
Histomorphometric comparative analysis between the oral mucosa of fibrous inflammatory hyperplasia and oral leukoplakia
Introduction
The squamous cell carcinoma is considered a public health problem throughout the world, it is the most common malignancy of the oral cavity and in most cases can be preceded by premalignant lesions including oral leukoplakia is the most common whose percentage malignant is 3% to 6% of the cases (1). The etiology of this change is still uncertain which makes it difficult to predict when they will undergo malignant transformation. Recent studies have evaluated the presence of mast cells in the inflammatory infiltrate of precancerous lesions and their participation through the release of pro-angiogenic factors in the tumorigenesis process these lesions via promotion angiogenesis (1-4). Understanding the mechanisms by which a lesion may undergo malignant transformation is important for the development of preventive care clinical strategies. The aim of this study is evaluate the inflammatory process in the oral leukoplakia compared to an inflammatory lesion correlating the inflammatory process with the mast cell population in the affected site and the vascularization of these lesions.
Methods
The study was submitted and approved by the Research Ethics Committee of the Amazonas State University (Registration number 736.008). All information about gender, age and the location of the lesions were obtained from the reports archive of the institution’s Pathology service. The sample consisted of 10 cases of oral leukoplakia (Group 1) and 10 cases of inflammatory fibrous hyperplasia (IFH) (Group 2). The sample selection was stablished following the inclusion/exclusion criteria:
- The reports with information about clinical characteristics, such as age, sex and location of the lesions;
- Biopsy reports with diagnosis of oral leukoplakia and IFH without association with other lesions;
- The cases diagnosed as oral leukoplakia should not be related to trauma at the biopsy site on the clinical description of the lesion;
- Cases diagnosed as IFH should be related to trauma (occlusal, parafunctional or unsatisfactory total prosthesis) on the clinical description of the lesion;
- Paraffin blocks with sufficient material for analysis.
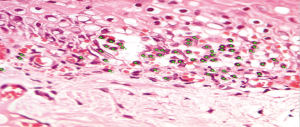
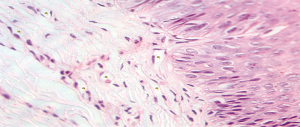
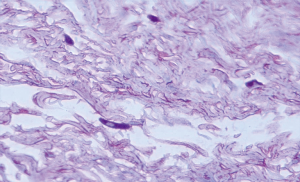
The selected samples were sectioned in 3 µm tissue sections, and slides were obtained and stained with toluidine blue and hematoxylin and eosin (HE). The inflammatory infiltrate, was quantified in HE stained sections under 100× magnification in 3 different focal fields per specimen. The inflammatory infiltrate was evaluated semiquantitatively and was considered strong (greater than 60 cells/field), moderate (30 to 60 cells/field), mild (less than 30 cells/field) or absent (5) as represented in Figure 1. The blood vessel count was performed quantitatively under 400× magnification as seen in Figure 2. For mast cell, identification and quantification the slides were stained with toluidine blue (0.1%) as represented in Figure 3. The mast cell and blood vessel count was performed with 400× magnification. A total of 10 fields per specimen were captured for mast cell and blood vessel quantification. The microscope used in the evaluation of the sample was Axio Lab A1 (Oberkochen, Germany) and the images captured with a Zeiss Axiocam 503 color camera (Oberkochen, Germany). Image analysis was performed using Image Java software (version 1.48v), with the Cell Counter plugin, on a 100% image zoom. A final consensus among examiners was made after independent evaluation.
The ANOVA test was applied for the variance between the groups using the statistical package Statistical Package for the Social Sciences (SPSS) 12.0. A P value of 0.05 was considered for statistical significance.
Results
Out of the 10 patients whose Oral leukoplakia samples were analyzed, 70% of the sample were male with a mean age of 51 years (Figure 4). The mean age of patients with IFH lesions was 50 years, with a female predilection (90%). The preferred localization of cases of oral leukoplakia was buccal mucosa (60%), tongue (20%) and lower lip (20%). Regarding IFH, it was found buccal mucosa (80%), tongue (10%) and lip (10%) (Table 1). The ANOVA test was used to compare the concentration of mast cells and blood vessels between the groups and showed a statistically significant difference (P<0.06). A higher concentration of mast cells per field was observed in IFH lesions when compared to oral leukoplakia. The blood vessels count was higher in oral leukoplakia cases, as shown in Table 2. Table 3 represents the presence and intensity of dysplasia found in the evaluated sample with oral leukoplakia. None of the cases showed severe dysplasia. A representation of one microscopic field with mast cells stained with toluene blue is available in Figure 3. Tables 4 and 5 present the means of mast cells and blood vessels per field in the evaluated sample.
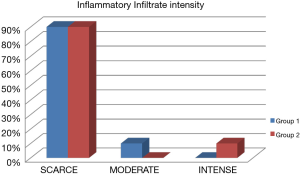
Table 1
| Group | Age in years | Average age | Gender | Location |
|---|---|---|---|---|
| 1 | 33–69 | 51 | Males (70%) | Buccal mucosa (60%); tongue (20%); lower lip mucosa (20%) |
| 2 | 14–65 | 50 | Females (90%) | Buccal mucosa (80%); tongue (10%); lip (10%) |
Table 2
| Variables | Group 1 | Group 2 | F | ANOVA |
|---|---|---|---|---|
| Mast cells | 2.67 | P<0.06 | ||
| Mean | 3.48 | 3.99 | ||
| SD | 2.75 | 2.68 | ||
| Blood vessels | 2.67 | |||
| Mean | 1.83 | 1.65 | ||
| SD | 1.74 | 1.69 |
Table 3
| Case | Dysplasia presence | Dysplasia grade | |
|---|---|---|---|
| Yes | No | ||
| I | X | ||
| II | X | ||
| III | X | Moderate | |
| IV | X | Moderate | |
| V | X | Mild | |
| VI | X | Mild | |
| VII | X | Mild | |
| VIII | X | Moderate | |
| IX | X | Mild | |
| X | X | Mild | |
Table 4
| Case | Mean per field | Dysplasia grade | |
|---|---|---|---|
| Mast cell | Blood vessels | ||
| I | 2.6 | 1.5 | – |
| II | 4.0 | 0.9 | – |
| III | 4.7 | 0.8 | Moderate |
| IV | 1.6 | 0.5 | Moderate |
| V | 2.5 | 1.1 | Mild |
| VI | 4.9 | 3.0 | Mild |
| VII | 2.8 | 3.2 | Mild |
| VIII | 3.9 | 1.9 | Moderate |
| IX | 3.6 | 2.1 | Mild |
| X | 4.2 | 3.3 | Mild |
Table 5
| Case | Mean per field | |
|---|---|---|
| Mast cell | Blood vessels | |
| I | 3.5 | 0.9 |
| II | 6.3 | 0.7 |
| III | 5.9 | 1.6 |
| IV | 4.2 | 2.8 |
| V | 5.3 | 2.9 |
| VI | 2.7 | 1.3 |
| VII | 5.5 | 0.4 |
| VIII | 1.6 | 0.7 |
| IX | 1.8 | 4.3 |
| X | 2.8 | 0.9 |
Discussion
Mast cells are derived from hematopoietic precursor cells, CD4+ cells from bone marrow, and they are widely distributed among tissues especially near blood vessels, nerves and subepithelial areas (6). It was first described for their role in the early, acute phase reactions of hypersensitivity (7). It can be activated by immunological and non-immunological stimuli, playing a critical role in the development of inflammation in early vaso-inductive event and the transition of acute inflammation to chronic inflammation (8). Once activated, these cells secrete vasoactive mediators and pro-inflammatory drugs such as histamine, serotonin, proteases (tryptase, chymase), cytokines (TNF, TNF-β, IL-3, IL-4, IL-5), chemokines, and factors endothelial growth factor (VGEF) (9). Under light microscopy the presence of granules in these cells give the characteristic metacromasia, color pattern with Toluidine Blue.
Studies have suggested that mast cells are important cells in the development of inflammatory diseases, releasing pro-inflammatory factors without degranulation, unlike in allergic and anaphylactic reactions in which large numbers of degranulated mast cells are observed (10).
Sudhakar et al. [2005] (11) have observed a lower concentration of mast cells in inflammatory lesions, but in cases of IFH it was noted an increase in mast cell number. The vascularization in most inflammatory lesions was more intense, suggesting that the continued presence of mast cells in IFH probably indicates the continuity of the inflammatory phenomenon. Once the inflammation and vascularization are established, the role of mast cells decreases.
In contrast, our research noticed in IFH lesions a higher presence of mast cells, sparse vascularization and inflammatory response relatively more intense compared to Oral leukoplakia lesions. The role of mast cells in neoplastic processes has also been suggested. It is believed that mast cells contribute to transformation and progression of these malignancies via angiogenesis. These cells showed a prominent role in the induction of angiogenesis of some tumors including oral tumors, esophageal, uterine cervix, colorectal and larynx (12).
The association of mast cells in the lesions with carcinogenic potential such as Oral leukoplakia has been the subject of many investigations. The present study aimed to evaluate the concentration of mast cells, blood vessels and correlate with the inflammatory infiltrate in Oral leukoplakia lesions comparing with IFH, chronic traumatic injury without a carcinogenic nature.
The series of oral leukoplakia lesions analyzed were mean age of 51 years with a higher prevalence in males (70%) in line with Pujari et al. [2013] (13). Oliveira-Neto et al. [2007] (14) however, observed a mean age of 49.9 years with prevalence of females (63.6%). The distribution of oral leukoplakia according to the level of dysplasia showed more cases of mild dysplasia and only 20% of cases without dysplasia, as Pujari et al. [2013] (13) showed in their survey 56% of cases with hyperkeratosis and 33% mild dysplasia.
Coussens et al. [1999] (15) while investigating the regulatory mechanisms of angiogenesis in mice, observed that hyperplastic areas in carcinomas induced by chymase produced by mast cells showed a modest increase in density and dilation of blood capillaries. Dysplastic areas of pre-malignant lesions contained a higher density of dilated capillaries in a region close to the basement membrane, infiltration of mast cells and activation of matrix metalloproteinases together with angiogenic transformation. It was suggested that neoplastic progression in this model involves the operation of an inflammatory response to tissue abnormality. Therefore the angiogenesis regulation during squamous epithelium carcinogenesis would be biphasic. Mast cells are recruited to rearrange the architecture of the stroma and angiogenesis is activated excessively. The tumor core would be self-sufficient and sustain neovascularization. This may explain the results of Michailidou et al. [2008] (3), who observed a direct relationship between concentration of mast cells and vasculature in normal oral mucosa, leukoplakia without dysplasia, leukoplakia with dysplasia and squamous cell carcinoma. It was observed a progressive increase in mast cell concentrations from normal oral mucosa comparing to OL with dysplasia and carcinoma.
Similar results were observed by Telagi et al. [2015] (16), and Sathyakumar et al. [2012] (17), the first, evaluated normal buccal mucosa tissue, submucosal fibrosis, oral epithelial dysplasia and squamous cell carcinoma. A higher concentration of mast cells in the studied lesions with emphasis on lesions with epithelial dysplasia and squamous cell carcinoma. The presence of atypical and degranulated mast cells was found in cases with the presence of moderate and intense inflammatory infiltrate. Pereira and Pinheiro [2019] (18), in a comparative study between potentially malignant lesions and oral squamous cell carcinoma, found that mast cell density was lower in oral leukoplakia when compared to lesions of (OSCC), which in turn presented a significant increase in mast cells and microvessels suggesting tumor progression and aggression through the positive regulation of angiogenesis by these cells. Sathyakumar et al. [2012] (17), reported the highest concentration of mast cells and blood vessels in oral leukoplakia lesions when compared to the normal oral mucosa. In contrast, Pujari et al. [2013] (13) found higher number of vessels in normal oral mucosa when compared to oral leukoplakia lesions.
The reduced number of mast cells in the lesions of Oral Leukoplakia found in our study may be related to the degree of dysplasia, which were predominantly mild. Oliveira-Neto et al. [2007] (14) noted a small number of mast cells in the lesions of Oral Leukoplakia and Squamous Cell Carcinoma. However, according to these authors, the decrease in the number of mast cells in oral leukoplakia could be due to the chronic presence of chemical carcinogens originating from tobacco, which together with alcohol habits are the main factor associated with the development of these lesions and squamous cell carcinoma.
It has thus been observed in our study most significant inflammatory response research and reduced vascularization in IFH lesions, whereas in oral leukoplakia lesions was found higher concentration of blood vessels and small number of mast cells which may represent a change in the microenvironment probably related to the etiology of these lesions.
Conclusions
Although most studies have shown a direct relationship between the presence of mast cells, vascularization and inflammation in premalignant lesions and oral squamous cell carcinoma, this study revealed an inverse relationship between the lower concentration of mast cells and increased vascularization in oral leukoplakia lesions. IFH showed a more intense inflammatory response, with a lower blood vessel count. The non-congruence of our results with other studies may be related to the sample size, degree of dysplasia in the oral leukoplakia lesions and differences in the applied methods. Further studies covering molecular events and including other lesions such as squamous cell carcinoma could lead to a better understanding of the process.
Acknowledgments
Funding: This work was supported by
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Shankargouda Patil, Sachin C. Sarode and Kamran Awan) for the series “Oral Pre-cancer and Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.12.23). The series “Oral Pre-cancer and Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was submitted and approved by the Research Ethics Committee of the Amazonas State University (Registration number 736.008). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wright JOC. Premalignancy, Oral cancer: clinical and pathological considerations. CRC Press Inc., 1988:45-8.
- Crivellato E, Nico B, Ribatti D. Mast cells and tumours angiogenesis: new insight from experimental carcinogenesis. Cancer Lett 2008;269:1-6. [Crossref] [PubMed]
- Michailidou EZ, Markopoulos AK, Antoniades DZ. Mast cells and angiogenesis in oral malignant and premalignant lesions. Open Dent J 2008;2:126-32. [Crossref] [PubMed]
- Nooshin M, Shahab B, Jahanshah SN, et al. Mast cell density and angiogenesis in oral dysplastic ephitelium and low-and high-grade oral squamous cell carcinoma. Act odont Scand 2010;68:300-4.
- Manzano AC, Altemani A, Martins AS, et al. Caracterizaçao imuno-histoquímica do infiltrado linfocitário em biópsia de carcinoma espinocelular da língua e soalho oral e sua implicaçao prognostica. Rev Bras Cir Cabeça Pescoço 2010;39:270-6.
- De Souza RO. Densidade de mastócitos e microdensidade vascular em displasias epiteliais e carcinomas orais. Fundação Oswaldo Cruz 2012.
- Da Silva EM, Jamus MC, Constance O. Mast cell function: a new vision of an old cell. J histochem cythochem 2014;62:698-738.
- Walsh LJ. Mast cells and oral inflammation. Crit Rev Oral Biol Med 2003;14:188-98. [Crossref] [PubMed]
- Brown JM, Wilson TM, Metcalfe DD. The mast cell and allergic diseases: role in pathogenesis and implication for therapy. Clin Exp Allergy 2008;38:4-18. [PubMed]
- Theoharides TC, Alysandratos KD, Angelidou A, et al. Mast cells and inflammation. Biochim biophys acta 2012;1822:21-33. [Crossref] [PubMed]
- Sudhakar R, Ramesh V, Balamurali PD, et al. Incidence of mast cells in oral inflammatory lesions: A pilot study. J Oral Maxillofac Pathol 2005;9:12-5. [Crossref]
- Benítez-Bribiesca L, Wong A, Utrera D, et al. The role of mast cell tryptase in neoangiogenesis of premalignant and malignant lesions of the uterine cervix. J Histochem Cytochem 2001;49:1061-2. [Crossref] [PubMed]
- Pujari RKV, Vanaki SS, Puranik RS, et al. Histomorphometric analysis of vascularity in normal buccal mucosa, leukoplakia and squamous cell carcinoma of buccal mucosa. J Oral Maxillofac Pathol 2013;17:334-9. [Crossref] [PubMed]
- Oliveira-Neto HH, Leite AF, Costa NL, et al. Decrease in mast cells in oral squamous cell carcinoma: Possible failure in the migration of these cells. Oral Oncol 2007;43:484-90. [Crossref] [PubMed]
- Coussens LM, Raymond WW, Bergers G, et al. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev 1999;13:1382-97. [Crossref] [PubMed]
- Telagi N, Ahmed Mujib BR, Kulkarni PG, et al. The master switch: Comparative study of mast cell in oral epithelial dysplasia, oral submucous fibrosis and oral squamous cells carcinoma and their association with inflammation and angiogenesis. J Oral Maxillofac Pathol 2015;19:25-9. [Crossref] [PubMed]
- Sathyakumar M, Sriram G, Saraswathi TR, et al. Immunohistochemical evaluation of mast cells and vascular endothelial proliferation in oral precancerous lesion-leukoplakia. J Oral Maxillofac Pathol 2012;16:343-8. [Crossref] [PubMed]
- Pereira NDS, Pinheiro NT. Histomorphometric comparative analysis between oral dysplastic potentially malignant disorder and oral squamous cell carcinoma. Eur J Dent 2019;13:1-4. [Crossref] [PubMed]


