
Long-term survival of an unresectable upper thoracic esophageal squamous cell carcinoma with severe dysphagia following nasogastric tube feeding and camrelizumab-containing therapy: a case report
Introduction
The incidence of esophageal cancer ranks seventh in the world (1). Esophageal squamous cell carcinoma is the predominant subtype in China (2). For upper thoracic ESCC, surgery is difficult and risky. Therefore, chemoradiotherapy, radiotherapy, and chemotherapy have been frequently performed in upper thoracic ESCC. However, the overall prognosis is poor (3,4). Therefore, exploring other effective and safe treatment is of great importance. Currently, the efficacy and safety of camrelizumab combined with chemotherapy or monotherapy with camrelizumab in ESCC are under investigation. In this case, comprehensive therapy regimens involved with nasogastric tube feeding and camrelizumab-containing therapy are first reported in ESCC with dysphagia, and clinical effect is remarkable. We present the following case in accordance with the CARE Guideline.
Case presentation
In May 2017, a 66-year-old man was admitted to a local hospital with severe dysphagia. The patient had no family history of genetic disease and was not treated at other hospital. Physical examination showed the patient’s Eastern Cooperative Oncology Group score was one. The dysphagia score was four. The gastroscope examination revealed an irregular bulge lesion 22 cm away from the incisor (Figure 1). Biopsy showed esophageal squamous cell carcinoma. No metastases were found on the chest and abdomen computed tomography (CT). He was diagnosed as upper thoracic ESCC.
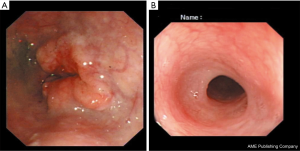
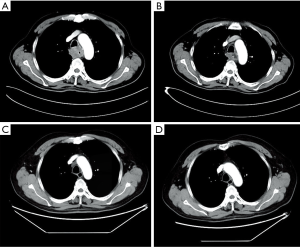
Considering the high risk of surgery, the patient received nasogastric tube placement and chemotherapy involving the administration of docetaxel (120 mg, day 1), nedaplatin (60 mg, days 2–3). After 3 cycles of chemotherapy, the lesion did not shrink obviously on chest enhanced CT (Figure 2A). In view of the good objective response rate (ORR) of camrelizumab in the phase 1 trial, the patient agreed to receive immunotherapy with camrelizumab. After 1 cycle of monotherapy with camrelizumab, the lesion shrank slightly based on chest CT (Figure 2B). Therefore, another 2 cycles of monotherapy with camrelizumab were administrated. To further improve the efficacy, the patient received 8 cycles of combination therapy with camrelizumab (200 mg day 1) and docetaxel (120 mg day 2) from November 2017. In January 2018, the nasogastric tube was removed. The patient can take some semi-liquid food. Then, 6 cycles of monotherapy with docetaxel as maintenance therapy were administrated. On August 17, 2018, there was no abnormal lesion found in the esophagus according to chest CT (Figure 2C). Dysphagia score improved to 1 score. On April 22, 2019, complete response was still observed on chest CT (Figure 2D). On November 14, 2019, the gastroscope examination indicated smooth mucosa of the esophagus (Figure 1B). Temporary grade II adverse event of reactive capillary hemangioma was observed during the treatment. By November 14, 2019, the PFS exceeded 28 months, and the patient is still being followed up. The treatment processes of the patient were shown in Figure 3.
Discussion
Camrelizumab is a PD-1 antibody developed by Jiangsu Hengrui Medicine Co. Ltd. It is approved in China for the treatment of recurrent or refractory classical Hodgkin's lymphoma. However, the anticancer activity of camrelizumab in esophageal cancer is under investigation. In the phase 1 clinical trial, 30 patients of advanced ESCC received monotherapy with camrelizumab. The ORR was 33.3%, and the median PFS was 3.6 months (5). In this case, camrelizumab-containing therapy achieved long-term PFS.
The incidence of reactive capillary hemangioma induced by camrelizumab is high. In the trials of ESCC and nasopharyngeal carcinoma, the incidence of reactive capillary hemangioma was 76.7% and 88%, respectively (5,6). However, the mechanism of this phenomenon is not clear. Some researchers found camrelizumab promotes hemangioma by activating vascular endothelial growth 2 (7). Based on the above research, camrelizumab combined with vascular endothelial growth 2 inhibitor may improve the phenomenon.
Dysphagia is the common symptom of advanced esophageal cancer and can result in malnutrition. Traditionally, the main methods to treat dysphagia caused by esophageal cancer include stent placement, dilation, and gastrostomy. Stent placement can immediately relieve the symptom, but stent-related complications also affect the quality of life, including chest pain, migration, recurrent dysphagia, hemorrhage, perforation, pneumonia (8). Esophageal dilation can also alleviate dysphagia, but perforation caused by dilation is life-threatening (9). Percutaneous endoscopic gastrostomy is a choice to fulfill nutritional requirements in patients with esophageal cancer. However, gastrostomy has risks of site infection, tumor seeding, anastomotic stricture, and anastomotic leak (10). Nasogastric tube feeding can also provide patient for essential nutrients. Importantly, nasogastric tube placement had fewer risks compared with stent placement, dilation, and gastrostomy.
In this paper, temporary nasogastric tube feeding provided the patient for essential nutrients and won the time for immunotherapy to work. Camrelizumab-containing therapy achieved complete response with long-term survival in upper thoracic ESCC patients. However, there are some limitations of this case. Firstly, the patient did not detect the expression of programmed cell death-ligand 1, which is considered as potential predictors of immunotherapy. Secondly, the patient received monotherapy with camrelizumab and combination therapy with camrelizumab and docetaxel, successively. So it is difficult to compare the efficacy between monotherapy with combination therapy. We expect more clinical trials to explore the efficacy of camrelizumab in upper thoracic ESCC patients in the future.
Conclusion
Comprehensive therapy regimens involved with nasogastric tube feeding and camrelizumab-containing therapy are effective and safe in upper thoracic ESCC patients with severe dysphagia.
Acknowledgments
The authors appreciate the patient and his family for their agreement to the publication of the report.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Lin Y, Totsuka Y, He Y, et al. Epidemiology of esophageal cancer in Japan and China. J Epidemiol 2013;23:233-42. [Crossref] [PubMed]
- Tu L, Sun L, Xu Y, et al. Paclitaxel and cisplatin combined with intensity-modulated radiotherapy for upper esophageal carcinoma. Radiat Oncol 2013;8:75. [Crossref] [PubMed]
- Zhang J, Zhang W, Zhang B, et al. Clinical results of intensity-modulated radiotherapy for 250 patients with cervical and upper thoracic esophageal carcinoma. Cancer Manag Res 2019;11:8285-94. [Crossref] [PubMed]
- Huang J, Xu B, Mo H, et al. Safety, Activity, and Biomarkers of SHR-1210, an Anti-PD-1 Antibody, for Patients with Advanced Esophageal Carcinoma. Clin Cancer Res 2018;24:1296-304. [Crossref] [PubMed]
- Fang W, Yang Y, Ma Y, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol 2018;19:1338-50. [Crossref] [PubMed]
- Finlay WJJ, Coleman JE, Edwards JS, et al. Anti-PD1 'SHR-1210' aberrantly targets pro-angiogenic receptors and this polyspecificity can be ablated by paratope refinement. MAbs 2019;11:26-44. [Crossref] [PubMed]
- Gao F, Xu YL, Liu YJ, et al. Outcomes of self-expandable metal stent placement for malignant oesophageal strictures. Clin Radiol 2020;75:156.e21-156.e27. [Crossref] [PubMed]
- Grooteman KV, Wong Kee Song LM, Vleggaar FP, et al. Non-adherence to the rule of 3 does not increase the risk of adverse events in esophageal dilation. Gastrointest Endosc 2017;85:332-7.e1. [Crossref] [PubMed]
- Siddique MZ, Mehmood S, Ismail M, et al. Pre-operative percutaneous endoscopic gastrostomy tube placement does not increase post-operative complications or mortality in oesophageal cancer. J Gastrointest Oncol 2019;10:492-8. [Crossref] [PubMed]



