
MAP kinase-interacting kinase 1 (MNK1) plays as a tumor suppressor in bladder cancer
Introduction
Bladder cancer (BCa) ranks as the tenth most common malignancy in 2018 worldwide, with estimated 549,000 new cases and 200,000 deaths each year (1). Despite the advances in surgery and other adjuvant treatment, the overall survival (OS) of BCa patients has not been significantly prolonged with 5-year survival rate lower than 50% in locally advanced BCa (2-5). Although some researches have brought kinds of promising results, there is still no specialized drug approved for treatment of BCa (6-11). Meanwhile, though PD-1/PD-L1 has been introduced in the recent EAU guideline for the treatment of metastatic BCa, its efficacy is still far from satisfactory. Hence, researches of targeted therapy are still the focus, which contains illuminating the potential molecular mechanisms in tumorigenesis as well as metastasis of BCa and finding some potential treatment target and effective new drugs.
MAP kinase-interacting kinase 1 (MNK1), which encodes a serine/threonine protein kinase that interacts with each other, is activated by ERK (extracellular regulated kinase) and p38 MAP (mitogen-activated protein) kinases, and is thought to play crucial roles in mediating a variety of physiological processes including inflammation, development, autophagy, apoptosis, oncogenesis, etc. (12,13). The MNK family, consisting of MNK1 and MNK2, was reported to play a crucial role in mRNA translation via their well-characterized substrate eukaryotic initiation factor 4E (eIF4E), which is a key determinant of the Ras/Raf/MEK/ERK and PI3K/Akt/mTOR signaling (14,15). Therefore, the MNK family is considered as key mediators of proinflammatory cytokines, cytokine signaling, oncogenic progression and drug resistance (14). So far, MNK1 has been reported to be highly expressed and function in multiple tumors, such as gliomas (16,17), Wilms’ tumor (18), hepatocellular carcinoma (19), epithelial ovarian cancer (20), breast cancer (21-23) and hematological malignancies (24). Moreover, inhibition of MNK1 suppresses the oncogenic potential of melanoma (25), ovarian cancer (20), non-small cell lung cancer (26), prostate cancer (27), leukemia (28), gliomas (29) and malignant peripheral nerve sheath tumors (30). However, MNK1’s expression pattern and biological roles in BCa remains unknown.
Therefore, the objective of the present study was to describe the expression pattern and biological function of MNK1 in BCa, and to explore its potential role in BCa prognosis.
Methods
Cell lines and cell culture
The human immortal normal bladder epithelial cell SV-HUC-1 and BCa cell lines (T24, TCCSUP, 5637, UMUC3, J82 and RT4) were purchased from the American Type Culture Collection, while BIU87 cell was acquired from China Center for Type Culture Collection (Wuhan, China). All cell lines were cultured in either DMEM or RPMI 1640 medium with 10% fetal bovine serum (16000044, Gibco, USA), 100 U/mL streptomycin and 100 U/mL penicillin in a 37 °C humidified atmosphere with 5% CO2.
Patients and specimens
One hundred and twenty-nine paraffin-embedded BCa tissues and 12 paired fresh BCa specimens and adjacent normal bladder tissues were obtained from patients who had undergone radical cystectomy from 2000 to 2013 in Sun Yat-sen University Cancer Center (SYSUCC). Paraffin-embedded tissues were subjected to immunohistochemistry (IHC) analysis. Fresh samples were collected immediately after resection, stored in liquid nitrogen and further used for quantitative real-time PCR (qPCR) as well as western blotting assays. All patients included in our study had a definite pathological diagnosis and follow-up data. The median follow-up time was 57.0 months (range, 0.2–197.0 months). None of these patients received new adjuvant chemotherapy. Clinical information included age, gender, tumor diameter, tumor number, histological grade, T status, N status and situation of progression was retrieved from electronic medical records system.
RNA extraction, reverse transcription and qPCR
Total RNA was extracted from cells and tissues by TRIzol reagent (10296028, Invitrogen, USA) according to manufacturer’s instructions and then reversely transcribed into cDNA using PrimeScript™ Master Mix (RR036A, TaKaRa, USA). cDNA was later run in standard qPCR instruments. Primer sequences used in our study were listed in Table 1. Expression data were normalized by GAPDH and showed as 2-(ΔCt of gene).
Table 1
| Target | Sequences (5'-3') |
|---|---|
| MNK1 forward primer | TGCTTGGAGAGGGAGCCTAT |
| MNK1 reverse primer | TGCCCTGCTTGTTTCTCGAT |
| GAPDH forward primer | GGAGCGAGATCCCTCCAAAAT |
| GAPDH reverse primer | GGCTGTTGTCATACTTCTCATGG |
| MNK1-shC | GCTTCGCGCCGTAGTCTTA |
| MNK1-sh1 | GCCAGAACAAGCTGTTTGAAA |
| MNK1-sh2 | GCAAGTATGAGTTTCCTGACA |
| MNK1-sh3 | GGAAAGCAATCACTTCTCACT |
| MNK1-sh4 | GCTTTATTTCATTTGGGATTT |
IHC analysis
IHC assays were carried out to detect MNK1 expression in a cohort of 129 BCa specimens by a similar method described previously (31). In brief, all paraffin-embedded tissues on 4 µm sections were deparaffinized by baking and immersing xylenes, rehydrated by gradient ethanol solutions, and extinguished endogenous peroxidase with 3% hydrogen peroxide. Sections were then submerged into sodium citrate buffer (PH 6.0) and subjected to antigenic retrieval using high pressure, followed by blocking nonspecific sites with 5% bovine serum albumin. Next, rabbit anti-MNK1 antibody (1:500 dilution, ab109102, Abcam, UK) was used to incubate the slides at 4 °C overnight. Meanwhile, PBS replaced the primary antibody as the negative control. In the next day, after being washed and subsequently processed with prediluted anti-rabbit secondary antibody, the slides were immunostained by diaminobenzidine (Dako, Denmark), counterstained with 10% Mayer’s hematoxylin, dehydrated, and finally mounted in Crystal Mount for observation under the microscope.
Two independent pathologists, who were blinded to the clinical parameters, separately scored and evaluated the staining results. The scores were determined based on the staining intensity and the proportion of positively stained cells. The staining intensity was graded as follows: 0 (negative staining), 1 (light yellow), 2 (yellow brown) and 3 (brown). The proportion of positively stained cells was classified as 0 (0%), 1 (1–24%), 2 (25–49%), 3 (50–74%) and 4 (≥75%). The final IHC score was calculated through multiplying score of staining intensity by that of positive proportion, and ultimately ranged from 0 to 12. As the median score of these 129 BCa tissues was 3, score ≤3 was defined as low MNK1 expression while score >3 as high MNK1 expression.
Protein extraction and western blotting analysis
Total protein from tissues or cultured cells were harvested by RIPA buffer (P0013B, Beyotime, USA) with the proteinase inhibitor cocktail (4693132001, Roche, Switzerland). Denatured protein samples in equal quantities were loaded on SDS-polyacrylamide gels, and transferred onto polyvinylidene difluoride membranes. The membrane was blocked with 5% defatted milk and then incubated with corresponding primary antibodies, followed by the horseradish peroxidase conjugated secondary antibody. Finally, proteins were visualized by ECL reagents (WP20005, Thermo Fisher Scientific, USA). Anti-MNK1 rabbit antibody (1:1,000 dilution, #2195, Cell Signaling Technology, USA) and anti-GAPDH mouse antibody (1:1,000 dilution, ProteinTech Group Inc, USA) were used in this assay.
Construction of the recombinant lentiviral vector
Commercialized lentiviral vectors shRNA against MNK1 (Table 1) and overexpressed MNK1 (Vector: pEZ-Lv105) were bought from GeneCopoeia (Rockville, USA). The stable BC cells were then picked out with 0.5 µg/mL puromycin for 7 days.
Cell counting kit-8 (CCK-8) assay
The viability of BCa cells was measured by CCK-8 reagent (C0038, Beyotime, USA). Briefly, a quantity of 2,000 BCa cells per well were seeded onto 96-well plates and then measured for each day according to the manufacturer’s instructions.
Xenograft assay
Fourteen four-week-old BALB/c female nude mice were purchased from Charles River Laboratories (Beijing, China), and then equally and randomly divided into two groups. A total of 6×106 TCCSUP cells (MNK1-shC or MNK1-sh2) were subcutaneously inoculated into the right back of each mouse. About 4 weeks later, mice were all sacrificed to determine the weight and volume of each tumor (diameter of subcutaneous tumor ≤1.5 cm), in which tumor volume was equal to 1/2 L × W2 (L: length, W: width).
Statistical analysis
All data were analyzed by SPSS v.20.0 statistical software (IBM, USA). Data derived from three independent experiments were expressed as mean ± standard deviation and compared by two-tailed Student’s t-test. Chi-square tests were performed to evaluate the associations between MNK1 expression and clinicopathological characteristics. Kaplan-Meier curves and log-rank tests were adopted to compare the difference in OS between various MNK1 expression level, and multivariate Cox regression analyses were used for evaluating the independent role of MNK1 in BCa prognosis. A P value <0.05 (two-sided) was considered as statistically significant.
Results
MNK1 was frequently down-regulated in BCa
To investigate MNK1’s expression in BCa, we first conducted bioinformatics analysis in human publicly available dataset Gene Expression Omnibus (GEO) (32). As shown in Figure 1A, there was no significant difference of MNK1 mRNA expression between para-carcinoma bladder epitheliums and primary BCa tissues, whereas MNK1 mRNA expression in recurrent BCa tissues was apparently lower than those in the para-carcinoma normal epithelia (P=0.002) or primary BCa specimens (P=0.010), which indicated that MNK1 deficiency happened in advanced BCa and might be a protective factor in BCa. Analogously, in our cohort of 12 paired fresh BCa tissues and the adjacent normal epithelia, MNK1 mRNA expression of BCa tissues was at lower level than the normal bladder epithelia (P=0.024, Figure 1B). Furthermore, in the western blotting assay, 11 of 12 human BCa tissues had obviously low MNK1 protein expression compared with the paired normal bladder tissues (P=0.004, Figure 1C). Consistently, compared to the immortal normal bladder cell SV-HUC-1, MNK1 protein expression was significantly lower in seven human BCa cell lines (Figure 1D). These preliminary data showed that in contrasts with other cancers, MNK1 was usually down-regulated in BCa.
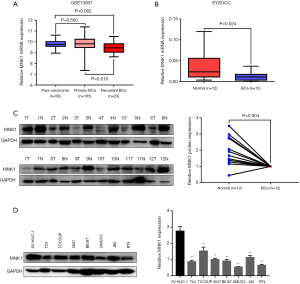
Low MNK1 expression was closely related to poor prognosis in BCa
To evaluate the clinical significance of MNK1 expression in BCa, we detected the protein expression level of MNK1 by IHC in a large cohort of 129 BCa patients. We found that MNK1 expressed at both the nuclear and the cytoplasm. Meanwhile, advanced BCa samples were generally examined with low expression of MNK1, whereas superficial BCa specimens or normal bladder epithelia with high level (Figure 2A,B). In association analysis between MNK1 expression and clinicopathological characteristics (Table 2), we found that 68/129 (52.7%) patients displayed low expression of MNK1, and low MNK1 expression was obviously associated with advanced T status (P=0.011). Nevertheless, no significant association was observed between MNK1 expression and other clinicopathological characteristics, including age, gender, tumor diameter, tumor number, histological grade, N status and BCa progression.
Table 2
| Parameters | MNK1 expression | P valuea | ||
|---|---|---|---|---|
| All | Low, n (%) | High, n (%) | ||
| Total | 129 | 61 (47.3) | 68 (52.7) | |
| Age (years) | 0.059 | |||
| ≤60 | 66 | 29 (43.9) | 37 (56.1) | |
| >60 | 63 | 32 (50.8) | 31 (49.2) | |
| Gender | 0.959 | |||
| Female | 15 | 7 (46.7) | 8 (53.3) | |
| Male | 114 | 54 (47.4) | 60 (52.6) | |
| Tumor diameter (cm) | 0.249 | |||
| ≤3 | 49 | 20 (40.8) | 29 (59.2) | |
| >3 | 80 | 41 (51.2) | 39 (48.8) | |
| Tumor number | 0.069 | |||
| 1 | 61 | 34 (55.7) | 27 (44.3) | |
| >1 | 68 | 27 (39.7) | 41 (60.3) | |
| Histological grade | 0.762 | |||
| Low | 16 | 7 (43.8) | 9 (56.2) | |
| High | 113 | 54 (47.8) | 59 (52.2) | |
| T status | 0.011* | |||
| T1−T2 | 68 | 25 (36.8) | 43 (63.2) | |
| T3−T4 | 61 | 36 (59.0) | 25 (41.0) | |
| N status | 0.334 | |||
| N− | 98 | 44 (44.9) | 54 (55.1) | |
| N+ | 31 | 17 (54.8) | 14 (45.2) | |
| Progression | 0.616 | |||
| No | 112 | 52 (46.4) | 60 (53.6) | |
| Yes | 17 | 9 (52.9) | 8 (47.1) | |
a, Chi-square test; *, P<0.05. BCa, bladder cancer.
Kaplan-Meier curves and log-rank test suggested that that BCa patients with low MNK1 expression had significantly shorter OS time than those with high MNK1 expression (P=0.002, median survival time: 33.0 vs. 80.0 months, Figure 2C). We also observed that patients who remained alive usually had high MNK1 expression (Figure 2D). In the subgroup analysis, the association between low MNK1 expression and poorer OS was only observed in T1-2 and N− BCa (P=0.004 and <0.001, Figure 2E). Multivariate Cox regression analysis suggested that tumors’ T status (P=0.018, hazard ratio: 2.35, 95% confidence intervals: 1.16–4.80) and MNK1 expression (P=0.037, hazard ratio: 0.49, 95% confidence intervals: 0.25–0.96) were independent prognostic factors in BCa patients (Table 3). Taken together, these above results indicated that low MNK1 expression suggested poor clinical outcome in BCa patients.
Table 3
| Parameters | Median OS (months) | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| Age (years) (>60 vs. ≤60) | 41.0 vs. 74.5 | 1.60 (0.88, 2.91) | 0.123 | 1.40 (0.74, 2.64) | 0.297 | |
| Gender (male vs. female) | 58.5 vs. 36.0 | 1.20 (0.43, 3.36) | 0.725 | 1.15 (0.40, 3.32) | 0.799 | |
| Tumor diameter (cm) (>3 vs. ≤3) | 60.0 vs. 44.0 | 1.16 (0.62, 2.16) | 0.642 | 1.04 (0.55, 1.97) | 0.901 | |
| Tumor number (>1 vs. 1) | 60.0 vs. 44.0 | 0.87 (0.48, 1.57) | 0.637 | 1.12 (0.60, 2.09) | 0.733 | |
| Histological grade (high vs. low) | 57.0 vs. 58.0 | 1.10 (0.43, 2.79) | 0.841 | 0.97 (0.37, 2.54) | 0.954 | |
| T status (T3−T4 vs. T1−T2) | 36.0 vs. 81.0 | 3.15 (1.68, 5.92) | <0.001* | 2.35 (1.16, 4.80) | 0.018* | |
| N status (N+ vs. N−) | 24.0 vs. 74.5 | 1.80 (0.92, 3.55) | 0.088 | 1.25 (0.59, 2.67) | 0.561 | |
| MNK1 expression (high vs. low) | 80.0 vs. 33.0 | 0.38 (0.21, 0.71) | 0.002* | 0.49 (0.25, 0.96) | 0.037* | |
*, P<0.05. BCa, bladder cancer; OS, overall survival; HR, hazard ratio; CI, confidence intervals.
Down-regulation of MNK1 promoted proliferation of BCa both in vitro and in vivo
To confirm the tumor-suppressing role of MNK1 in BCa progression, we then performed cellular experiments. MNK1 stable knockdown and over-expression were established in TCCSUP and T24 cell lines (Figure 3A). CCK-8 assays showed that down-regulation of MNK1 displayed a significantly enhanced proliferation pattern compared with control cells, while MNK1 up-regulation showed an attenuated proliferation ability (Figure 3B), which meant that MNK1 impaired BCa cells proliferation in vitro.
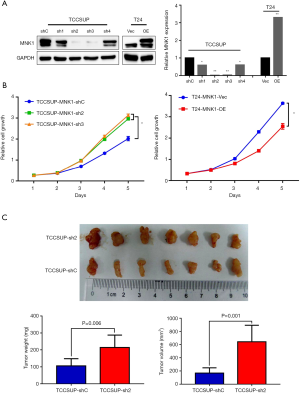
To further investigate the effects of MNK1 in BCa tumorigenesis in vivo, xenograft assays were performed by subcutaneously injecting MNK1 knockdown TCCSUP cells or the control cells into BALB/c-nu mice. We observed that mice infected with MNK1-silenced cells generated heavier in weight and bigger in size tumors than those in the TCCSUP-control mice (Figure 3C), which indicated that down-regulation of MNK1 apparently promoted tumor growth in vivo. In conclusion, knockdown of MNK1 strengthened the proliferation ability of BCa cells both in vitro and in vivo.
Discussion
Up to now, BCa has been considered to be a high economic-burdensome malignancy which is causing direct threat to patients’ health. For one thing, non-muscle-invasive BCa (NMIBC) is apt to recur or progress into muscle-invasive BCa (MIBC), which forces patients to suffer repeated and invasive cystoscopy, as well as the postoperative complications like ureteral fistula or urinary tract infection in radical cystectomy for MIBC (3,5). For another, considerable MIBC would finally develop distant metastasis causing to an extremely poor prognosis despite adding multiple therapeutic methods including adjuvant chemotherapy or radiotherapy (2). However, tiny minority of the biomarkers found in basic researches have been truly applied for diagnosis and monitoring of BCa due to the complicated biological behavior of BCa. Hence, it is still urgent to identify effective biomarkers for diagnosing and monitoring BCa.
MNK1, which is activated by the ERK and p38 MAP kinases, plays an important role in autophagy, oncogenesis and drug resistance (12-14). To date, MNK1 has been found to be over-expressed in various cancers, including breast cancer, hepatocellular carcinoma and ovarian cancer, etc. Among these studies, most focused on that MNK1 played roles in MNKs/eIF4E axis to affect translational regulation and then modulated tumorigenesis and tumor progression (23,28,33,34), because eIF4E was the intersection point of the Ras/Raf/MEK/ERK and PI3K/Akt/mTOR signal path (15), which was well known to be related to initiation and development of tumors (35). For now, inhibitor of MNK1 like CGP57380 (S7421, Selleck, USA) have appeared and shown to have anti-tumor potentials (26,33). However, whether or not MNK1 plays an oncogenic role in BCa like other cancers remains unclear and deserves exploration.
To the best of our knowledge, it is the first study that explored the relationship between MNK1 expression and BCa prognosis. In the current study, we found that MNK1 was down-regulated in BCa tissues and cell lines, especially at the advanced stage, which was different from other tumors. Low expression of MNK1 indicated a shorter OS of BCa patients and was negatively correlated to advanced T status. Besides, we found that MNK1 expression was an independent predictive marker for BCa patients especially who had a better prognostic value in T1-2 and N−. Taken together, these findings indicated that low MNK1 expression was associated with tumor progression and could be a protective element and valuable prognostic factor of BCa. Moreover, we found that silence of MNK1 could enhance the proliferation ability of BCa cells both in vitro and in vivo, which indicated that inhibitors of MNK1 might have anti-cancer potential for BCa. When it comes to the unusual role of MNK1 in BCa compared with other cancers, we consider that MNK1 might function in a distinct and dominant signaling in BCa, which mainly accounts for its ultimate inverse role. Meanwhile, it is not a rare event that a gene might play diverse parts in different types of cancer.
However, there is no doubt that limitations exist in our study. For example, the role of MNK1 in cell migration or invasion of BCa needs to be illuminated in vitro and in vivo assays. Besides, more in-depth tumorigenesis mechanism of MNK1 in BCa remains to be further disclosed.
Conclusions
Our present study suggested that the deficiency of MNK1, frequently seen in advanced BCa, was significantly associated with poor prognosis of BCa patients and could serve as a potential prognostic biomarker in BCa. MNK1 could weaken proliferation of BCa cells and played as a suppressor gene in BCa.
Acknowledgments
The authors would like to thank all our participants in this study.
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.67). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of Sun Yat-sen University Cancer Center (B2018-061). All patients signed written informed consent for surgery and for the study. The study was carried out in accordance with the principles expressed in the Declaration of Helsinki as revised in 2013. All procedures involving animals had got the approval from the animal ethics committee of our center (L102012017110J).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Choi W, Czerniak B, Ochoa A, et al. Intrinsic basal and luminal subtypes of muscle-invasive bladder cancer. Nat Rev Urol 2014;11:400-10. [Crossref] [PubMed]
- Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer 2015;15:25-41. [Crossref] [PubMed]
- Alfred Witjes J, Lebret T, Comperat EM, et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur Urol 2017;71:462-75. [Crossref] [PubMed]
- Prasad SM, Decastro GJ, Steinberg GD, et al. Urothelial carcinoma of the bladder: definition, treatment and future efforts. Nat Rev Urol 2011;8:631-42. [Crossref] [PubMed]
- Xie R, Chen X, Chen Z, et al. Polypyrimidine tract binding protein 1 promotes lymphatic metastasis and proliferation of bladder cancer via alternative splicing of MEIS2 and PKM. Cancer Lett 2019;449:31-44. [Crossref] [PubMed]
- Xiao KH, Teng K, Ye YL, et al. Kinesin family member C1 accelerates bladder cancer cell proliferation and induces epithelial-mesenchymal transition via Akt/GSK3beta signaling. Cancer Sci 2019;110:2822-33. [Crossref] [PubMed]
- Chen Z, Chen X, Xie R, et al. DANCR Promotes Metastasis and Proliferation in Bladder Cancer Cells by Enhancing IL-11-STAT3 Signaling and CCND1 Expression. Mol Ther 2019;27:326-41. [Crossref] [PubMed]
- Chen X, Xie R, Gu P, et al. Long Noncoding RNA LBCS Inhibits Self-Renewal and Chemoresistance of Bladder Cancer Stem Cells through Epigenetic Silencing of SOX2. Clin Cancer Res 2019;25:1389-403. [Crossref] [PubMed]
- Chen M, Wu R, Li G, et al. Motor neuron and pancreas homeobox 1/HLXB9 promotes sustained proliferation in bladder cancer by upregulating CCNE1/2. J Exp Clin Cancer Res 2018;37:154. [Crossref] [PubMed]
- Chen X, Gu P, Xie R, et al. Heterogeneous nuclear ribonucleoprotein K is associated with poor prognosis and regulates proliferation and apoptosis in bladder cancer. J Cell Mol Med 2017;21:1266-79. [Crossref] [PubMed]
- Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 2004;68:320-44. [Crossref] [PubMed]
- Buxade M, Parra-Palau JL, Proud CG. The Mnks: MAP kinase-interacting kinases (MAP kinase signal-integrating kinases). Front Biosci 2008;13:5359-73. [Crossref] [PubMed]
- Joshi S, Platanias LC. Mnk kinase pathway: Cellular functions and biological outcomes. World J Biol Chem 2014;5:321-33. [Crossref] [PubMed]
- Hou J, Lam F, Proud C, et al. Targeting Mnks for cancer therapy. Oncotarget 2012;3:118-31. [Crossref] [PubMed]
- Grzmil M, Morin P Jr, Lino MM, et al. MAP kinase-interacting kinase 1 regulates SMAD2-dependent TGF-beta signaling pathway in human glioblastoma. Cancer Res 2011;71:2392-402. [Crossref] [PubMed]
- Grzmil M, Huber RM, Hess D, et al. MNK1 pathway activity maintains protein synthesis in rapalog-treated gliomas. J Clin Invest 2014;124:742-54. [Crossref] [PubMed]
- Liu K, He B, Xu J, et al. miR-483-5p Targets MKNK1 to Suppress Wilms' Tumor Cell Proliferation and Apoptosis In Vitro and In Vivo. Med Sci Monit 2019;25:1459-68. [Crossref] [PubMed]
- Wang X, Wang Y, Zhang Q, et al. MAP Kinase-Interacting Kinase 1 Promotes Proliferation and Invasion of Hepatocellular Carcinoma and Is an Unfavorable Prognostic Biomarker. Med Sci Monit 2018;24:1759-67. [Crossref] [PubMed]
- Hou S, Du P, Wang P, et al. Significance of MNK1 in prognostic prediction and chemotherapy development of epithelial ovarian cancer. Clin Transl Oncol 2017;19:1107-16. [Crossref] [PubMed]
- Pinto-Diez C, Garcia-Recio EM, Perez-Morgado MI, et al. Increased expression of MNK1b, the spliced isoform of MNK1, predicts poor prognosis and is associated with triple-negative breast cancer. Oncotarget 2018;9:13501-16. [Crossref] [PubMed]
- Chrestensen CA, Shuman JK, Eschenroeder A, et al. MNK1 and MNK2 regulation in HER2-overexpressing breast cancer lines. J Biol Chem 2007;282:4243-52. [Crossref] [PubMed]
- Wheater MJ, Johnson PW, Blaydes JP. The role of MNK proteins and eIF4E phosphorylation in breast cancer cell proliferation and survival. Cancer Biol Ther 2010;10:728-35. [Crossref] [PubMed]
- Worch J, Tickenbrock L, Schwable J, et al. The serine-threonine kinase MNK1 is post-translationally stabilized by PML-RARalpha and regulates differentiation of hematopoietic cells. Oncogene 2004;23:9162-72. [Crossref] [PubMed]
- Zhan Y, Guo J, Yang W, et al. MNK1/2 inhibition limits oncogenicity and metastasis of KIT-mutant melanoma. J Clin Invest 2017;127:4179-92. [Crossref] [PubMed]
- Wen QY, Wang WY, Luo JD, et al. CGP57380 enhances efficacy of RAD001 in non-small cell lung cancer through abrogating mTOR inhibition-induced phosphorylation of eIF4E and activating mitochondrial apoptotic pathway. Oncotarget 2016;7:27787-801. [Crossref] [PubMed]
- D'Abronzo LS, Bose S, Crapuchettes ME, et al. The androgen receptor is a negative regulator of eIF4E phosphorylation at S209: implications for the use of mTOR inhibitors in advanced prostate cancer. Oncogene 2017;36:6359-73. [Crossref] [PubMed]
- Lim S, Saw TY, Zhang M, et al. Targeting of the MNK-eIF4E axis in blast crisis chronic myeloid leukemia inhibits leukemia stem cell function. Proc Natl Acad Sci U S A 2013;110:E2298-307. [Crossref] [PubMed]
- Ueda T, Sasaki M, Elia AJ, et al. Combined deficiency for MAP kinase-interacting kinase 1 and 2 (Mnk1 and Mnk2) delays tumor development. Proc Natl Acad Sci U S A 2010;107:13984-90. [Crossref] [PubMed]
- Lock R, Ingraham R, Maertens O, et al. Cotargeting MNK and MEK kinases induces the regression of NF1-mutant cancers. J Clin Invest 2016;126:2181-90. [Crossref] [PubMed]
- Gu P, Chen X, Xie R, et al. lncRNA HOXD-AS1 Regulates Proliferation and Chemo-Resistance of Castration-Resistant Prostate Cancer via Recruiting WDR5. Mol Ther 2017;25:1959-73. [Crossref] [PubMed]
- Clough E, Barrett T. The Gene Expression Omnibus Database. Methods Mol Biol 2016;1418:93-110. [Crossref] [PubMed]
- Liu S, Zha J, Lei M. Inhibiting ERK/Mnk/eIF4E broadly sensitizes ovarian cancer response to chemotherapy. Clin Transl Oncol 2018;20:374-81. [Crossref] [PubMed]
- Waskiewicz AJ, Johnson JC, Penn B, et al. Phosphorylation of the cap-binding protein eukaryotic translation initiation factor 4E by protein kinase Mnk1 in vivo. Mol Cell Biol 1999;19:1871-80. [Crossref] [PubMed]
- Wendel HG, Silva RL, Malina A, et al. Dissecting eIF4E action in tumorigenesis. Genes Dev 2007;21:3232-7. [Crossref] [PubMed]


