
Extrapancreatic solid pseudopapillary neoplasm: report of a unique case of primary posterior mediastinum origin and review of the literature
Introduction
Solid pseudopapillary neoplasm (SPN) is an uncommon and low grade malignant tumor that traditionally occurs in pancreas. SPN accounts for 0.3% to 2.7% of all pancreatic exocrine tumors (1). SPNs have been seen in men and women aged 2 to 85 years but are most frequently associated with younger women aged 20 to 30 years, with a slight predilection for the pancreatic head and tail (2,3). Extrapancreatic SPN is extremely rare. The first extrapancreatic SPN was reported by Ishikawa et al. in 1990 (4). To our knowledge, only approximately 50 cases of extrapancreatic SPNs have been reported so far in the English literature (5). Testis/paratesticular area and ovary are the most common location of extrapancreatic SPN (6,7), followed by retroperitoneum, mesentery, omentum, and so on. SPN in mediastinum has not been reported in the literature. Herein, we report an additional extrapancreatic SPN in a 62-year-old woman, and this is the first case of mediastinal SPN.
Case presentation
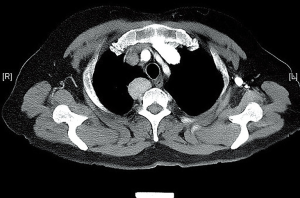
A 62-year-old asymptomatic female was admitted to The Affiliated Hospital of Qingdao University because of a mass in posterior mediastinum detected incidentally by chest computerized tomographic (CT) scan during her annual checkup. The CT scan revealed a 30 mm solid nodule with well-defined outline in right posterior mediastinum (Figure 1). The later abdominal CT scan showed no mass in the pancreas.
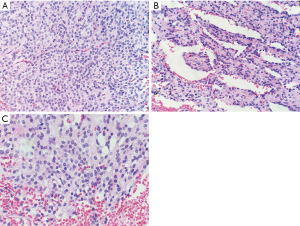
A video-assisted thoracoscope tumorectomy was performed. Grossly, the tumor showed a 3.0×2.4×2.1 cm well-defined reddish-brown nodule with multiple foci of hemorrhage on the cut surface. Microscopic examination of the tumor revealed an apparently well-defined but nonencapsulated neoplasm. The tumor was composed of solid cellular sheets and nests of cells with an epithelioid appearance (Figure 2A). Some pseudopapillary areas could also be identified in the tumor (Figure 2B). The tumor cells were interrupted by some delicate fibrous septa. Capillary network and blood sinus-like structures could be found in the tumor. The tumor cells had round to oval nuclei with finely dispersed chromatin. The cytoplasm was rich and lightly eosinophilic. In some foci, the tumor cells had vacuolated cytoplasm (Figure 2C). Mitotic figures and necrosis were not seen. The resection margins are free of tumor.
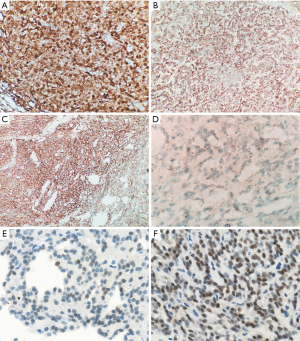
Immunohistochemical staining was performed to confirm the diagnosis. The tumor cells showed nuclear and cytoplasmic staining for β-catenin and S-100 protein, nuclear staining for cyclin D1 and TFE3, focal nuclear staining for SOX-11, membranous staining for CD56 and CD10, paranuclear dot-like staining for CD99, cytoplasmic staining for vimentin and membranous and cytoplasmic staining for CD34 (Figure 3). They were negative for cytokeratin (AE1/AE3), E-cadherin, WT-1, synaptophysin, chromogranin, neuron specific endolase (NSE), CD31, ERG protein, glial fibrillary acidic protein (GFAP), signal transducer and activators of transcription 6 (STAT-6), smooth muscle actin (SMA), α-inhibin, epithelial membrane antigen (EMA), and progesterone receptor. Ki-67 stained 1% to 2% of the tumor cells.
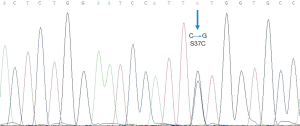
DNA sequencing chromatogram demonstrates a TCT (serine)→TGT (cysteine) mutation in codon 37 in exon 3 of the β-catenin (CTNNB1) gene (Figure 4).
The patient only underwent tumor resection, not chemotherapy or radiotherapy. The patient has been followed up for 5 months with no recurrence or metastasis.
Discussion
SPN is a mysterious entity, whose cell of origin has yet to be revealed. Two basic hypothesises for the SPN origin has been proposed: (I) pancreatic progenitor cells and (II) genital ridge-related cells (8,9). In some extrapancreatic SPNs, ectopic pancreatic tissue can be observed (4,10,11). Like intrapancreatic SPNs, these cases might origin from the pancreatic progenitor cells in the ectopic pancreatic tissue. In most cases, like the current one, there are no ectopic pancreatic tissue identified. The hypothesis of genital ridge-related cells might explain why the SPNs are lacking of ectopic pancreatic tissue. Ectopic genital ridge-related stem cells might be the origin of our case. Mediastinal pancreatic ectopia is very rare, and all of those cases reported were in the anterior mediastinum (12). In the current case, the tumor is located in posterior mediastinum, where the pancreatic ectopia has not been reported. Therefore, the second hypothesis is more suitable for our case.
Any single feature of SPNs is rather nondescript, overlapping with other tumors, such as neuroendocrine tumors. Nevertheless, the constellation of histologic features is characteristic, especially when one or more of the following features are present: myxohyaline cores in the pseudopapillae, eosinophilic hyaline globules, vacuolated cells, and foamy cells (13). In our case, above features are inconspicuous, except only a few vacuolated cells. Because of the rarity of extrapancreatic SPNs, a panel of immunohistochemical stain should be made for diagnosis and differential diagnosis. The tumor is usually negative for chromogranin and E-cadherin. A high frequency of positivity for progesterone receptor, α1-antitrypsin, CD10, and CD56 has been reported, however, these markers are not very specific (1). The only distinctive immunohistochemical marker is aberrant nuclear staining for β-catenin, which results from mutation in the β-catenin (CTNNB1) gene (14). Although nuclear staining can occasionally occur in some mimickers (such as some aggressive gastrointestinal or pancreatic neuroendocrine tumors) (15), this aberrant staining can provide a strong support for the diagnosis of SPN based on the histologic features. CD99 was recommended as a useful marker for differentiating SPTs from other tumors by its unique paranuclear dot-like staining pattern (16,17). CD99 is a 32 kD membranocytoplasmic glycoprotein encoded by the MIC2 gene located on the pseudoautosomal regions of both X chromosome and Y chromosome (18). CD99 is characteristically expressed in Ewing’s sarcomas/PNETs and lymphoblastic lymphoma/leukemia as strong membranous staining. CD99 paranuclear dot-like staining strong supports SPT, although this pattern can occur in some colonic adenomas and adenocarcinomas (19). Recently, some researchers found that SOX11 and TFE3 could be an additional diagnostic marker of SPN in differential diagnosis (20-22). All SPNs showed immunoreaction for SOX11 (20,21), and most cases of SPNs were positive for TFE3 (20-22).
Genetically, almost all SPNs show mutations in the β-catenin (CTNNB1) gene (14,23). Because of the weak expression of SOX11 and the aberrant location in our case, DNA sequencing is necessary for the diagnosis. Our case shows a mutation in exon 3 of the β-catenin (CTNNB1) gene, which supports the diagnosis of SPN. The role of β-catenin in SPN tumorigenesis has been confirmed in vivo murine models of pancreas (24). In this model, conditional activation of β-catenin in pancreas tissue can yield tumors with histomorphology identical to SPN in humans. β-catenin (CTNNB1) mutation can affect Wnt signaling pathways as well as self-renewal capability of stem cells, which drives tumorigenesis (25).
In conclusion, we report a rare case of extrapancreatic SPN, and this is the first case of mediastinal SPN. The accurate diagnosis was a challenge for pathologists because of its abnormal location. Extrapancreatic SPN is a tumor with low malignant potential, but some cases can show metastasis and cause death especially in male (26). Complete surgical resection is the preferred treatment for SPN.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.58). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bosman FT, Carneiro F, Hruban RH, et al. WHO classification of tumours of the digestive system. Lyon: International Agency For Research On Cancer, 2010.
- Yu PF, Hu ZH, Wang XB, et al. Solid pseudopapillary tumor of the pancreas: a review of 553 cases in Chinese literature. World J Gastroenterol 2010;16:1209-14. [Crossref] [PubMed]
- Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg 2005;200:965-72. [Crossref] [PubMed]
- Ishikawa O, Ishiguro S, Ohhigashi H, et al. Solid and papillary neoplasm arising from an ectopic pancreas in the mesocolon. Am J Gastroenterol 1990;85:597-601. [PubMed]
- Gurzu S, Bara T, Sincu M, et al. Solid pseudopapillary neoplasm of pancreas: Two case reports. Medicine (Baltimore) 2019;98:e16455. [Crossref] [PubMed]
- Michalova K, Michal M, Sedivcova M, et al. Solid pseudopapillary neoplasm (SPN) of the testis: Comprehensive mutational analysis of 6 testicular and 8 pancreatic SPNs. Ann Diagn Pathol 2018;35:42-7. [Crossref] [PubMed]
- Gahlot GP, Mridha AR, Sable M, et al. Solid pseudopapillary neoplasm of the ovary with metastases to the omentum and regional lymph nodes. Indian J Pathol Microbiol 2016;59:348-50. [Crossref] [PubMed]
- Mao C, Guvendi M, Domenico DR, et al. Papillary cystic and solid tumors of the pancreas: a pancreatic embryonic tumor? Studies of three cases and cumulative review of the world's literature. Surgery 1995;118:821-8. [Crossref] [PubMed]
- Kosmahl M, Seada LS, Janig U, et al. Solid-pseudopapillary tumor of the pancreas: its origin revisited. Virchows Arch 2000;436:473-80. [Crossref] [PubMed]
- Tornoczky T, Kalman E, Jakso P, et al. Solid and papillary epithelial neoplasm arising in heterotopic pancreatic tissue of the mesocolon. J Clin Pathol 2001;54:241-5. [Crossref] [PubMed]
- Zhu H, Xia D, Wang B, et al. Extrapancreatic solid pseudopapillary neoplasm: Report of a case of primary retroperitoneal origin and review of the literature. Oncol Lett 2013;5:1501-4. [Crossref] [PubMed]
- Koh HM, Chang JW, Jeong SY, et al. Ectopic Pancreas Presenting as a Solid Mediastinal Mass. Int J Surg Pathol 2015;23:585-8. [Crossref] [PubMed]
- Cheuk W, Beavon I, Chui DT, et al. Extrapancreatic solid pseudopapillary neoplasm: report of a case of primary ovarian origin and review of the literature. Int J Gynecol Pathol 2011;30:539-43. [Crossref] [PubMed]
- Abraham SC, Klimstra DS, Wilentz RE, et al. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am J Pathol 2002;160:1361-9. [Crossref] [PubMed]
- Hervieu V, Lepinasse F, Gouysse G, et al. Expression of beta-catenin in gastroenteropancreatic endocrine tumours: a study of 229 cases. J Clin Pathol 2006;59:1300-4. [Crossref] [PubMed]
- Guo Y, Yuan F, Deng H, et al. Paranuclear dot-like immunostaining for CD99: a unique staining pattern for diagnosing solid-pseudopapillary neoplasm of the pancreas. Am J Surg Pathol 2011;35:799-806. [Crossref] [PubMed]
- Li L, Li J, Hao C, et al. Immunohistochemical evaluation of solid pseudopapillary tumors of the pancreas: the expression pattern of CD99 is highly unique. Cancer Lett 2011;310:9-14. [Crossref] [PubMed]
- Kovar H, Dworzak M, Strehl S, et al. Overexpression of the pseudoautosomal gene MIC2 in Ewing's sarcoma and peripheral primitive neuroectodermal tumor. Oncogene 1990;5:1067-70. [PubMed]
- Makhoul R, Schwartz AM, Williams R, et al. Paranuclear dot-like immunostaining for CD99: presence in colonic adenomas and adenocarcinomas. Am J Surg Pathol 2011;35:1749-50. [Crossref] [PubMed]
- Foo WC, Harrison G, Zhang X. Immunocytochemistry for SOX-11 and TFE3 as diagnostic markers for solid pseudopapillary neoplasms of the pancreas in FNA biopsies. Cancer Cytopathol 2017;125:831-7. [Crossref] [PubMed]
- Harrison G, Hemmerich A, Guy C, et al. Overexpression of SOX11 and TFE3 in Solid-Pseudopapillary Neoplasms of the Pancreas. Am J Clin Pathol 2017;149:67-75. [Crossref] [PubMed]
- Jiang Y, Xie J, Wang B, et al. TFE3 is a diagnostic marker for solid pseudopapillary neoplasms of the pancreas. Hum Pathol 2018;81:166-75. [Crossref] [PubMed]
- Springer S, Wang Y, Dal Molin M, et al. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology 2015;149:1501-10. [Crossref] [PubMed]
- Heiser PW, Cano DA, Landsman L, et al. Stabilization of beta-catenin induces pancreas tumor formation. Gastroenterology 2008;135:1288-300. [Crossref] [PubMed]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell 2006;127:469-80. [Crossref] [PubMed]
- Wu H, Huang YF, Liu XH, et al. Extrapancreatic solid pseudopapillary neoplasm followed by multiple metastases: Case report. World J Gastrointest Oncol 2017;9:497-501. [Crossref] [PubMed]


