05) (Figure 6A). This pattern was to some extent reproduced in 5 mg/kg Epiblastin A-treated CRC-002-based PDX tumor mice models (Figure 6B). Furthermore, using DLD1-derived tumor xenograft models treated with or without Epiblastin A, we demonstrated that similar to its effect in the PDX models, 5 mg/kg Epiblastin A markedly reduced tumor growth in the treated mice compared to the vehicle-treated control group (P<0.05), had no apparent effect on the mice body weights, and caused a 2.18-fold reduction in median tumor weight (P=0.04) over the time-course of the animal study (Figure 7A). Moreover, immunohistochemical staining of samples from the DLD1 tumor xenografts showed that the hitherto enhanced expression of CK1α was profoundly suppressed in the 5 mg/kg Epiblastin A-treated mice (1.42-fold, P=0.02) (Figure 7B).
Discussion
The overexpression of the serine/threonine kinase, CK1α, is increasingly implicated in cancer development, progression and prognosis, as evidenced by recent documentation of its role in the sustenance of oncogenic pathways including the Janus kinase/signal transducer and activator of transcription (JAK/STAT), phosphatidylinositol-4,5-biphosphate 3-kinase (PI3K)/AKT, and nuclear factor kappa B (NF-κB) (17), mediation of oncogenic autophagy in RAS-driven malignancies (18,19), negative modulation of p53 and maintenance of invasive phenotype (20,21). Representing a tumorigenic weak-spot, CK1α is apparently an attractive molecular target for effective anticancer therapy, including in CRC. Consistent with this understanding and recent demonstration of the inhibitory effect of CK1α inhibition on acquired drug resistance in epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (11), it is not out of place that the present study demonstrates that the Epiblastin A-induced pharmacologic targeting of CK1α inhibits primary and metastatic CRC in in vitro, ex vivo, in mice cell-line-derived, and PDX models.
Early diagnosis and adoption of effective targeted therapies based on our current knowledge of CRC biology and molecular characteristics prove to be essential to any successful treatment of patients with CRC; thus, considering the cumulative scientific evidences suggesting that the intratumoral heterogeneity and genetic constitution of CRC determine their response to targeted therapies and clinical outcome, in the present study, we demonstrated that CRC cells aberrantly express the serine/threonine kinase CK1α at mRNA and protein levels (Figure 1), and that this high CK1α expression is implicated in the poor clinical outcome of patients with CRC (Figure 2). This is consistent with the findings of another study which showed a positive correlation between serine threonine kinase 1 (STYK1) and disease progression, and established a functional association between the former and clinical outcome of patients with CRC as demonstrated by significantly shorter disease-specific survival (DSS) amongst patients with high STYK1 compared to those with low STYK1 expression (22). It is worth mentioning that our findings are also corroborated by those of Richter et al. (23) showing that the overexpression of CK1α correlates with poor survival in CRC based on Cox proportional hazard regression analysis of CK1α and selected clinicopathological variables in a cohort of 283 CRC patients, and published while the present study was in submission.
Furthermore, based on evidence supporting the designation of Epiblastin A as a potent and specific small molecule inhibitor of CK1α at IC50≤10 µM (14), we demonstrated that the pharmacological inhibition of CK1α using Epiblastin A significantly suppressed the viability of primary CRC (HT29), transforming growth factor (TGF)-β1/β2—rich RAS-mutant CRC (HCT116), and Dukes’ type C p53-mutant CRC cells without cytotoxicity to normal colonic cells, in vitro (Figure 3). These finding have translational significance not only because of the proven success of recently developed small-molecule kinase inhibitors in the treatment of various types of cancer, with approximately 150 kinase-targeting drugs in clinical phase trials and about 40 kinase inhibitors already approved by the United States FDA for treatment of malignancies including for lung and breast cancers (8-10), but also because our findings show that the therapeutic efficacy of Epiblastin A was not affected or limited by the presence and/or activity of RAS or TGF-β, against contemporary knowledge that RAS signaling drives the switch from a tumor-suppressive to tumor-promoting role of TGF-β, leading to enhanced tumor growth and metastatic dissemination of primary tumors (24), coupled with the fact that the oncogenic RAS and TGF-β signaling induce the progression of malignancies via activation of the transcriptional program of a predominant amino-terminal-truncated isoform of the master regulator of gene expression in epithelial cells, p63 (ΔNp63) (25).
Ensuring clinical relevance, our findings were not restricted to in vitro data alone, as we also showed that Epiblastin A effectively inhibits CK1α expression in CRC clinical and PDX tumor samples, ex vivo (Figures 4,5), and that Epiblastin A inhibits tumor growth in CRC PDX and cell-line-derived tumor xenograft models by suppressing CK1α expression, in vivo (Figure 6). While human cancer-derived cell lines remain the most broadly used study models for cancer biology and preclinical cancer therapy hypothetical efficacy testing, the reliability of this model remains debatable and their clinical relevance continue to be questioned (26,27). Thus, our findings replicating the in vitro Epiblastin A CK1α-mediated anti-CRC activity ex vivo and in vivo using both cell-line-derived and PDX tumor xenograft models addresses the question of clinical relevance, and is consistent with our growing understanding that “cancer tissue represents a promising approach for the pre-clinical evaluation of conventional and immune-mediated treatments and provides a platform for testing of innovative treatments” (28).
In conclusion, our findings indicate that Epiblastin A inhibits primary and metastatic CRC by targeting CK1α in vitro, ex vivo, and in mice cell-line-derived and PDX models. Thus, we establish the potential clinical relevance of the use of Epiblastin A as an effective anticancer treatment option in patients with CRC, regardless of nature or genetic constitution.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol for the study was reviewed and approved by the Hwa Mei Hospital, University of Chinese Academy of Sciences review board and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941-53. [Crossref] [PubMed]
- Duineveld LA, van Asselt KM, Bemelman WA, et al. Symptomatic and asymptomatic colon cancer recurrence: a multicenter cohort study. Ann Fam Med 2016;14:215-20. [Crossref] [PubMed]
- Sevá-Pereira G, Cypreste RN, Oliveira Filho JJ, et al. Recurrence pattern of rectal cancer after surgical treatment. Analysis of 122 patients in a tertiary care center. Journal of Coloproctology (Rio de Janeiro) 2018;38:18-23. [Crossref]
- Krarup PM, Nordholm-Carstensen A, Jorgensen LN, et al. Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann Surg 2014;259:930-8. [Crossref] [PubMed]
- Wong CK, Law WL, Wan YF, et al. Health-related quality of life and risk of colorectal cancer recurrence and All-cause death among advanced stages of colorectal cancer 1-year after diagnosis. BMC Cancer 2014;14:337. [Crossref] [PubMed]
- Maeda H, Kashiwabara K, Aoyama T, et al. Hazard rate of tumor recurrence over time in patients with colon cancer: implications for postoperative surveillance from three Japanese Foundation for Multidisciplinary Treatment of Cancer (JFMC) clinical trials. J Cancer 2017;8:4057-64. [Crossref] [PubMed]
- García-Aranda M, Redondo M. Targeting receptor kinases in colorectal cancer. Cancers (Basel) 2019; [Crossref] [PubMed]
- Bhullar KS, Lagarón NO, McGowan EM, et al. Kinase-targeted cancer therapies: progress, challenges and future directions. Mol Cancer 2018;17:48. [Crossref] [PubMed]
- Gross S, Rahal R, Stransky N, et al. Targeting cancer with kinase inhibitors. J Clin Invest 2015;125:1780-9. [Crossref] [PubMed]
- Jiang S, Zhang M, Sun J, et al. Casein kinase 1α: biological mechanisms and theranostic potential. Cell Commun Signal 2018;16:23. [Crossref] [PubMed]
- Stamos JL, Weis WI. The β-catenin destruction complex. Cold Spring Harb Perspect Biol 2013;5:a007898. [Crossref] [PubMed]
- Huart AS, MacLaine NJ, Meek DW, et al. CK1alpha plays a central role in mediating MDM2 control of p53 and E2F-1 protein stability. J Biol Chem 2009;284:32384-94. [Crossref] [PubMed]
- Ursu A, Illich DJ, Takemoto Y, et al. Epiblastin A induces reprogramming of epiblast stem cells into embryonic stem cells by inhibition of casein kinase 1. Cell Chem Biol 2016;23:494-507. [Crossref] [PubMed]
- Köstler WJ, Zielinski CC. Targeting receptor tyrosine kinases in cancer. In: Wheeler DL, Yarden Y. Receptor tyrosine kinases: structure, functions and role in human disease. New York: Springer, 2015:225-78.
- Voigt W. Sulforhodamine B assay and chemosensitivity. Methods Mol Med 2005;110:39-48. [PubMed]
- Manni S, Carrino M, Piazza F. Role of protein kinases CK1α and CK2 in multiple myeloma: regulation of pivotal survival and stress-managing pathways. J Hematol Oncol 2017;10:157. [Crossref] [PubMed]
- Lee SY, Kim H, Li CM, et al. Casein kinase-1γ1 and 3 stimulate tumor necrosis factor-induced necroptosis through RIPK3. Cell Death Dis 2019;10:923. [Crossref] [PubMed]
- Zhang F, Virshup DM, Cheong JK. Oncogenic RAS-induced CK1α drives nuclear FOXO proteolysis. Oncogene 2018;37:363-76. [Crossref] [PubMed]
- Mazzoldi EL, Pastò A, Ceppelli E, et al. Casein kinase 1 delta regulates cell proliferation, response to chemotherapy and migration in human ovarian cancer cells. Front Oncol 2019;9:1211. [Crossref] [PubMed]
- Janovska P, Verner J, Kohoutek J, et al. Casein kinase 1 is a therapeutic target in chronic lymphocytic leukemia. Blood 2018;131:1206-18. [Crossref] [PubMed]
- Chen S, Wang Q, Wang L, et al. REGγ deficiency suppresses tumor progression via stabilizing CK1ε in renal cell carcinoma. Cell Death Dis 2018;9:627. [Crossref] [PubMed]
- Richter J, Kretz AL, Lemke J, et al. CK1α overexpression correlates with poor survival in colorectal cancer. BMC Cancer 2018;18:140. [Crossref] [PubMed]
- Grusch M, Petz M, Metzner T, et al. The crosstalk of RAS with the TGF-β family during carcinoma progression and its implications for targeted cancer therapy. Curr Cancer Drug Targets 2010;10:849-57. [Crossref] [PubMed]
- Vasilaki E, Morikawa M, Koinuma D, et al. Ras and TGF-β signaling enhance cancer progression by promoting the ΔNp63 transcriptional program. Sci Signal 2016;9:ra84. [Crossref] [PubMed]
- Gillet JP, Varma S, Gottesman MM. The clinical relevance of cancer cell lines. J Natl Cancer Inst 2013;105:452-8. [Crossref] [PubMed]
- Gillet JP, Calcagno AM, Varma S, et al. Redefining the relevance of established cancer cell lines to the study of mechanisms of clinical anti-cancer drug resistance. Proc Natl Acad Sci U S A 2011;108:18708-13. [Crossref] [PubMed]
- Muraro MG, Muenst S, Mele V, et al. Ex-vivo assessment of drug response on breast cancer primary tissue with preserved microenvironments. Oncoimmunology 2017;6:e1331798. [Crossref] [PubMed]


